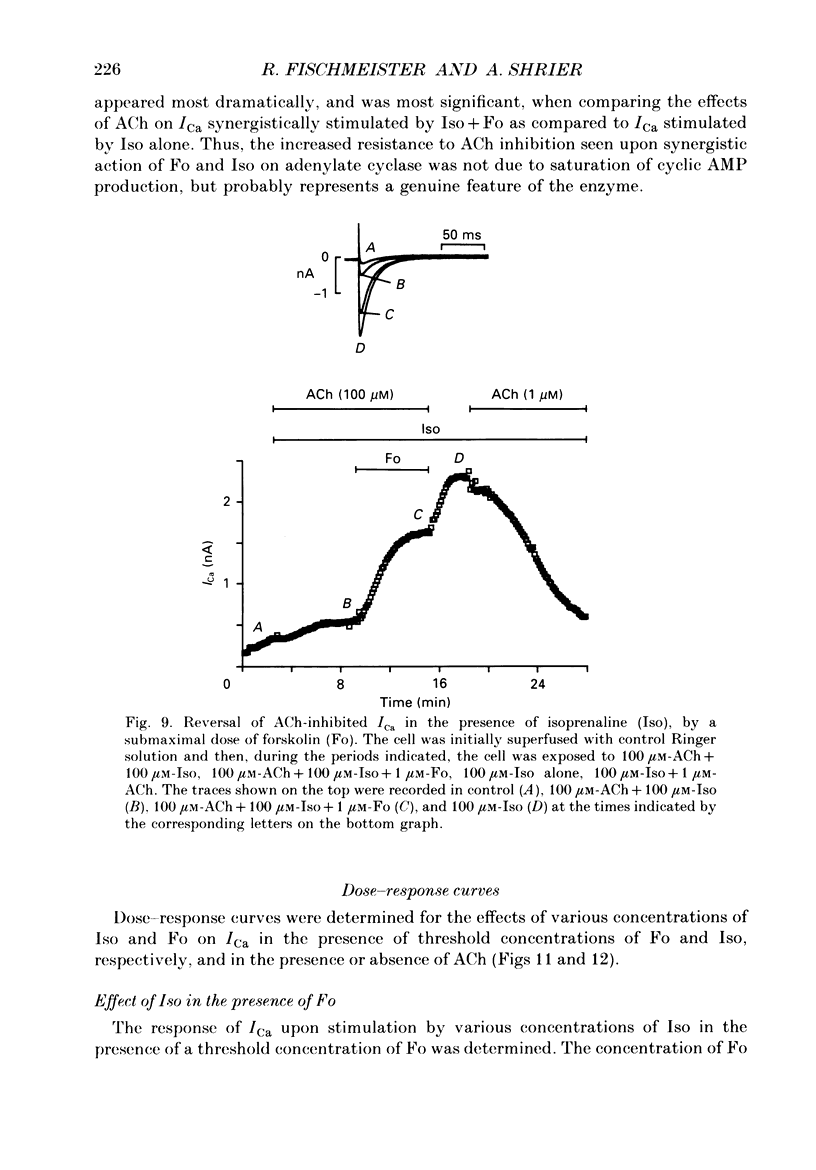
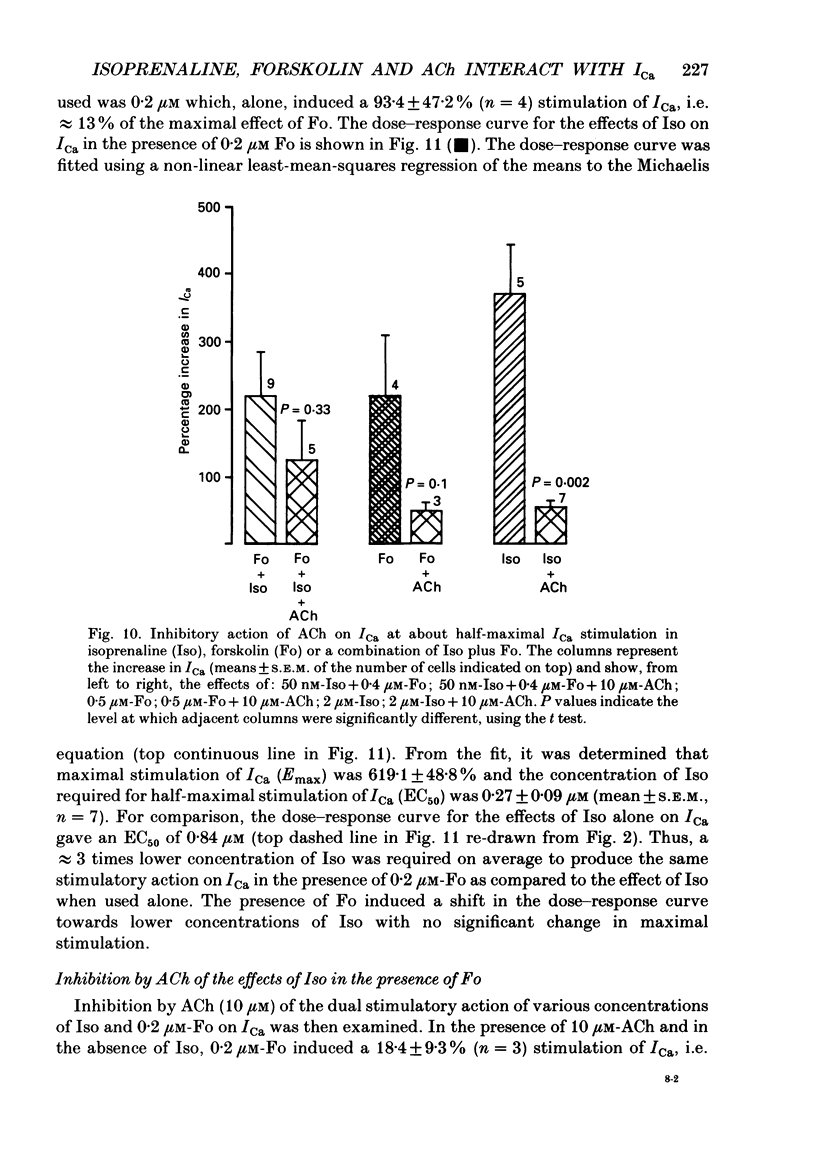
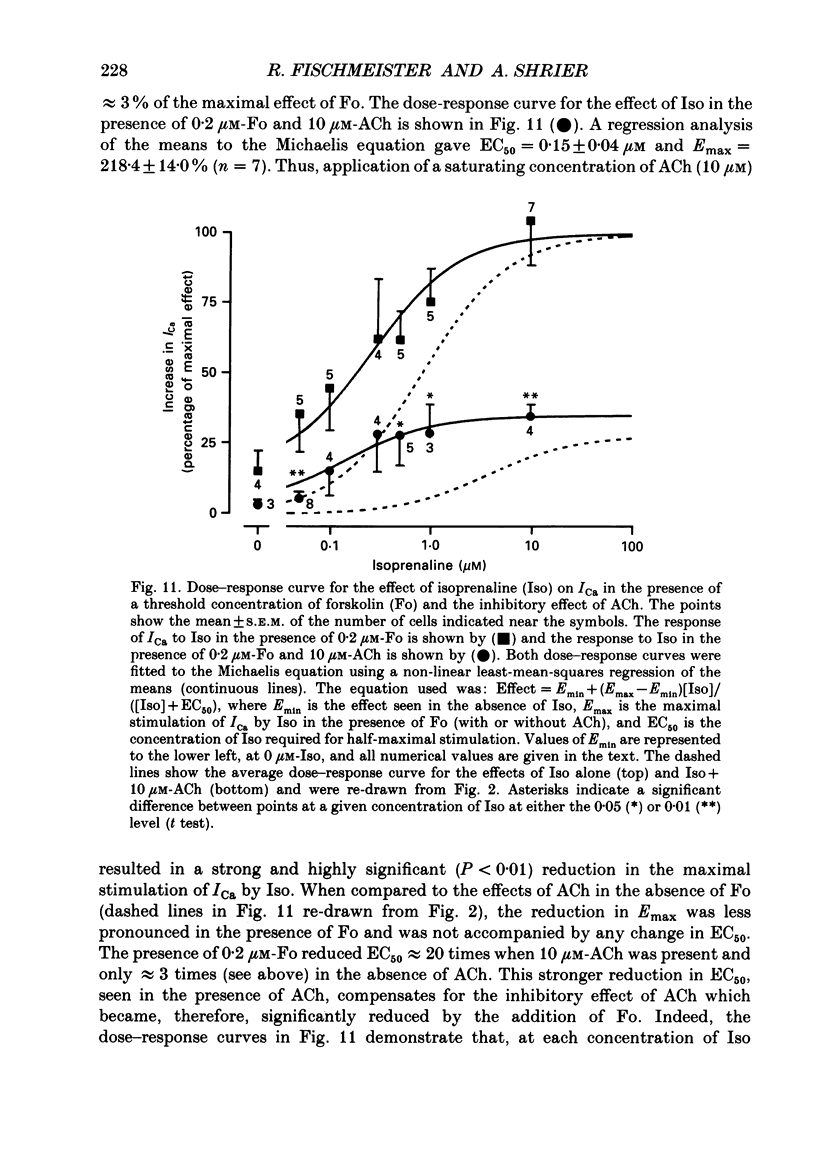
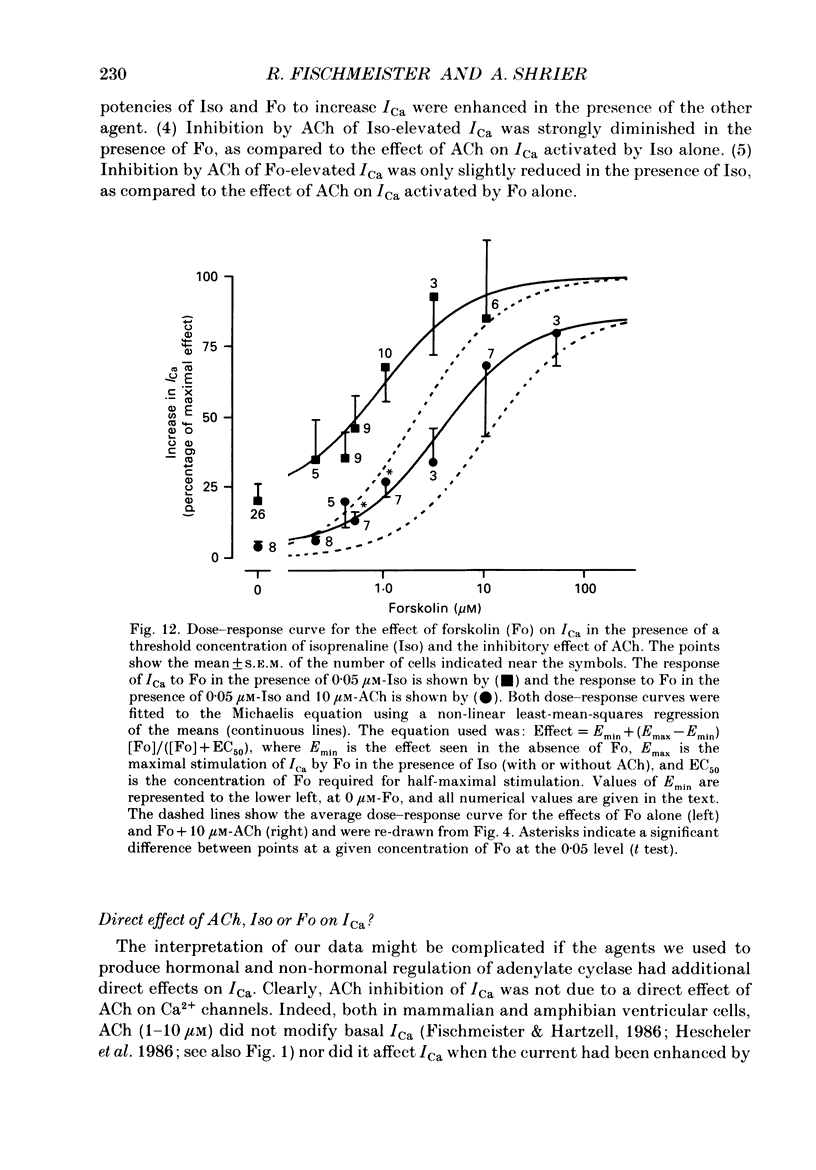
Abstract
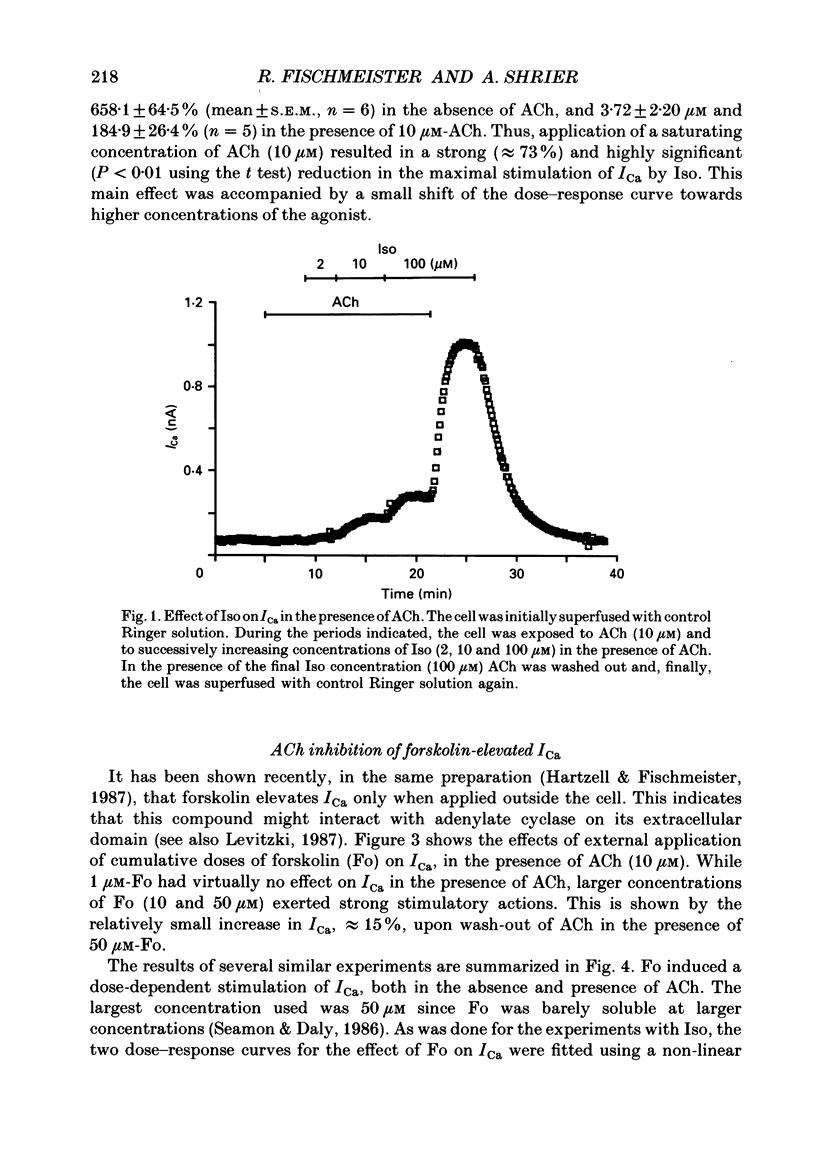
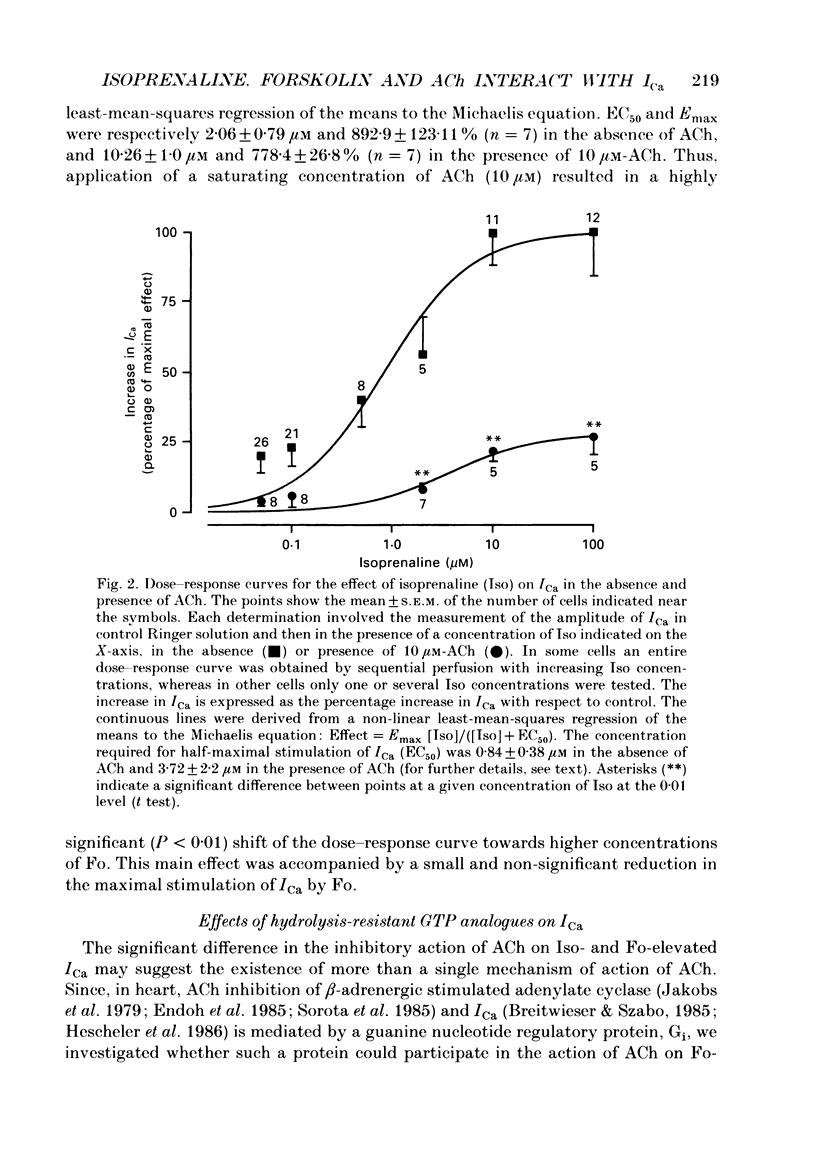
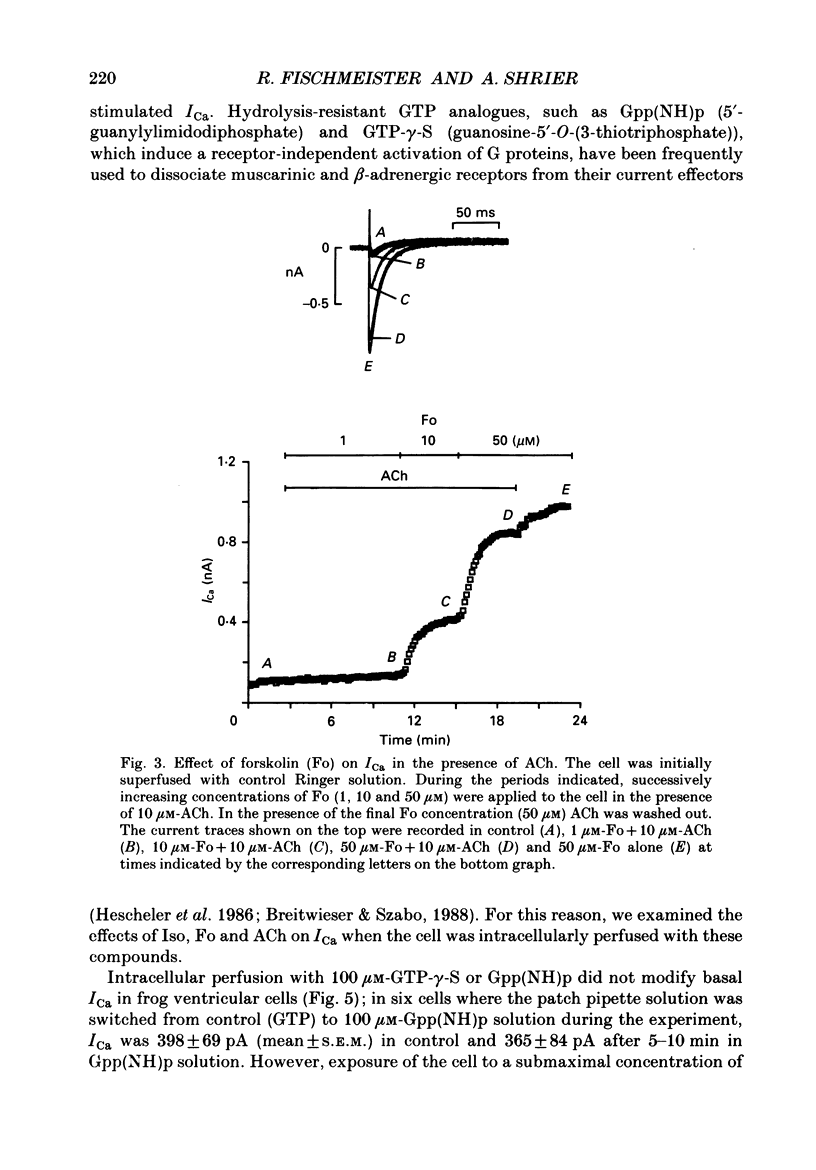
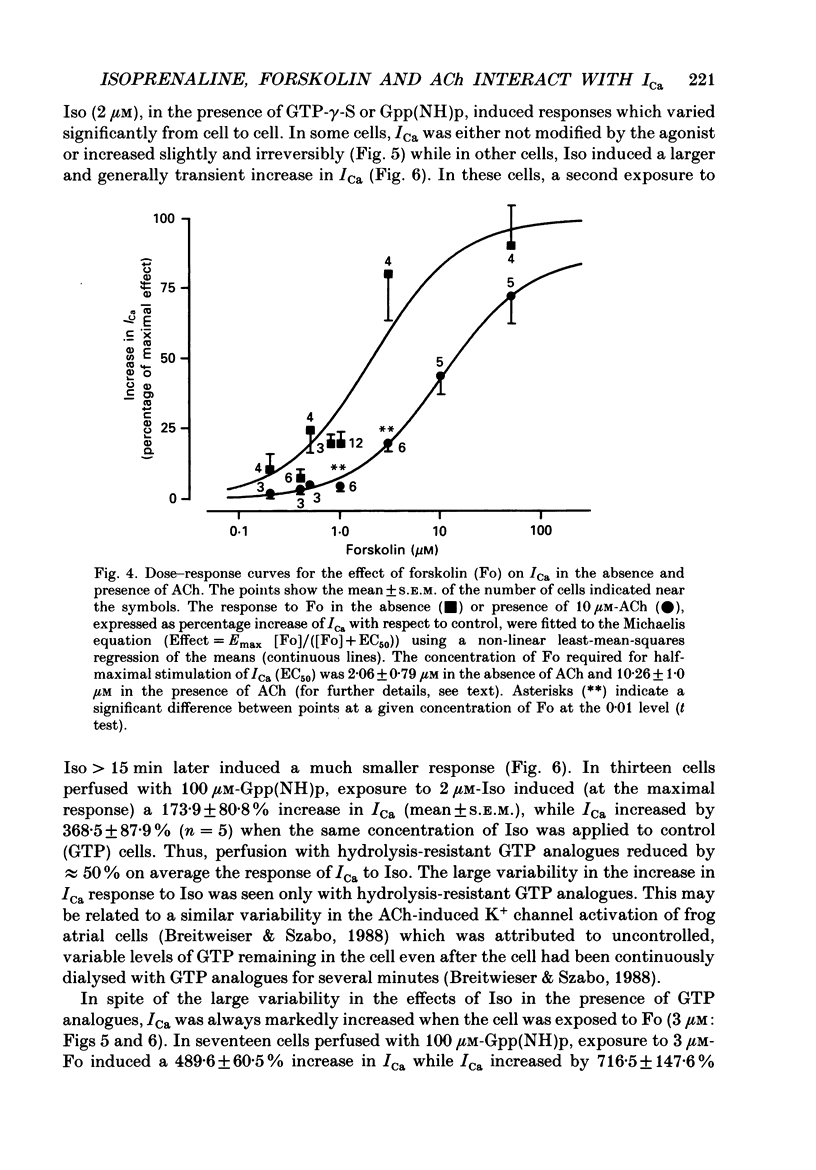
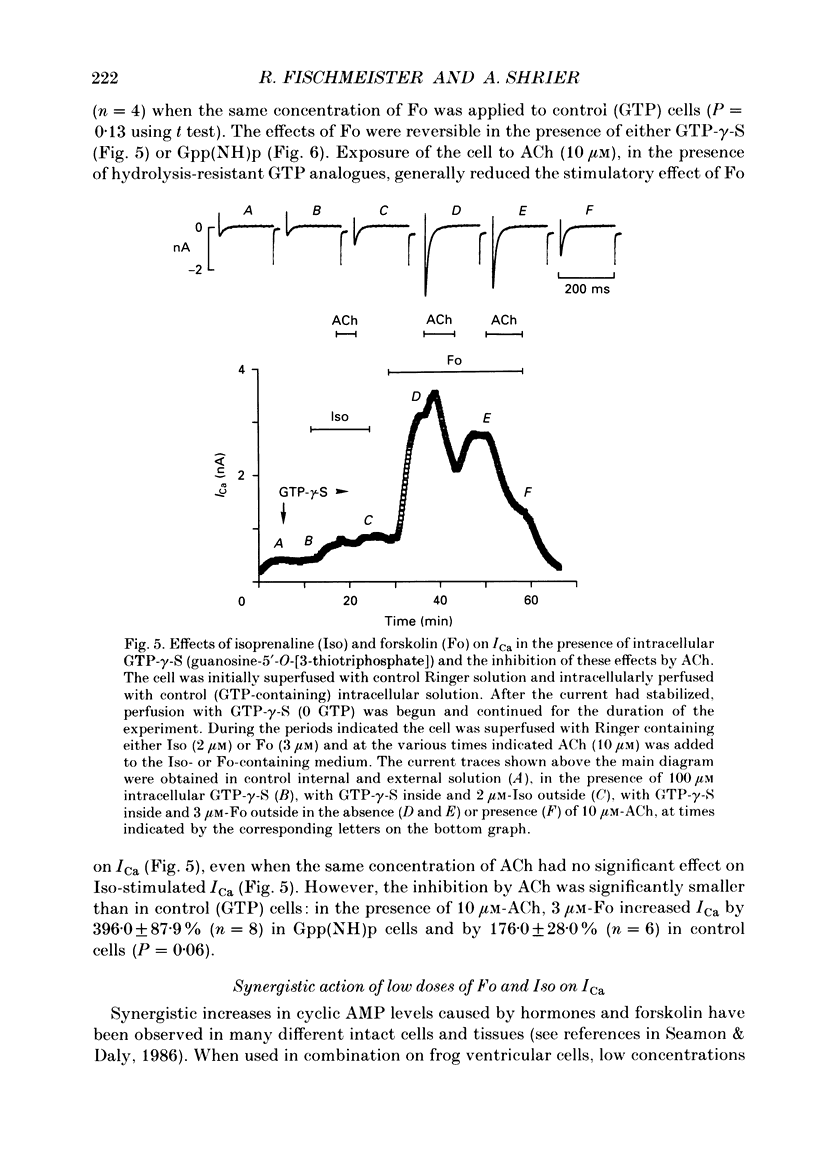
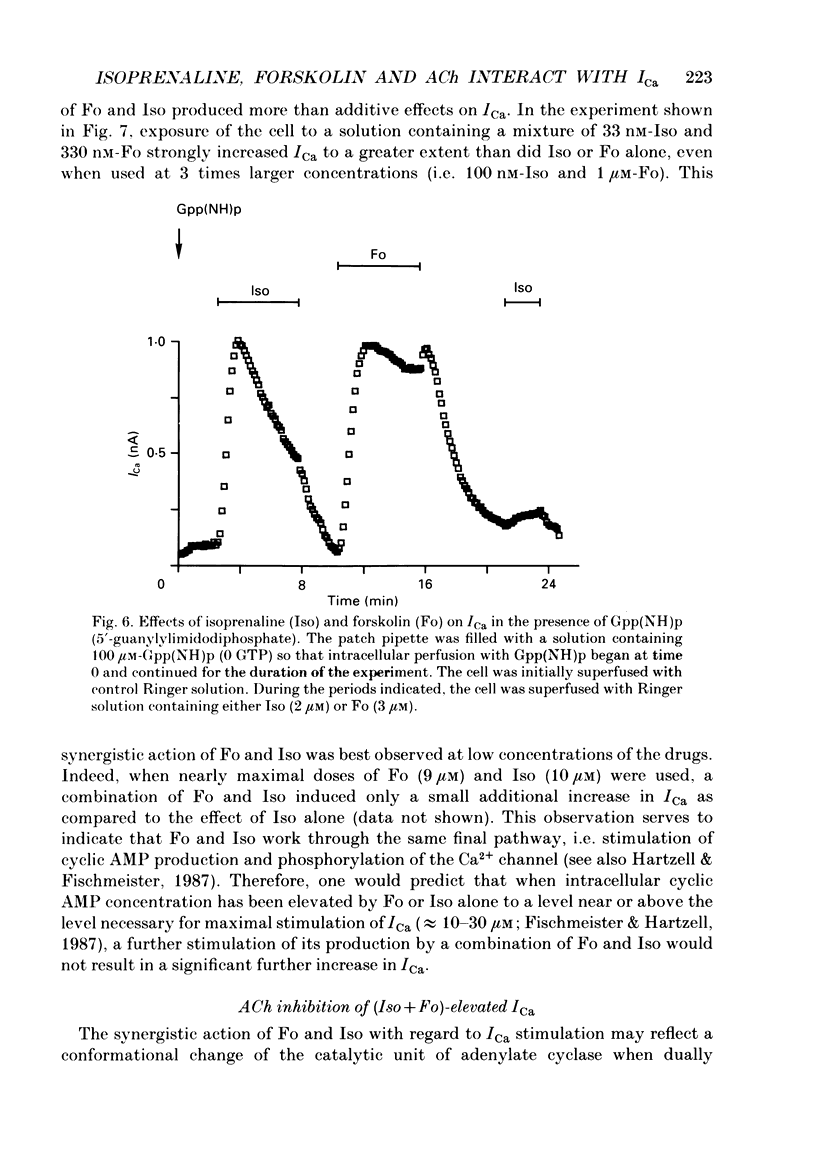
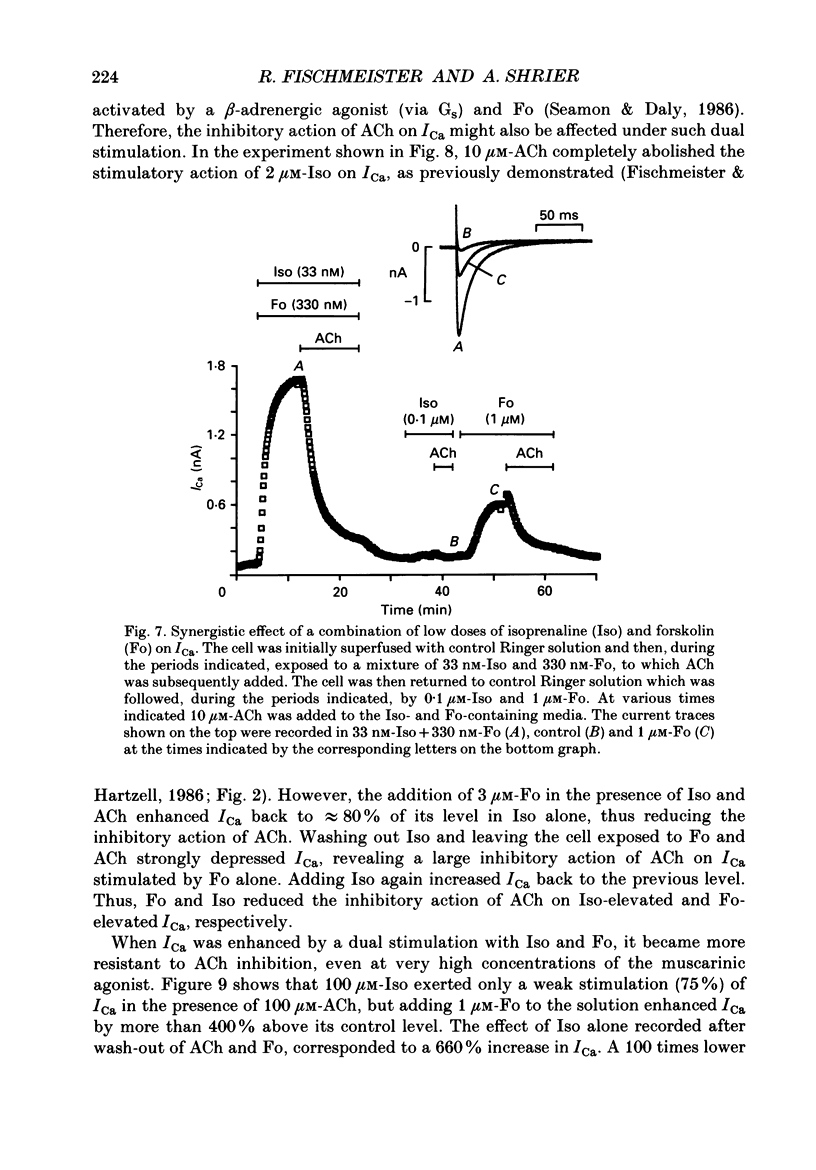
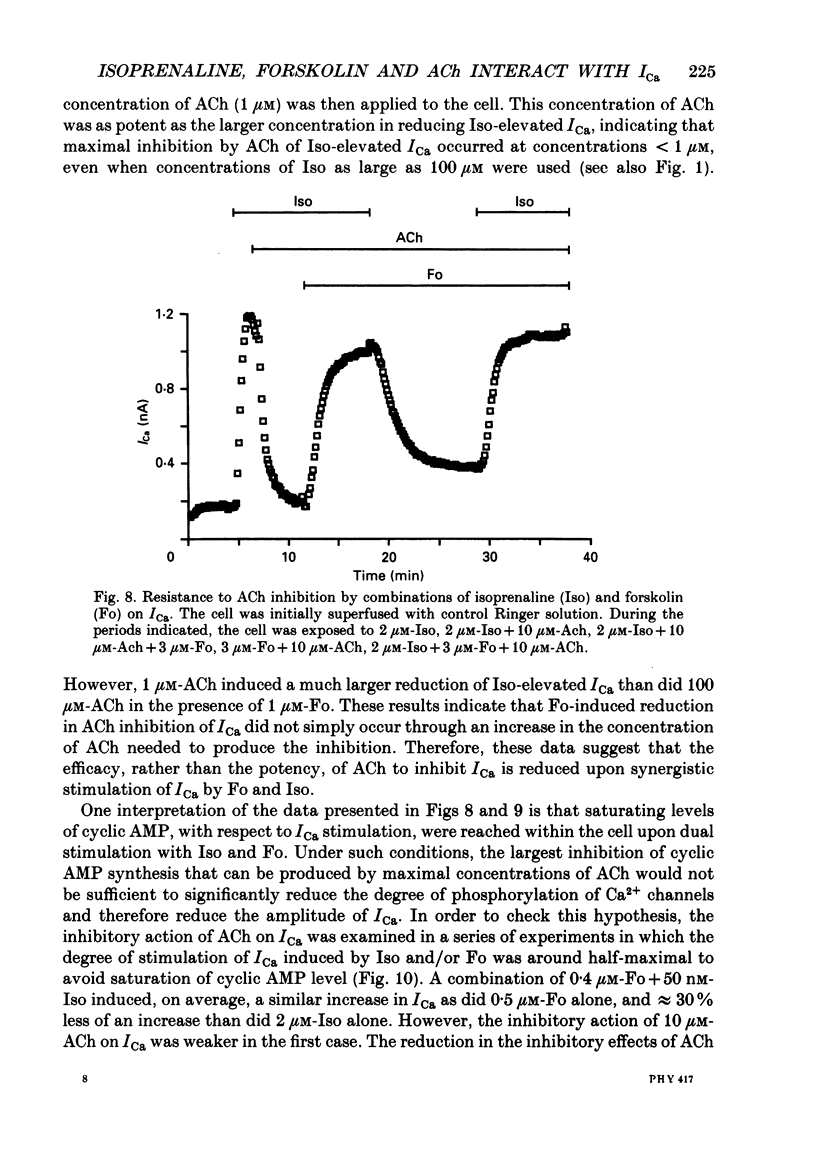
1. Calcium currents (ICa) were measured in single cells isolated from frog ventricle using the whole-cell patch-clamp technique and a perfused pipette. The dose-dependent stimulatory effects of isoprenaline (Iso, 0.1-100 microM) and forskolin (Fo. 0.1-50 microM) on ICa were determined in the presence and absence of acetylcholine (ACh, 10 microM) and/or threshold concentrations of Fo (0.2 microM) and Iso (0.05 microM), respectively. EC50 (i.e. concentration of Iso or Fo at which the response was 50% of the maximum) and Emax (i.e. maximal stimulation of Ica expressed as percentage increase in ICa with respect to control) were measured under each condition. 2. ACh increased EC50 for the stimulatory action of Iso on ICa from 0.84 to 3.72 microM while it reduced Emax from 658 to 185%. Thus, ACh mainly reduced the efficacy of Iso to stimulate ICa. 3. ACh increased EC50 for the stimulatory action of Fo on ICa from 2.06 to 10.26 microM but only slightly reduced Emax from 893 to 778%. Thus, ACh mainly reduced the potency of Fo to stimulate ICa. 4. Intracellular perfusion with 100 microM of hydrolysis-resistant GTP analogues, GTP-gamma-S [guanosine-5'-O-(3-thiotriphosphate)] and Gpp (NH)p (5'-guanylylimido-diphosphate), had no effect on basal ICa but reduced by greater than 50% the stimulatory effect of 2 microM-Iso on ICa. 5. In the presence of Gpp(NH)p or GTP-gamma-S, Fo (3 microM) reversibly increased ICa by 490%, as compared to a 717% increase in control (GTP) intracellular solution. Although ACh could still inhibit Fo-stimulated ICa, the degree of inhibition was significantly smaller than in the presence of GTP. 6. Extracellular perfusion with low concentrations of a combination of Iso (33 nM) and Fo (330 nM) enhanced ICa to a much greater extent than did either agent alone at 3 times higher concentrations. Thus, low concentrations of Iso and Fo appear to increase ICa in a synergistic fashion. 7. ICa stimulated by a combination of Iso and Fo appeared to be more resistant to inhibition by ACh than when stimulated by either alone. It was the efficacy, rather than the potency, of ACh to inhibit ICa that was reduced upon dual stimulation of ICa. 8. In the presence of 0.2 microM-Fo, EC50 and Emax for the effects of Iso on ICa were 0.27 microM and 619%, respectively. By comparison with the effects of Iso alone, Fo reduced EC50 approximately 3 times with no significant change in maximal stimulation.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argibay J. A., Fischmeister R., Hartzell H. C. Inactivation, reactivation and pacing dependence of calcium current in frog cardiocytes: correlation with current density. J Physiol. 1988 Jul;401:201–226. doi: 10.1113/jphysiol.1988.sp017158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L., Codina J., Mattera R., Cerione R. A., Hildebrandt J. D., Sunyer T., Rojas F. J., Caron M. G., Lefkowitz R. J., Iyengar R. Regulation of hormone receptors and adenylyl cyclases by guanine nucleotide binding N proteins. Recent Prog Horm Res. 1985;41:41–99. doi: 10.1016/b978-0-12-571141-8.50006-8. [DOI] [PubMed] [Google Scholar]
- Bouhelal R., Guillon G., Homburger V., Bockaert J. Forskolin-induced change of the size of adenylate cyclase. J Biol Chem. 1985 Sep 15;260(20):10901–10904. [PubMed] [Google Scholar]
- Brandwein H. J., Lewicki J. A., Waldman S. A., Murad F. Effect of GTP analogues on purified soluble guanylate cyclase. J Biol Chem. 1982 Feb 10;257(3):1309–1311. [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Mechanism of muscarinic receptor-induced K+ channel activation as revealed by hydrolysis-resistant GTP analogues. J Gen Physiol. 1988 Apr;91(4):469–493. doi: 10.1085/jgp.91.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Uncoupling of cardiac muscarinic and beta-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985 Oct 10;317(6037):538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- Bristow M. R., Ginsburg R., Strosberg A., Montgomery W., Minobe W. Pharmacology and inotropic potential of forskolin in the human heart. J Clin Invest. 1984 Jul;74(1):212–223. doi: 10.1172/JCI111404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Birnbaumer L. Direct G protein gating of ion channels. Am J Physiol. 1988 Mar;254(3 Pt 2):H401–H410. doi: 10.1152/ajpheart.1988.254.3.H401. [DOI] [PubMed] [Google Scholar]
- Brown J. H. Depolarization-induced inhibition of cyclic AMP accumulation: cholinergic-adrenergic antagonism in murine atria. Mol Pharmacol. 1979 Nov;16(3):841–850. [PubMed] [Google Scholar]
- Buxton I. L., Brunton L. L. Action of the cardiac alpha 1-adrenergic receptor. Activation of cyclic AMP degradation. J Biol Chem. 1985 Jun 10;260(11):6733–6737. [PubMed] [Google Scholar]
- Cachelin A. B., de Peyer J. E., Kokubun S., Reuter H. Ca2+ channel modulation by 8-bromocyclic AMP in cultured heart cells. Nature. 1983 Aug 4;304(5925):462–464. doi: 10.1038/304462a0. [DOI] [PubMed] [Google Scholar]
- Coombs J., Thompson S. Forskolin's effect on transient K current in nudibranch neurons is not reproduced by cAMP. J Neurosci. 1987 Feb;7(2):443–452. doi: 10.1523/JNEUROSCI.07-02-00443.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh M., Maruyama M., Iijima T. Attenuation of muscarinic cholinergic inhibition by islet-activating protein in the heart. Am J Physiol. 1985 Aug;249(2 Pt 2):H309–H320. doi: 10.1152/ajpheart.1985.249.2.H309. [DOI] [PubMed] [Google Scholar]
- England P. J., Shahid M. Effects of forskolin on contractile responses and protein phosphorylation in the isolated perfused rat heart. Biochem J. 1987 Sep 15;246(3):687–695. doi: 10.1042/bj2460687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov A. K., Porotikov V. I. Effect of forskolin on action potential, slow inward current and tension of frog atrial fibers. J Physiol (Paris) 1985;80(3):163–167. [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Cyclic guanosine 3',5'-monophosphate regulates the calcium current in single cells from frog ventricle. J Physiol. 1987 Jun;387:453–472. doi: 10.1113/jphysiol.1987.sp016584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Mechanism of action of acetylcholine on calcium current in single cells from frog ventricle. J Physiol. 1986 Jul;376:183–202. doi: 10.1113/jphysiol.1986.sp016148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J. W., Strawbridge R. A., Watanabe A. M. Muscarinic receptor regulation of cardiac adenylate cyclase activity. J Mol Cell Cardiol. 1987 Jan;19(1):47–61. doi: 10.1016/s0022-2828(87)80544-8. [DOI] [PubMed] [Google Scholar]
- Flitney F. W., Singh J. Evidence that cyclic GMP may regulate cyclic AMP metabolism in the isolated frog ventricle. J Mol Cell Cardiol. 1981 Nov;13(11):963–979. doi: 10.1016/0022-2828(81)90472-7. [DOI] [PubMed] [Google Scholar]
- Garnier D. L'acétylcholine et le myocarde: aspects électrophysiologiques. J Physiol (Paris) 1987;82(2):145–159. [PubMed] [Google Scholar]
- George W. J., Polson J. B., O'Toole A. G., Goldberg N. D. Elevation of guanosine 3',5'-cyclic phosphate in rat heart after perfusion with acetylcholine. Proc Natl Acad Sci U S A. 1970 Jun;66(2):398–403. doi: 10.1073/pnas.66.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Godt R. E., Lindley B. D. Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog. J Gen Physiol. 1982 Aug;80(2):279–297. doi: 10.1085/jgp.80.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R., Johnston D. Noradrenaline and beta-adrenoceptor agonists increase activity of voltage-dependent calcium channels in hippocampal neurons. Nature. 1987 Jun 18;327(6123):620–622. doi: 10.1038/327620a0. [DOI] [PubMed] [Google Scholar]
- Hancock A. A., DeLean A. L., Lefkowitz R. J. Quantitative resolution of beta-adrenergic receptor subtypes by selective ligand binding: application of a computerized model fitting technique. Mol Pharmacol. 1979 Jul;16(1):1–9. [PubMed] [Google Scholar]
- Hartzell H. C. Distribution of muscarinic acetylcholine receptors and presynaptic nerve terminals in amphibian heart. J Cell Biol. 1980 Jul;86(1):6–20. doi: 10.1083/jcb.86.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Fischmeister R. Effect of forskolin and acetylcholine on calcium current in single isolated cardiac myocytes. Mol Pharmacol. 1987 Nov;32(5):639–645. [PubMed] [Google Scholar]
- Hartzell H. C., Fischmeister R. Opposite effects of cyclic GMP and cyclic AMP on Ca2+ current in single heart cells. Nature. 1986 Sep 18;323(6085):273–275. doi: 10.1038/323273a0. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C., Simmons M. A. Comparison of effects of acetylcholine on calcium and potassium currents in frog atrium and ventricle. J Physiol. 1987 Aug;389:411–422. doi: 10.1113/jphysiol.1987.sp016663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeki O., Ui M. Modification by islet-activating protein of receptor-mediated regulation of cyclic AMP accumulation in isolated rat heart cells. J Biol Chem. 1981 Mar 25;256(6):2856–2862. [PubMed] [Google Scholar]
- Hescheler J., Kameyama M., Trautwein W. On the mechanism of muscarinic inhibition of the cardiac Ca current. Pflugers Arch. 1986 Aug;407(2):182–189. doi: 10.1007/BF00580674. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Garber S. S., Aldrich R. W. Effect of forskolin on voltage-gated K+ channels is independent of adenylate cyclase activation. Science. 1988 Jun 17;240(4859):1652–1655. doi: 10.1126/science.2454506. [DOI] [PubMed] [Google Scholar]
- Hudson T. H., Fain J. N. Forskolin-activated adenylate cyclase. Inhibition by guanyl-5'-yl imidodiphosphate. J Biol Chem. 1983 Aug 25;258(16):9755–9761. [PubMed] [Google Scholar]
- Iijima T., Irisawa H., Kameyama M. Membrane currents and their modification by acetylcholine in isolated single atrial cells of the guinea-pig. J Physiol. 1985 Feb;359:485–501. doi: 10.1113/jphysiol.1985.sp015598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel P. A., Stengel D., Ferry N., Hanoune J. Regulation of adenylate cyclase of human platelet membranes by forskolin. J Biol Chem. 1982 Jul 10;257(13):7485–7490. [PubMed] [Google Scholar]
- Jakobs K. H., Aktories K., Schultz G. GTP-dependent inhibition of cardiac adenylate cyclase by muscarinic cholinergic agonists. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):113–119. doi: 10.1007/BF00500275. [DOI] [PubMed] [Google Scholar]
- Joost H. G., Habberfield A. D., Simpson I. A., Laurenza A., Seamon K. B. Activation of adenylate cyclase and inhibition of glucose transport in rat adipocytes by forskolin analogues: structural determinants for distinct sites of action. Mol Pharmacol. 1988 Apr;33(4):449–453. [PubMed] [Google Scholar]
- Kameyama M., Hescheler J., Hofmann F., Trautwein W. Modulation of Ca current during the phosphorylation cycle in the guinea pig heart. Pflugers Arch. 1986 Aug;407(2):123–128. doi: 10.1007/BF00580662. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hofmann F., Trautwein W. On the mechanism of beta-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflugers Arch. 1985 Oct;405(3):285–293. doi: 10.1007/BF00582573. [DOI] [PubMed] [Google Scholar]
- Katada T., Oinuma M., Ui M. Mechanisms for inhibition of the catalytic activity of adenylate cyclase by the guanine nucleotide-binding proteins serving as the substrate of islet-activating protein, pertussis toxin. J Biol Chem. 1986 Apr 15;261(11):5215–5221. [PubMed] [Google Scholar]
- Krause D., Lee S. C., Deutsch C. Forskolin effects on the voltage-gated K+ conductance of human T cells. Pflugers Arch. 1988 Jul;412(1-2):133–140. doi: 10.1007/BF00583742. [DOI] [PubMed] [Google Scholar]
- Lacerda A. E., Rampe D., Brown A. M. Effects of protein kinase C activators on cardiac Ca2+ channels. Nature. 1988 Sep 15;335(6187):249–251. doi: 10.1038/335249a0. [DOI] [PubMed] [Google Scholar]
- Levi R. C., Alloatti G., Fischmeister R. Cyclic GMP regulates the Ca-channel current in guinea pig ventricular myocytes. Pflugers Arch. 1989 Apr;413(6):685–687. doi: 10.1007/BF00581823. [DOI] [PubMed] [Google Scholar]
- Levitzki A. Beta-adrenergic receptors and their mode of coupling to adenylate cyclase. Physiol Rev. 1986 Jul;66(3):819–854. doi: 10.1152/physrev.1986.66.3.819. [DOI] [PubMed] [Google Scholar]
- Linden J., Hollen C. E., Patel A. The mechanism by which adenosine and cholinergic agents reduce contractility in rat myocardium. Correlation with cyclic adenosine monophosphate and receptor densities. Circ Res. 1985 May;56(5):728–735. doi: 10.1161/01.res.56.5.728. [DOI] [PubMed] [Google Scholar]
- Lindner E., Metzger H. The action of forskolin on muscle cells is modified by hormones, calcium ions and calcium antagonists. Arzneimittelforschung. 1983;33(10):1436–1441. [PubMed] [Google Scholar]
- Löffelholz K., Pappano A. J. The parasympathetic neuroeffector junction of the heart. Pharmacol Rev. 1985 Mar;37(1):1–24. [PubMed] [Google Scholar]
- MURAD F., CHI Y. M., RALL T. W., SUTHERLAND E. W. Adenyl cyclase. III. The effect of catecholamines and choline esters on the formation of adenosine 3',5'-phosphate by preparations from cardiac muscle and liver. J Biol Chem. 1962 Apr;237:1233–1238. [PubMed] [Google Scholar]
- MacLeod K. M., Diamond J. Effects of the cyclic GMP lowering agent LY83583 on the interaction of carbachol with forskolin in rabbit isolated cardiac preparations. J Pharmacol Exp Ther. 1986 Jul;238(1):313–318. [PubMed] [Google Scholar]
- Martin J. M., Subers E. M., Halvorsen S. W., Nathanson N. M. Functional and physical properties of chick atrial and ventricular GTP-binding proteins: relationship to muscarinic acetylcholine receptor-mediated responses. J Pharmacol Exp Ther. 1987 Feb;240(2):683–688. [PubMed] [Google Scholar]
- McAfee D. A., Whiting G. J., Siegel B. Neurotransmitter and cyclic nucleotide modulation of frog cardiac contractility. J Mol Cell Cardiol. 1978 Aug;10(8):705–716. doi: 10.1016/0022-2828(78)90405-4. [DOI] [PubMed] [Google Scholar]
- Metzger H., Lindner E. The positive inotropic-acting forskolin, a potent adenylate cyclase activator. Arzneimittelforschung. 1981;31(8):1248–1250. [PubMed] [Google Scholar]
- Mokhtari A., Do Khac L., Harbon S. Forskolin alters sensitivity of the cAMP-generating system to stimulatory as well as to inhibitory agonists. A study with intact human platelets and guinea pig myometrium. Eur J Biochem. 1988 Sep 1;176(1):131–137. doi: 10.1111/j.1432-1033.1988.tb14260.x. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Clapham D. E. Roles of G protein subunits in transmembrane signalling. Nature. 1988 May 12;333(6169):129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Rardon D. P., Pappano A. J. Carbachol inhibits electrophysiological effects of cyclic AMP in ventricular myocytes. Am J Physiol. 1986 Sep;251(3 Pt 2):H601–H611. doi: 10.1152/ajpheart.1986.251.3.H601. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Rodger I. W., Shahid M. Forskolin, cyclic nucleotides and positive inotropism in isolated papillary muscles of the rabbit. Br J Pharmacol. 1984 Jan;81(1):151–159. doi: 10.1111/j.1476-5381.1984.tb10755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. J., Sawyer B. D., Truex L. L., Marshall W. S., Fleisch J. H. LY83583: an agent that lowers intracellular levels of cyclic guanosine 3',5'-monophosphate. J Pharmacol Exp Ther. 1985 Mar;232(3):764–769. [PubMed] [Google Scholar]
- Schrader J., Baumann G., Gerlach E. Adenosine as inhibitor of myocardial effects of catecholamines. Pflugers Arch. 1977 Nov 25;372(1):29–35. doi: 10.1007/BF00582203. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: its biological and chemical properties. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:1–150. [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Guanosine 5'-(beta, gamma-imido)triphosphate inhibition of forskolin-activated adenylate cyclase is mediated by the putative inhibitory guanine nucleotide regulatory protein. J Biol Chem. 1982 Oct 10;257(19):11591–11596. [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamon K., Daly J. W. Activation of adenylate cyclase by the diterpene forskolin does not require the guanine nucleotide regulatory protein. J Biol Chem. 1981 Oct 10;256(19):9799–9801. [PubMed] [Google Scholar]
- Sorota S., Tsuji Y., Tajima T., Pappano A. J. Pertussis toxin treatment blocks hyperpolarization by muscarinic agonists in chick atrium. Circ Res. 1985 Nov;57(5):748–758. doi: 10.1161/01.res.57.5.748. [DOI] [PubMed] [Google Scholar]
- Späh F. Forskolin, a new positive inotropic agent, and its influence on myocardial electrogenic cation movements. J Cardiovasc Pharmacol. 1984 Jan-Feb;6(1):99–106. [PubMed] [Google Scholar]
- Stiles G. L., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptors: biochemical mechanisms of physiological regulation. Physiol Rev. 1984 Apr;64(2):661–743. doi: 10.1152/physrev.1984.64.2.661. [DOI] [PubMed] [Google Scholar]
- Trautwein W., Cavalié A. Cardiac calcium channels and their control by neurotransmitters and drugs. J Am Coll Cardiol. 1985 Dec;6(6):1409–1416. doi: 10.1016/s0735-1097(85)80233-3. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Adrenaline-like effects of intracellular iontophoresis of cyclic AMP in cardiac Purkinje fibres. Nat New Biol. 1973 Sep 26;245(143):120–122. doi: 10.1038/newbio245120a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Bean B. P., Hess P., Lansman J. B., Nilius B., Nowycky M. C. Mechanisms of calcium channel modulation by beta-adrenergic agents and dihydropyridine calcium agonists. J Mol Cell Cardiol. 1986 Jul;18(7):691–710. doi: 10.1016/s0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Cyclic AMP and contractile activity in heart. Adv Cyclic Nucleotide Res. 1977;8:363–420. [PubMed] [Google Scholar]
- Tsien R. W., Giles W., Greengard P. Cyclic AMP mediates the effects of adrenaline on cardiac purkinje fibres. Nat New Biol. 1972 Dec 6;240(101):181–183. doi: 10.1038/newbio240181a0. [DOI] [PubMed] [Google Scholar]
- Vassort G., Rougier O., Garnier D., Sauviat M. P., Coraboeuf E., Gargouïl Y. M. Effects of adrenaline on membrane inward currents during the cardiac action potential. Pflugers Arch. 1969;309(1):70–81. doi: 10.1007/BF00592283. [DOI] [PubMed] [Google Scholar]
- Wagoner P. K., Pallotta B. S. Modulation of acetylcholine receptor desensitization by forskolin is independent of cAMP. Science. 1988 Jun 17;240(4859):1655–1657. doi: 10.1126/science.2454507. [DOI] [PubMed] [Google Scholar]
- West G. A., Isenberg G., Belardinelli L. Antagonism of forskolin effects by adenosine in isolated hearts and ventricular myocytes. Am J Physiol. 1986 May;250(5 Pt 2):H769–H777. doi: 10.1152/ajpheart.1986.250.5.H769. [DOI] [PubMed] [Google Scholar]
- White R. E., Hartzell H. C. Effects of intracellular free magnesium on calcium current in isolated cardiac myocytes. Science. 1988 Feb 12;239(4841 Pt 1):778–780. doi: 10.1126/science.2448878. [DOI] [PubMed] [Google Scholar]
- Yamashita A., Kurokawa T., Higashi K., Dan'ura T., Ishibashi S. Forskolin stabilizes a functionally coupled state between activated guanine nucleotide-binding stimulatory regulatory protein, Ns, and catalytic protein of adenylate cyclase system in rat erythrocytes. Biochem Biophys Res Commun. 1986 May 29;137(1):190–194. doi: 10.1016/0006-291x(86)91194-0. [DOI] [PubMed] [Google Scholar]
- Yatani A., Codina J., Imoto Y., Reeves J. P., Birnbaumer L., Brown A. M. A G protein directly regulates mammalian cardiac calcium channels. Science. 1987 Nov 27;238(4831):1288–1292. doi: 10.1126/science.2446390. [DOI] [PubMed] [Google Scholar]
- Zünkler B. J., Trube G., Ohno-Shosaku T. Forskolin-induced block of delayed rectifying K+ channels in pancreatic beta-cells is not mediated by cAMP. Pflugers Arch. 1988 Jun;411(6):613–619. doi: 10.1007/BF00580856. [DOI] [PubMed] [Google Scholar]



