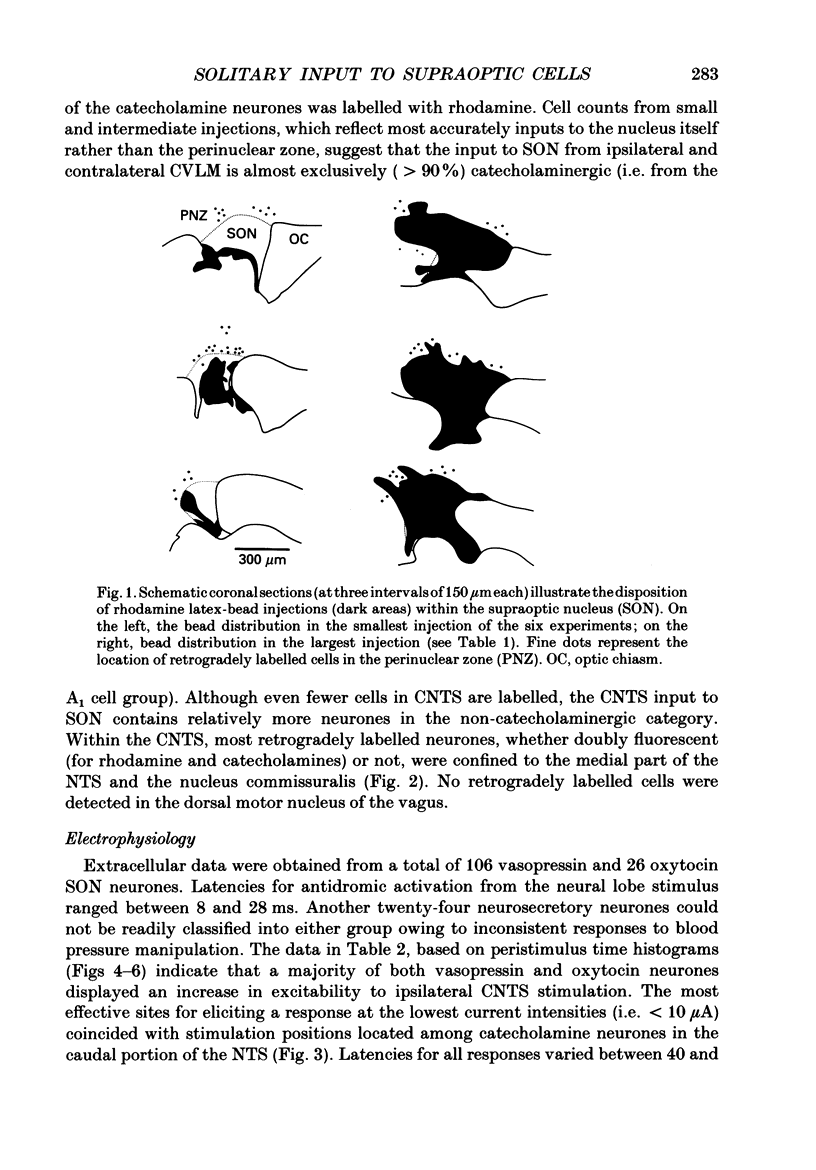
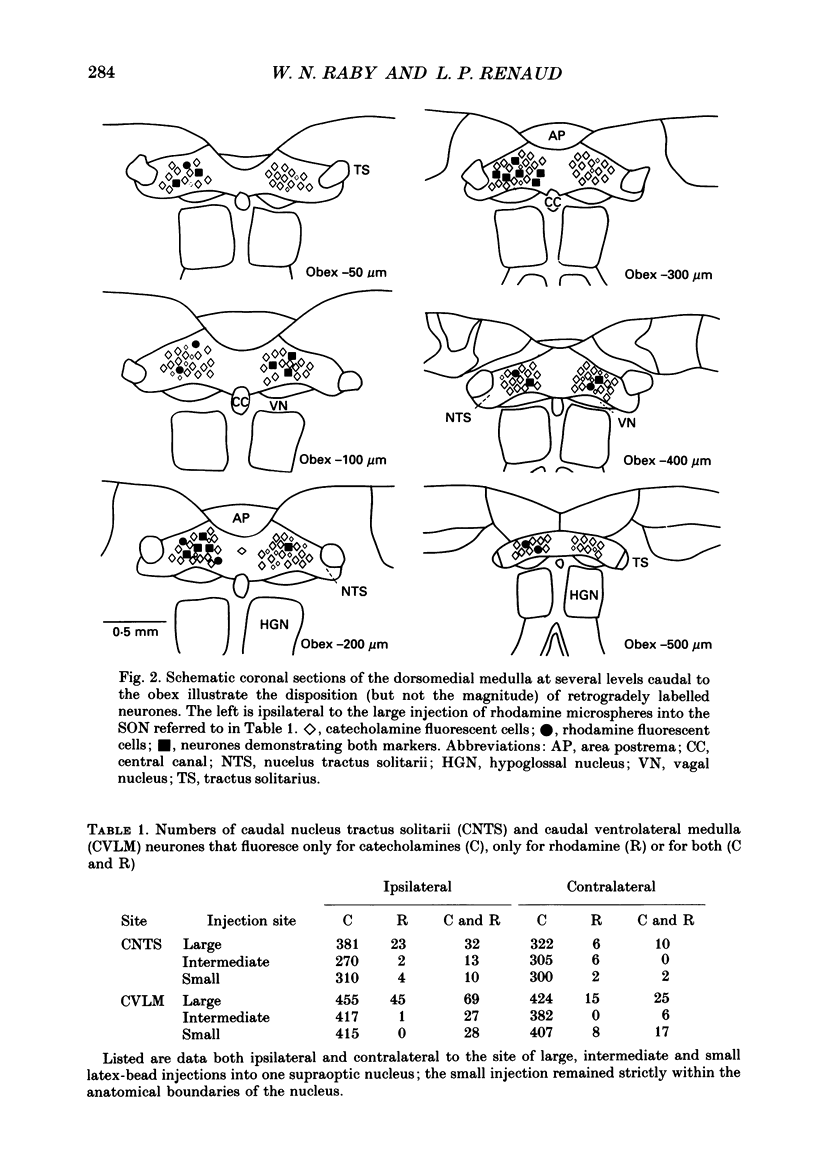
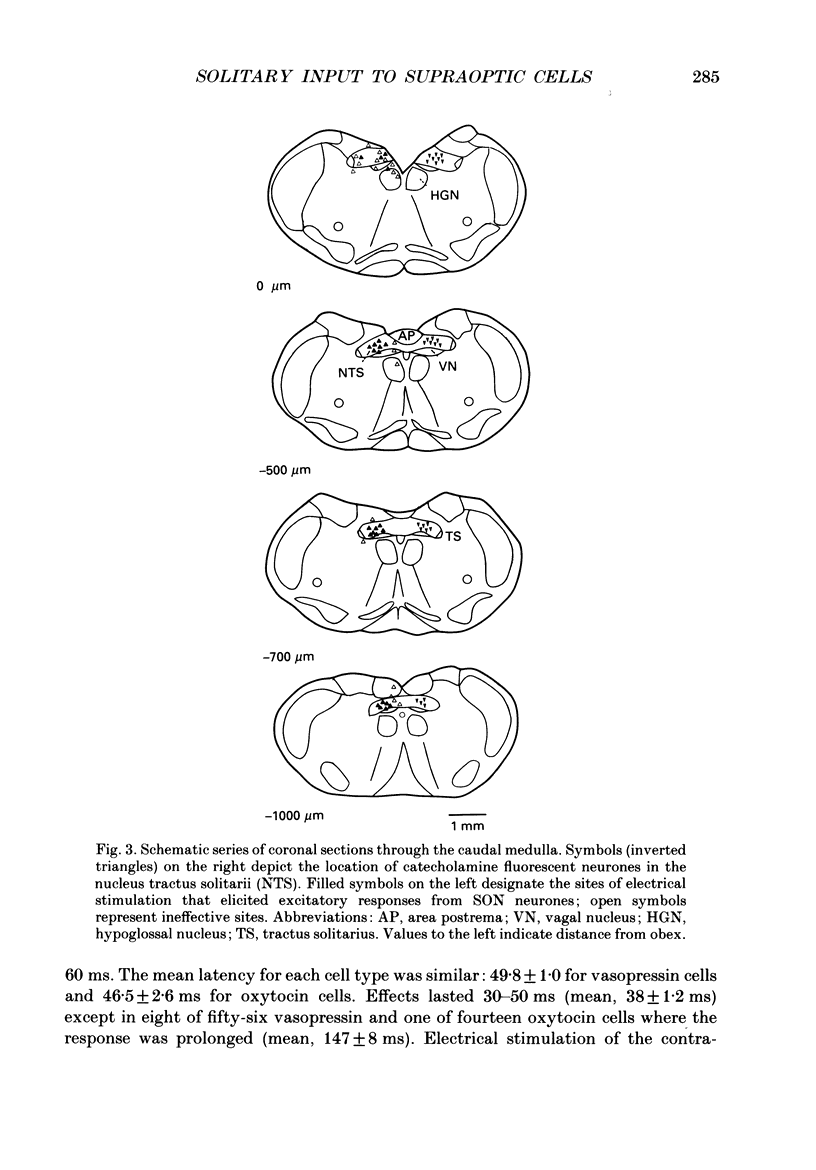
Abstract
1. This study utilized retrograde anatomical tracer techniques and in vivo extracellular electrophysiological studies to examine caudal ventrolateral and dorsomedial medulla afferents to supraoptic nucleus neurosecretory neurones in male Long-Evans rats. 2. In one series of experiments, pentobarbitone-anaesthetized animals were subjected to ventral exposure of the hypothalamus and rhodamine-tagged latex microspheres (0.05-0.2 microliter) were injected into one supraoptic nucleus. Following perfusion with paraformaldehyde-glutaraldehyde 18-24 h later, cell counts were obtained of rhodamine- and/or catecholamine-labelled neurones in the caudal ventrolateral and dorsomedial medulla both ipsi- and contralateral to the injection site. 3. In the caudal ventrolateral medulla, each injection labelled fewer than 15% of the catecholaminergic neurones; with small injections, most (68-100%) of the rhodamine-labelled neurones also displayed catecholamine histofluorescence. In the caudal nucleus tractus solitarii, one-half to one-third as many rhodamine-labelled cells were observed, but a higher percentage (13-100%) of these were non-catecholaminergic. 4. Extracellular recordings were obtained from antidromically identified supraoptic neurones classified as vasopressin (n = 106) or oxytocin (n = 26) secreting. Single cathodal pulses (0.2 ms duration, 0.02-0.08 mA) applied in the caudal half of the ipsilateral nucleus tractus solitarii evoked a transient (30-50 ms) activation of 63% of both vasopressin- and oxytocin-secreting neurones. Mean latencies (+/- S.E.M.) for vasopressin and oxytocin cells were 49.8 +/- 1.0 and 46.5 +/- 2.4 ms respectively; these were not significantly different. Similar responses were noted to contralateral stimuli applied to four vasopressin and two oxytocin cells. 5. Vasopressin neurones activated by caudal nucleus tractus solitarii stimulation displayed similar patterns of response to stimulation in the caudal ventrolateral medulla. However, latencies from the nucleus solitarius (mean 47.6 +/- 1.4 ms; n = 59) were significantly longer (P less than 0.05) than from the ventrolateral medulla (41.5 +/- 2.0 ms; n = 17). In eight out of eleven vasopressin neurones tested, interruption of synaptic transmission through the ventrolateral medulla reduced or abolished the caudal nucleus tractus solitarii-evoked excitation but had no effect on their response to baroreceptor activation. This manoeuvre affected zero out of five oxytocin cells similarly excited by nucleus solitarius stimulation. 6. These observations indicate that visceral input mediated through the nucleus tractus solitarii is transmitted differentially to supraoptic vasopressin- and oxytocin-secreting neurones.
Full text
PDF



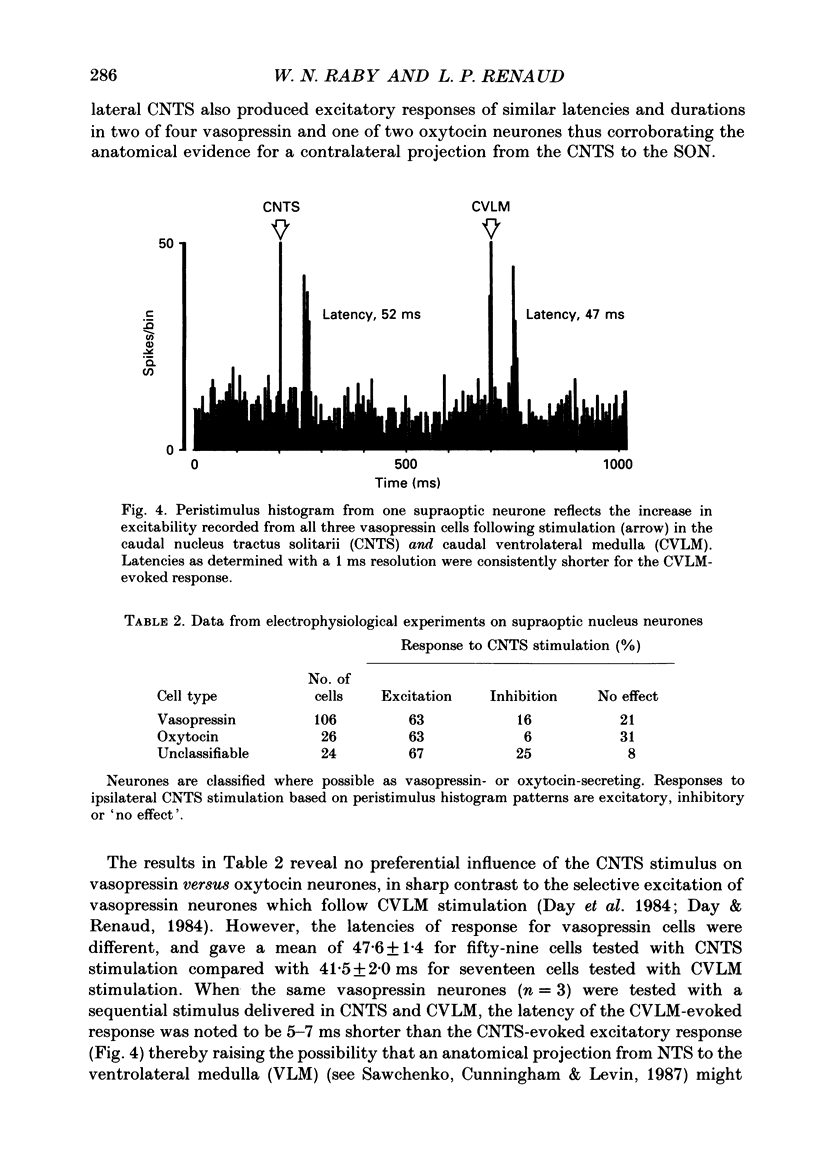
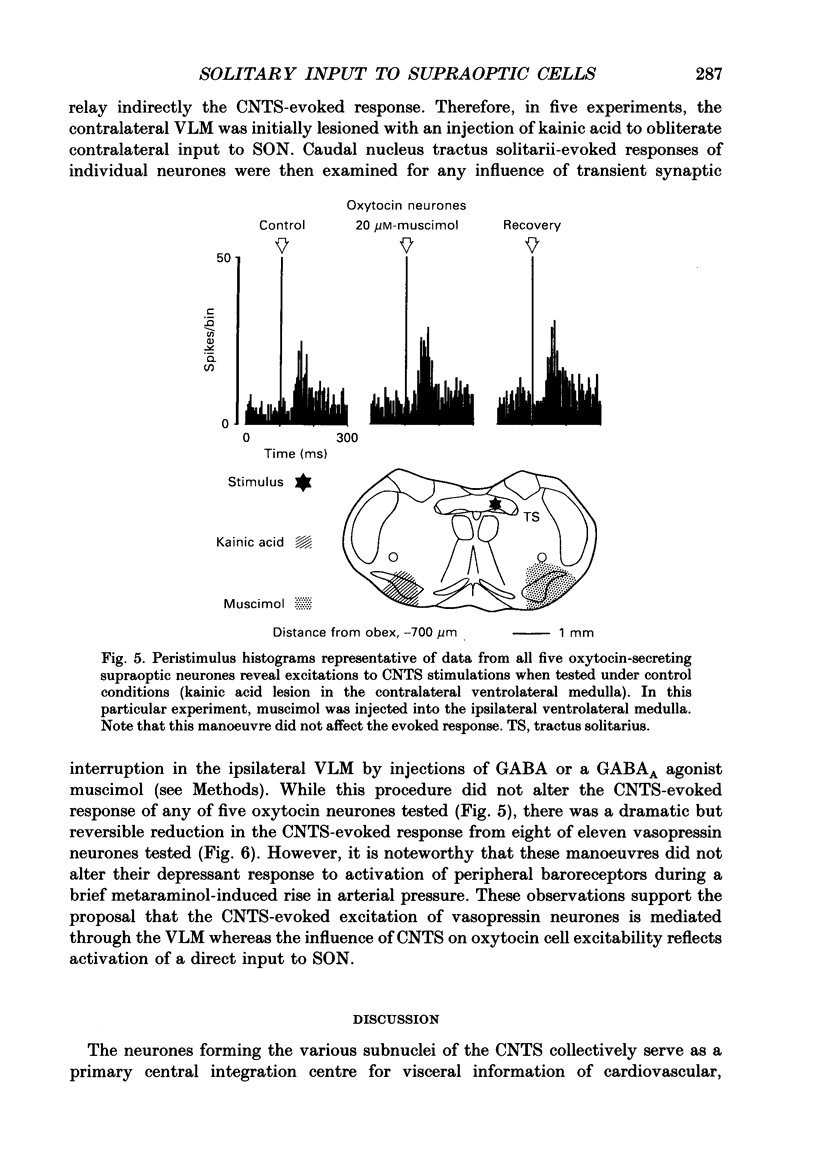
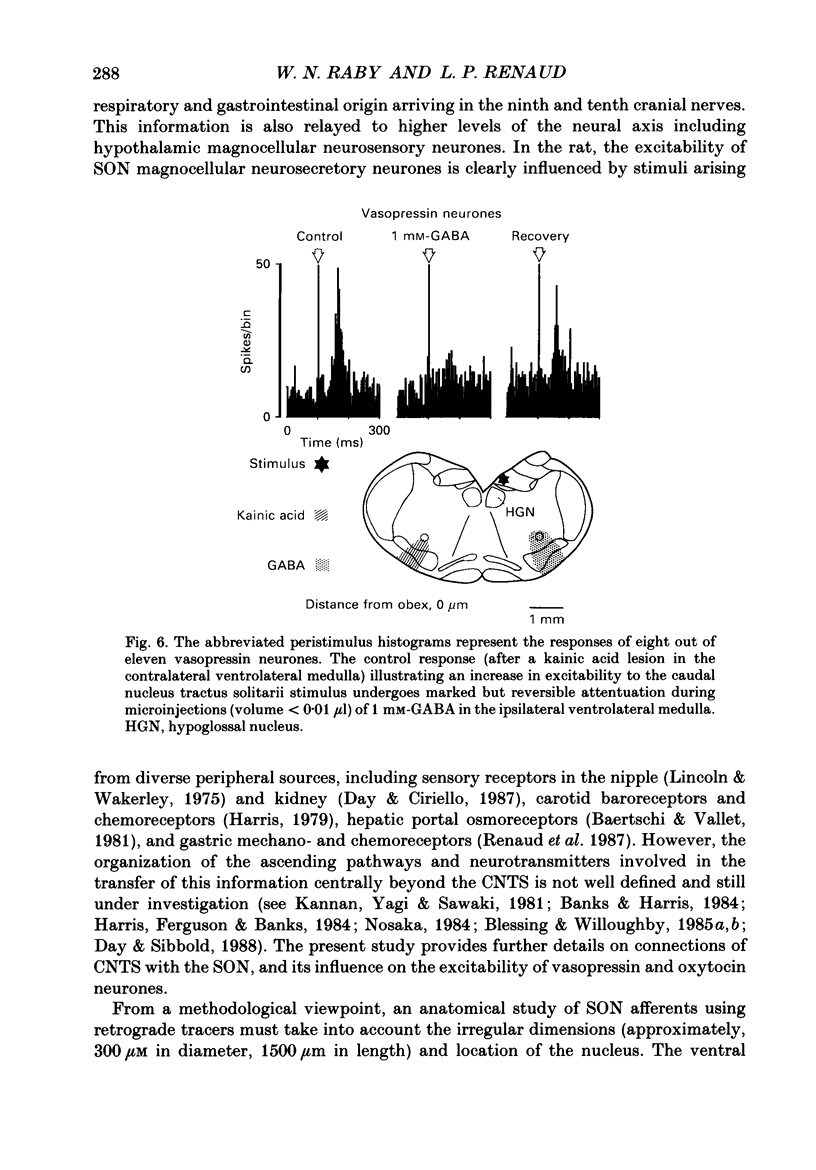






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong W. E., Gallagher M. J., Sladek C. D. Noradrenergic stimulation of supraoptic neuronal activity and vasopressin release in vitro: mediation by an alpha 1-receptor. Brain Res. 1986 Feb 12;365(1):192–197. doi: 10.1016/0006-8993(86)90739-0. [DOI] [PubMed] [Google Scholar]
- Arnauld E., Czernichow P., Fumoux F., Vincent J. D. The effects of hypotension and hypovolaemia on the liberation of vasopressin during haemorrhage in the unanaesthetized monkey (Macaca mulatta). Pflugers Arch. 1977 Nov 23;371(3):193–200. doi: 10.1007/BF00586258. [DOI] [PubMed] [Google Scholar]
- Baertschi A. J., Vallet P. G. Osmosensitivity of the hepatic portal vein area and vasopressin release in rats. J Physiol. 1981 Jun;315:217–230. doi: 10.1113/jphysiol.1981.sp013743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw E. V., Evans M. H. Measurement of current spread from microelectrodes when stimulating within the nervous system. Exp Brain Res. 1976 Jun 30;25(4):391–400. doi: 10.1007/BF00241729. [DOI] [PubMed] [Google Scholar]
- Banks D., Harris M. C. Activation within dorsal medullary nuclei following stimulation in the hypothalamic paraventricular nucleus in rats. Pflugers Arch. 1987 May;408(6):619–627. doi: 10.1007/BF00581165. [DOI] [PubMed] [Google Scholar]
- Banks D., Harris M. C. Lesions of the locus coeruleus abolish baroreceptor-induced depression of supraoptic neurones in the rat. J Physiol. 1984 Oct;355:383–398. doi: 10.1113/jphysiol.1984.sp015425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing W. W., Willoughby J. O. Excitation of neuronal function in rabbit caudal ventrolateral medulla elevates plasma vasopressin. Neurosci Lett. 1985 Jul 31;58(2):189–194. doi: 10.1016/0304-3940(85)90162-4. [DOI] [PubMed] [Google Scholar]
- Blessing W. W., Willoughby J. O. Inhibiting the rabbit caudal ventrolateral medulla prevents baroreceptor-initiated secretion of vasopressin. J Physiol. 1985 Oct;367:253–265. doi: 10.1113/jphysiol.1985.sp015823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriello J., Caverson M. M. Direct pathway from neurons in the ventrolateral medulla relaying cardiovascular afferent information to the supraoptic nucleus in the cat. Brain Res. 1984 Feb 6;292(2):221–228. doi: 10.1016/0006-8993(84)90758-3. [DOI] [PubMed] [Google Scholar]
- Clark B. J., Silva MR Jr E. An afferent pathway for the selective release of vasopressin in response to carotid occlusion and haemorrhage in the cat. J Physiol. 1967 Aug;191(3):529–542. doi: 10.1113/jphysiol.1967.sp008266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett P., Smithson K. G., Hatton G. I. Immunoreactivity to vasopressin- but not oxytocin-associated neurophysin antiserum in phasic neurons of rat hypothalamic paraventricular nucleus. Brain Res. 1986 Jan 1;362(1):7–16. doi: 10.1016/0006-8993(86)91392-2. [DOI] [PubMed] [Google Scholar]
- Cowley A. W., Jr, Liard J. F., Guyton A. C. Role of baroreceptor reflex in daily control of arterial blood pressure and other variables in dogs. Circ Res. 1973 May;32(5):564–576. doi: 10.1161/01.res.32.5.564. [DOI] [PubMed] [Google Scholar]
- Critchley J. A., Ellis P., Ungar A. The reflex release of adrenaline and noradrenaline from the adrenal glands of cats and dogs. J Physiol. 1980 Jan;298:71–78. doi: 10.1113/jphysiol.1980.sp013067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham E. T., Jr, Sawchenko P. E. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol. 1988 Aug 1;274(1):60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- Day T. A., Ciriello J. Effects of renal receptor activation on neurosecretory vasopressin cells. Am J Physiol. 1987 Aug;253(2 Pt 2):R234–R241. doi: 10.1152/ajpregu.1987.253.2.R234. [DOI] [PubMed] [Google Scholar]
- Day T. A., Ferguson A. V., Renaud L. P. Facilitatory influence of noradrenergic afferents on the excitability of rat paraventricular nucleus neurosecretory cells. J Physiol. 1984 Oct;355:237–249. doi: 10.1113/jphysiol.1984.sp015416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day T. A., Randle J. C., Renaud L. P. Opposing alpha- and beta-adrenergic mechanisms mediate dose-dependent actions of noradrenaline on supraoptic vasopressin neurones in vivo. Brain Res. 1985 Dec 9;358(1-2):171–179. doi: 10.1016/0006-8993(85)90961-8. [DOI] [PubMed] [Google Scholar]
- Day T. A., Renaud L. P. Electrophysiological evidence that noradrenergic afferents selectively facilitate the activity of supraoptic vasopressin neurons. Brain Res. 1984 Jun 15;303(2):233–240. doi: 10.1016/0006-8993(84)91209-5. [DOI] [PubMed] [Google Scholar]
- Day T. A., Sibbald J. R. Solitary nucleus excitation of supraoptic vasopressin cells via adrenergic afferents. Am J Physiol. 1988 Apr;254(4 Pt 2):R711–R716. doi: 10.1152/ajpregu.1988.254.4.R711. [DOI] [PubMed] [Google Scholar]
- FUXE K. EVIDENCE FOR THE EXISTENCE OF MONOAMINE NEURONS IN THE CENTRAL NERVOUS SYSTEM. IV. DISTRIBUTION OF MONOAMINE NERVE TERMINALS IN THE CENTRAL NERVOUS SYSTEM. Acta Physiol Scand Suppl. 1965:SUPPL 247–247:37+. [PubMed] [Google Scholar]
- Fater D. C., Sundet W. D., Schultz H. D., Goetz K. L. Arterial baroreceptors have minimal physiological effects on adrenal medullary secretion. Am J Physiol. 1983 Feb;244(2):H194–H200. doi: 10.1152/ajpheart.1983.244.2.H194. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Heath J. W., Costa M. Aqueous aldehyde (Faglu) methods for the fluorescence histochemical localization of catecholamines and for ultrastructural studies of central nervous tissue. Histochemistry. 1978 Sep 28;57(4):285–295. doi: 10.1007/BF00492664. [DOI] [PubMed] [Google Scholar]
- Gross R., Kirchheim H., Ruffmann K. Effect of carotid occlusion and of perfusion pressure on renal function in conscious dogs. Circ Res. 1981 Jun;48(6 Pt 1):777–784. doi: 10.1161/01.res.48.6.777. [DOI] [PubMed] [Google Scholar]
- Harris M. C. Effects of chemoreceptor and baroreceptor stimulation on the discharge of hypothalamic supraoptic neurones in rats. J Endocrinol. 1979 Jul;82(1):115–125. doi: 10.1677/joe.0.0820115. [DOI] [PubMed] [Google Scholar]
- Harris M. C., Ferguson A. V., Banks D. The afferent pathway for carotid body chemoreceptor input to the hypothalamic supraoptic nucleus in the rat. Pflugers Arch. 1984 Jan;400(1):80–87. doi: 10.1007/BF00670540. [DOI] [PubMed] [Google Scholar]
- Hornby P. J., Piekut D. T. Catecholamine distribution and relationship to magnocellular neurons in the paraventricular nucleus of the rat. Cell Tissue Res. 1987 May;248(2):239–246. doi: 10.1007/BF00218190. [DOI] [PubMed] [Google Scholar]
- Housley G. D., Martin-Body R. L., Dawson N. J., Sinclair J. D. Brain stem projections of the glossopharyngeal nerve and its carotid sinus branch in the rat. Neuroscience. 1987 Jul;22(1):237–250. doi: 10.1016/0306-4522(87)90214-4. [DOI] [PubMed] [Google Scholar]
- Jhamandas J. H., Renaud L. P. A gamma-aminobutyric-acid-mediated baroreceptor input to supraoptic vasopressin neurones in the rat. J Physiol. 1986 Dec;381:595–606. doi: 10.1113/jphysiol.1986.sp016345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMM D. E., LEVINSKY N. G. THE MECHANISM OF DENERVATION NATRIURESIS. J Clin Invest. 1965 Jan;44:93–102. doi: 10.1172/JCI105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia M., Fuxe K., Hökfelt T., Johansson O., Lang R., Ganten D., Cuello C., Terenius L. Distribution of neuropeptide immunoreactive nerve terminals within the subnuclei of the nucleus of the tractus solitarius of the rat. J Comp Neurol. 1984 Jan 20;222(3):409–444. doi: 10.1002/cne.902220308. [DOI] [PubMed] [Google Scholar]
- Kannan H., Yagi K., Sawaki Y. Pontine neurones: electrophysiological evidence of mediating carotid baroreceptor inputs to supraoptic neurones in rats. Exp Brain Res. 1981;42(3-4):362–370. doi: 10.1007/BF00237501. [DOI] [PubMed] [Google Scholar]
- Karim F., Kaufman S., Kappagoda C. T. Effect of stimulating right atrial receptors on renal blood flow. Can J Physiol Pharmacol. 1982 Dec;60(12):1672–1679. doi: 10.1139/y82-245. [DOI] [PubMed] [Google Scholar]
- Karim F., Mackay D. U., Kappagoda C. T. Influence of carotid sinus pressure on atrial receptors and renal blood flow. Am J Physiol. 1982 Feb;242(2):H220–H226. doi: 10.1152/ajpheart.1982.242.2.H220. [DOI] [PubMed] [Google Scholar]
- Karim F., Poucher S. M., Summerill R. A. The effects of stimulating carotid chemoreceptors on renal haemodynamics and function in dogs. J Physiol. 1987 Nov;392:451–462. doi: 10.1113/jphysiol.1987.sp016790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim F., Poucher S. M., Summerill R. A. The reflex effects of changes in carotid sinus pressure upon renal function in dogs. J Physiol. 1984 Oct;355:557–566. doi: 10.1113/jphysiol.1984.sp015438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L. C., Burkhalter A., Dreyer W. J. Fluorescent latex microspheres as a retrograde neuronal marker for in vivo and in vitro studies of visual cortex. Nature. 1984 Aug 9;310(5977):498–500. doi: 10.1038/310498a0. [DOI] [PubMed] [Google Scholar]
- Kezdi P., Geller E. Baroreceptor control of postganglionic sympathetic nerve discharge. Am J Physiol. 1968 Mar;214(3):427–435. doi: 10.1152/ajplegacy.1968.214.3.427. [DOI] [PubMed] [Google Scholar]
- Kopp U., DiBona G. F. Interaction of renal beta 1-adrenoceptors and prostaglandins in reflex renin release. Am J Physiol. 1983 Apr;244(4):F418–F424. doi: 10.1152/ajprenal.1983.244.4.F418. [DOI] [PubMed] [Google Scholar]
- Lightman S. L., Todd K., Everitt B. J. Ascending noradrenergic projections from the brainstem: evidence for a major role in the regulation of blood pressure and vasopressin secretion. Exp Brain Res. 1984;55(1):145–151. doi: 10.1007/BF00240508. [DOI] [PubMed] [Google Scholar]
- Lincoln D. W., Wakerley J. B. Factors governing the periodic activation of supraoptic and paraventricular neurosecretory cells during suckling in the rat. J Physiol. 1975 Sep;250(2):443–461. doi: 10.1113/jphysiol.1975.sp011064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden R. J., Mary D. A., Weatherill D. The responses in renal nerves to stimulation of atrial receptors, carotid sinus baroreceptors and carotid chemoreceptors. Q J Exp Physiol. 1981 Apr;66(2):179–191. doi: 10.1113/expphysiol.1981.sp002544. [DOI] [PubMed] [Google Scholar]
- Mancia G., Shepherd J. T., Donald D. E. Interplay among carotid sinus, cardiopulmonary, and carotid body reflexes in dogs. Am J Physiol. 1976 Jan;230(1):19–24. doi: 10.1152/ajplegacy.1976.230.1.19. [DOI] [PubMed] [Google Scholar]
- Mancia G., Shepherd J. T., Donald D. E. Role of cardiac, pulmonary, and carotid mechanoreceptors in the control of hind-limb and renal circulation in dogs. Circ Res. 1975 Aug;37(2):200–208. doi: 10.1161/01.res.37.2.200. [DOI] [PubMed] [Google Scholar]
- McAllen R. M., Blessing W. W. Neurons (presumably A1-cells) projecting from the caudal ventrolateral medulla to the region of the supraoptic nucleus respond to baroreceptor inputs in the rabbit. Neurosci Lett. 1987 Jan 27;73(3):247–252. doi: 10.1016/0304-3940(87)90253-9. [DOI] [PubMed] [Google Scholar]
- McKellar S., Loewy A. D. Efferent projections of the A1 catecholamine cell group in the rat: an autoradiographic study. Brain Res. 1982 Jun 3;241(1):11–29. doi: 10.1016/0006-8993(82)91224-0. [DOI] [PubMed] [Google Scholar]
- McNeill T. H., Sladek J. R., Jr Simultaneous monoamine histofluorescence and neuropeptide immunocytochemistry: II. Correlative distribution of catecholamine varicosities and magnocellular neurosecretory neurons in the rat supraoptic and paraventricular nuclei. J Comp Neurol. 1980 Oct 15;193(4):1023–1033. doi: 10.1002/cne.901930414. [DOI] [PubMed] [Google Scholar]
- Mimran A., Deschodt G. The role of the renin-angiotensin system in the hormonal and renal responses to tilt in normal man. Ren Physiol. 1983;6(1):36–42. doi: 10.1159/000172879. [DOI] [PubMed] [Google Scholar]
- Moore S. D., Guyenet P. G. An electrophysiological study of the forebrain projection of nucleus commissuralis: preliminary identification of presumed A2 catecholaminergic neurons. Brain Res. 1983 Mar 21;263(2):211–222. doi: 10.1016/0006-8993(83)90314-1. [DOI] [PubMed] [Google Scholar]
- Moore S. D., Guyenet P. G. Effect of blood pressure on A2 noradrenergic neurons. Brain Res. 1985 Jul 8;338(1):169–172. doi: 10.1016/0006-8993(85)90262-8. [DOI] [PubMed] [Google Scholar]
- Nosaka S. Solitary nucleus neurons transmitting vagal visceral input to the forebrain via a direct pathway in rats. Exp Neurol. 1984 Sep;85(3):493–505. doi: 10.1016/0014-4886(84)90026-8. [DOI] [PubMed] [Google Scholar]
- O'Connor W. J., Summerill R. A. Sodium excretion in normal conscious dogs. Cardiovasc Res. 1979 Jan;13(1):22–30. doi: 10.1093/cvr/13.1.22. [DOI] [PubMed] [Google Scholar]
- Oberg B., Thorén P. Circulatory responses to stimulation of left ventricular receptors in the cat. Acta Physiol Scand. 1973 May;88(1):8–22. doi: 10.1111/j.1748-1716.1973.tb05429.x. [DOI] [PubMed] [Google Scholar]
- Osborn J. L., Francisco L. L., DiBona G. F. Effect of renal nerve stimulation on renal blood flow autoregulation and antinatriuresis during reductions in renal perfusion pressure. Proc Soc Exp Biol Med. 1981 Oct;168(1):77–81. doi: 10.3181/00379727-168-41238. [DOI] [PubMed] [Google Scholar]
- PERLMUTT J. H. Reflex antidiuresis after occlusion of common carotid arteries in hydrated dogs. Am J Physiol. 1963 Feb;204:197–201. doi: 10.1152/ajplegacy.1963.204.2.197. [DOI] [PubMed] [Google Scholar]
- Powis D. A., Donald D. E. Involvement of renal alpha- and beta-adrenoceptors in release of renin by carotid baroreflex. Am J Physiol. 1979 Apr;236(4):H580–H585. doi: 10.1152/ajpheart.1979.236.4.H580. [DOI] [PubMed] [Google Scholar]
- Prosnitz E. H., Zambraski E. J., DiBona G. F. Mechanism of intrarenal blood flow redistribution after carotid artery occlusion. Am J Physiol. 1977 Feb;232(2):F167–F172. doi: 10.1152/ajprenal.1977.232.2.F167. [DOI] [PubMed] [Google Scholar]
- Purtock R. V., von Colditz J. H., Seagard J. L., Igler F. O., Zuperku E. J., Kampine J. P. Reflex effects of thoracic sympathetic afferent nerve stimulation on the kidney. Am J Physiol. 1977 Nov;233(5):H580–H586. doi: 10.1152/ajpheart.1977.233.5.H580. [DOI] [PubMed] [Google Scholar]
- Randle J. C., Bourque C. W., Renaud L. P. Alpha 1-adrenergic receptor activation depolarizes rat supraoptic neurosecretory neurons in vitro. Am J Physiol. 1986 Sep;251(3 Pt 2):R569–R574. doi: 10.1152/ajpregu.1986.251.3.R569. [DOI] [PubMed] [Google Scholar]
- Randle J. C., Bourque C. W., Renaud L. P. Serial reconstruction of Lucifer yellow-labeled supraoptic nucleus neurons in perfused rat hypothalamic explants. Neuroscience. 1986 Feb;17(2):453–467. doi: 10.1016/0306-4522(86)90259-9. [DOI] [PubMed] [Google Scholar]
- Randle J. C., Mazurek M., Kneifel D., Dufresne J., Renaud L. P. Alpha 1-adrenergic receptor activation releases vasopressin and oxytocin from perfused rat hypothalamic explants. Neurosci Lett. 1986 Apr 11;65(2):219–223. doi: 10.1016/0304-3940(86)90308-3. [DOI] [PubMed] [Google Scholar]
- Raybould H. E., Gayton R. J., Dockray G. J. CNS effects of circulating CCK8: involvement of brainstem neurones responding to gastric distension. Brain Res. 1985 Sep 2;342(1):187–190. doi: 10.1016/0006-8993(85)91373-3. [DOI] [PubMed] [Google Scholar]
- SHARE L. EFFECTS OF CAROTID OCCLUSION AND LEFT ATRIAL DISTENTION ON PLASMA VASOPRESSIN TITER. Am J Physiol. 1965 Feb;208:219–223. doi: 10.1152/ajplegacy.1965.208.2.219. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Benoit R., Brown M. R. Somatostatin 28-immunoreactive inputs to the paraventricular and supraoptic nuclei: principal origin from non-aminergic neurons in the nucleus of the solitary tract. J Chem Neuroanat. 1988 Mar-Apr;1(2):81–94. [PubMed] [Google Scholar]
- Sawchenko P. E., Plotsky P. M., Pfeiffer S. W., Cunningham E. T., Jr, Vaughan J., Rivier J., Vale W. Inhibin beta in central neural pathways involved in the control of oxytocin secretion. Nature. 1988 Aug 18;334(6183):615–617. doi: 10.1038/334615a0. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W. Central noradrenergic pathways for the integration of hypothalamic neuroendocrine and autonomic responses. Science. 1981 Nov 6;214(4521):685–687. doi: 10.1126/science.7292008. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W. The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. J Comp Neurol. 1983 Aug 1;218(2):121–144. doi: 10.1002/cne.902180202. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. 1982 Nov;257(3):275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- Share L., Levy M. N. Carotid sinus pulse pressure, a determinant of plasma antidiuretic hormone concentration. Am J Physiol. 1966 Sep;211(3):721–724. doi: 10.1152/ajplegacy.1966.211.3.721. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sawchenko P. E., Bérod A., Hartman B. K., Helle K. B., Vanorden D. E. An immunohistochemical study of the organization of catecholaminergic cells and terminal fields in the paraventricular and supraoptic nuclei of the hypothalamus. J Comp Neurol. 1981 Feb 20;196(2):271–285. doi: 10.1002/cne.901960207. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sawchenko P. E. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Tribollet E., Armstrong W. E., Dubois-Dauphin M., Dreifuss J. J. Extra-hypothalamic afferent inputs to the supraoptic nucleus area of the rat as determined by retrograde and anterograde tracing techniques. Neuroscience. 1985 May;15(1):135–148. doi: 10.1016/0306-4522(85)90128-9. [DOI] [PubMed] [Google Scholar]
- Willoughby J. O., Jervois P. M., Menadue M. F., Blessing W. W. Noradrenaline, by activation of alpha-1-adrenoceptors in the region of the supraoptic nucleus, causes secretion of vasopressin in the unanaesthetized rat. Neuroendocrinology. 1987 Mar;45(3):219–226. doi: 10.1159/000124729. [DOI] [PubMed] [Google Scholar]
- Yamane Y., Nakai M., Yamamoto J., Umeda Y., Ogino K. Release of vasopressin by electrical stimulation of the intermediate portion of the nucleus of the tractus solitarius in rats with cervical spinal cordotomy and vagotomy. Brain Res. 1984 Dec 24;324(2):358–360. doi: 10.1016/0006-8993(84)90049-0. [DOI] [PubMed] [Google Scholar]
- Yamashita H., Inenaga K., Kannan H. Depolarizing effect of noradrenaline on neurons of the rat supraoptic nucleus in vitro. Brain Res. 1987 Mar 10;405(2):348–352. doi: 10.1016/0006-8993(87)90304-0. [DOI] [PubMed] [Google Scholar]
- Yamashita H., Inenaga K., Kawata M., Sano Y. Phasically firing neurons in the supraoptic nucleus of the rat hypothalamus: immunocytochemical and electrophysiological studies. Neurosci Lett. 1983 May 27;37(1):87–92. doi: 10.1016/0304-3940(83)90509-8. [DOI] [PubMed] [Google Scholar]
- Záborszky L., Léránth C., Makara G. B., Palkovits M. Quantitative studies on the supraoptic nucleus in the rat. II. Afferent fiber connections. Exp Brain Res. 1975 May 22;22(5):525–540. doi: 10.1007/BF00237352. [DOI] [PubMed] [Google Scholar]