Abstract
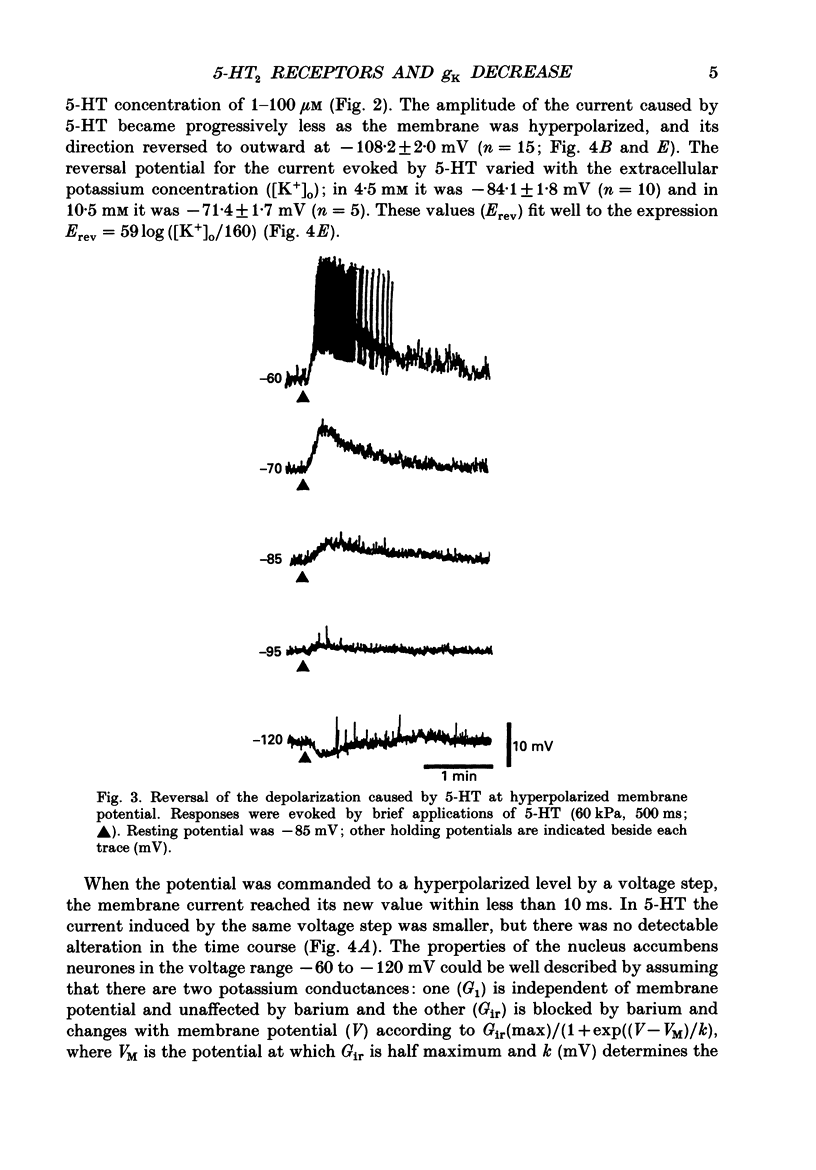
1. Intracellular recordings were made from neurones in the nucleus accumbens in slices from the rat brain maintained in vitro. 2. 5-Hydroxytryptamine (5-HT.1-100 microM) depolarized 170 of 203 (84%) neurones and caused them to discharge action potentials. The depolarization was associated with an increase in the input resistance, and was reversed in polarity by conditioning hyperpolarization; this reversal potential was linearly related to the logarithm of the extracellular potassium concentration. 3. Application of 5-HT to neurones voltage-clamped near their resting potential (typically about -80 mV) caused an inward current and a decrease in the slope conductance. The current caused by 5-HT reversed polarity at the potassium equilibrium potential. Analysis with an equivalent circuit model of the neurone at steady state indicated that 5-HT selectively reduced the inward rectifier potassium conductance. 4. The depolarization caused by 5-HT persisted in tetrodotoxin (1 microM). It was reduced but not abolished by a solution that contained lower levels of calcium (0.24 instead of 2.4 mM), higher levels of magnesium (5 instead of 1.2 mM), and cobalt (2 mM). 5. The depolarization caused by 5-HT was competitively antagonized by the 5-HT2 antagonists ketanserin and mianserin with dissociation equilibrium constants of 3 and 45 nM respectively: spiperone (300 nM) also blocked the action of 5-HT. The depolarization was not mimicked or blocked by a number of other agonists and antagonists selective for the 5-HT1 and 5-HT3 receptor types.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanian G. K., Lakoski J. M. Hyperpolarization of serotonergic neurons by serotonin and LSD: studies in brain slices showing increased K+ conductance. Brain Res. 1984 Jul 2;305(1):181–185. doi: 10.1016/0006-8993(84)91137-5. [DOI] [PubMed] [Google Scholar]
- Akasu T., Hasuo H., Tokimasa T. Activation of 5-HT3 receptor subtypes causes rapid excitation of rabbit parasympathetic neurones. Br J Pharmacol. 1987 Jul;91(3):453–455. doi: 10.1111/j.1476-5381.1987.tb11236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade R., Malenka R. C., Nicoll R. A. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986 Dec 5;234(4781):1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- Andrade R., Nicoll R. A. Pharmacologically distinct actions of serotonin on single pyramidal neurones of the rat hippocampus recorded in vitro. J Physiol. 1987 Dec;394:99–124. doi: 10.1113/jphysiol.1987.sp016862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobillier P., Seguin S., Petitjean F., Salvert D., Touret M., Jouvet M. The raphe nuclei of the cat brain stem: a topographical atlas of their efferent projections as revealed by autoradiography. Brain Res. 1976 Sep 3;113(3):449–486. doi: 10.1016/0006-8993(76)90050-0. [DOI] [PubMed] [Google Scholar]
- Bradley P. B., Engel G., Feniuk W., Fozard J. R., Humphrey P. P., Middlemiss D. N., Mylecharane E. J., Richardson B. P., Saxena P. R. Proposals for the classification and nomenclature of functional receptors for 5-hydroxytryptamine. Neuropharmacology. 1986 Jun;25(6):563–576. doi: 10.1016/0028-3908(86)90207-8. [DOI] [PubMed] [Google Scholar]
- Carter C. J., Pycock C. J. A study of the sites of interaction between dopamine and 5-hydroxytryptamine for the production of fluphenazine-induced catalepsy. Naunyn Schmiedebergs Arch Pharmacol. 1978 Sep;304(2):135–139. doi: 10.1007/BF00495549. [DOI] [PubMed] [Google Scholar]
- Colino A., Halliwell J. V. Differential modulation of three separate K-conductances in hippocampal CA1 neurons by serotonin. Nature. 1987 Jul 2;328(6125):73–77. doi: 10.1038/328073a0. [DOI] [PubMed] [Google Scholar]
- Conn P. J., Sanders-Bush E. Regulation of serotonin-stimulated phosphoinositide hydrolysis: relation to the serotonin 5-HT-2 binding site. J Neurosci. 1986 Dec;6(12):3669–3675. doi: 10.1523/JNEUROSCI.06-12-03669.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costall B., Fortune D. H., Naylor R. J., Mardsen C. D., Pycock C. Serotonergic involvement with neuroleptic catalepsy. Neuropharmacology. 1975 Nov;14(11):859–868. doi: 10.1016/0028-3908(75)90114-8. [DOI] [PubMed] [Google Scholar]
- Davies M. F., Deisz R. A., Prince D. A., Peroutka S. J. Two distinct effects of 5-hydroxytryptamine on single cortical neurons. Brain Res. 1987 Oct 13;423(1-2):347–352. doi: 10.1016/0006-8993(87)90861-4. [DOI] [PubMed] [Google Scholar]
- Galligan J. J., Surprenant A., Tonini M., North R. A. Differential localization of 5-HT1 receptors on myenteric and submucosal neurons. Am J Physiol. 1988 Nov;255(5 Pt 1):G603–G611. doi: 10.1152/ajpgi.1988.255.5.G603. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984 Oct 4;311(5985):461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- Higashi H., Nishi S. 5-Hydroxytryptamine receptors of visceral primary afferent neurones on rabbit nodose ganglia. J Physiol. 1982 Feb;323:543–567. doi: 10.1113/jphysiol.1982.sp014091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland S. J., Jordan C. C. Pharmacological characterization of 5-hydroxytryptamine-induced hyperpolarization of the rat superior cervical ganglion. Br J Pharmacol. 1987 Oct;92(2):417–427. doi: 10.1111/j.1476-5381.1987.tb11338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson E. A., Kelly P. H., Schultz L. Effects of serotonergic activity in nucleus accumbens septi on drug-induced circling. Neuropharmacology. 1985 Aug;24(8):721–727. doi: 10.1016/0028-3908(85)90005-x. [DOI] [PubMed] [Google Scholar]
- Johnson S. M., Katayama Y., North R. A. Multiple actions of 5-hydroxytryptamine on myenteric neurones of the guinea-pig ileum. J Physiol. 1980 Jul;304:459–470. doi: 10.1113/jphysiol.1980.sp013336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M., Shinnick-Gallagher P., Gallagher J. P. Effect of serotonin and serotonin analogues on passive membrane properties of lateral septal neurons in vitro. Brain Res. 1987 Aug 4;417(1):99–107. doi: 10.1016/0006-8993(87)90183-1. [DOI] [PubMed] [Google Scholar]
- Kilpatrick G. J., Jones B. J., Tyers M. B. Identification and distribution of 5-HT3 receptors in rat brain using radioligand binding. Nature. 1987 Dec 24;330(6150):746–748. doi: 10.1038/330746a0. [DOI] [PubMed] [Google Scholar]
- Kiraly M., Ma R. C., Dun N. J. Serotonin mediates a slow excitatory potential in mammalian celiac ganglia. Brain Res. 1983 Sep 26;275(2):378–383. doi: 10.1016/0006-8993(83)91002-8. [DOI] [PubMed] [Google Scholar]
- Mawe G. M., Branchek T. A., Gershon M. D. Peripheral neural serotonin receptors: identification and characterization with specific antagonists and agonists. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9799–9803. doi: 10.1073/pnas.83.24.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. J., Richardson T. L., Fibiger H. C., McLennan H. Anatomical and electrophysiological identification of a projection from the mesencephalic raphe to the caudate-putamen in the rat. Brain Res. 1975 Oct 24;97(1):133–136. doi: 10.1016/0006-8993(75)90920-8. [DOI] [PubMed] [Google Scholar]
- Mogenson G. J., Jones D. L., Yim C. Y. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14(2-3):69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Parent A., Descarries L., Beaudet A. Organization of ascending serotonin systems in the adult rat brain. A radioautographic study after intraventricular administration of [3H]5-hydroxytryptamine. Neuroscience. 1981;6(2):115–138. doi: 10.1016/0306-4522(81)90050-6. [DOI] [PubMed] [Google Scholar]
- Pijnenburg A. J., Honig W. M., Van der Heyden J. A., Van Rossum J. M. Effects of chemical stimulation of the mesolimbic dopamine system upon locomotor activity. Eur J Pharmacol. 1976 Jan;35(1):45–58. doi: 10.1016/0014-2999(76)90299-5. [DOI] [PubMed] [Google Scholar]
- Pycock C. J. Turning behaviour in animals. Neuroscience. 1980;5(3):461–514. doi: 10.1016/0306-4522(80)90048-2. [DOI] [PubMed] [Google Scholar]
- Saavedra J. M., Brownstein M., Palkovits M. Serotonin distribution in the limbic system of the rat. Brain Res. 1974 Oct 25;79(3):437–441. doi: 10.1016/0006-8993(74)90441-7. [DOI] [PubMed] [Google Scholar]
- Segal M. The action of serotonin in the rat hippocampal slice preparation. J Physiol. 1980 Jun;303:423–439. doi: 10.1113/jphysiol.1980.sp013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. J Physiol. 1978 Jul;280:169–191. doi: 10.1113/jphysiol.1978.sp012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R., Nakajima Y., Yamaguchi K. Substance P raises neuronal membrane excitability by reducing inward rectification. Nature. 1985 Jun 6;315(6019):498–501. doi: 10.1038/315498a0. [DOI] [PubMed] [Google Scholar]
- Steinbusch H. W. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6(4):557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Surprenant A., Crist J. Electrophysiological characterization of functionally distinct 5-hydroxytryptamine receptors on guinea-pig submucous plexus. Neuroscience. 1988 Jan;24(1):283–295. doi: 10.1016/0306-4522(88)90331-4. [DOI] [PubMed] [Google Scholar]
- Uchimura N., Higashi H., Nishi S. Hyperpolarizing and depolarizing actions of dopamine via D-1 and D-2 receptors on nucleus accumbens neurons. Brain Res. 1986 Jun 11;375(2):368–372. doi: 10.1016/0006-8993(86)90760-2. [DOI] [PubMed] [Google Scholar]
- Vandermaelen C. P., Aghajanian G. K. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat brain slices. Brain Res. 1983 Dec 19;289(1-2):109–119. doi: 10.1016/0006-8993(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Vandermaelen C. P., Aghajanian G. K. Intracellular studies on the effects of systemic administration of serotonin agonists on rat facial motoneurons. Eur J Pharmacol. 1982 Feb 26;78(2):233–236. doi: 10.1016/0014-2999(82)90242-4. [DOI] [PubMed] [Google Scholar]
- Wallis D. I., North R. A. The action of 5-hydroxytryptamine on single neurones of the rabbit superior cervical ganglion. Neuropharmacology. 1978 Dec;17(12):1023–1028. doi: 10.1016/0028-3908(78)90028-x. [DOI] [PubMed] [Google Scholar]
- Williams J. T., Colmers W. F., Pan Z. Z. Voltage- and ligand-activated inwardly rectifying currents in dorsal raphe neurons in vitro. J Neurosci. 1988 Sep;8(9):3499–3506. doi: 10.1523/JNEUROSCI.08-09-03499.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. T., North R. A., Tokimasa T. Inward rectification of resting and opiate-activated potassium currents in rat locus coeruleus neurons. J Neurosci. 1988 Nov;8(11):4299–4306. doi: 10.1523/JNEUROSCI.08-11-04299.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. D., Mayer C. J. Serotonergic activation of tonic-type enteric neurons in guinea pig small bowel. J Neurophysiol. 1979 Mar;42(2):582–593. doi: 10.1152/jn.1979.42.2.582. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Higashi H. 5-Hydroxytryptamine mediates inhibitory postsynaptic potentials in rat dorsal raphe neurons. Neurosci Lett. 1985 Jan 7;53(1):69–74. doi: 10.1016/0304-3940(85)90099-0. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Nishi S. Intracellular recordings from lateral horn cells fo the spinal cord in vitro. J Auton Nerv Syst. 1982 Jul;6(1):5–11. doi: 10.1016/0165-1838(82)90017-0. [DOI] [PubMed] [Google Scholar]


