Abstract
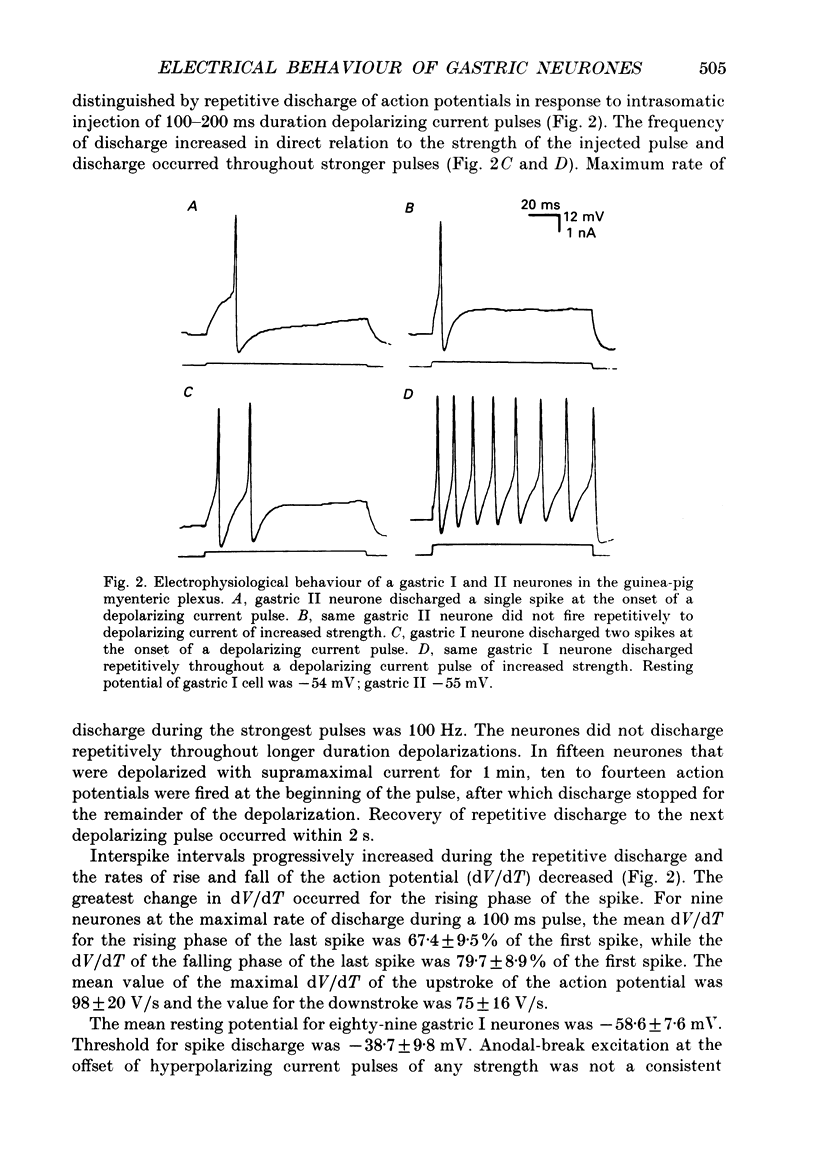
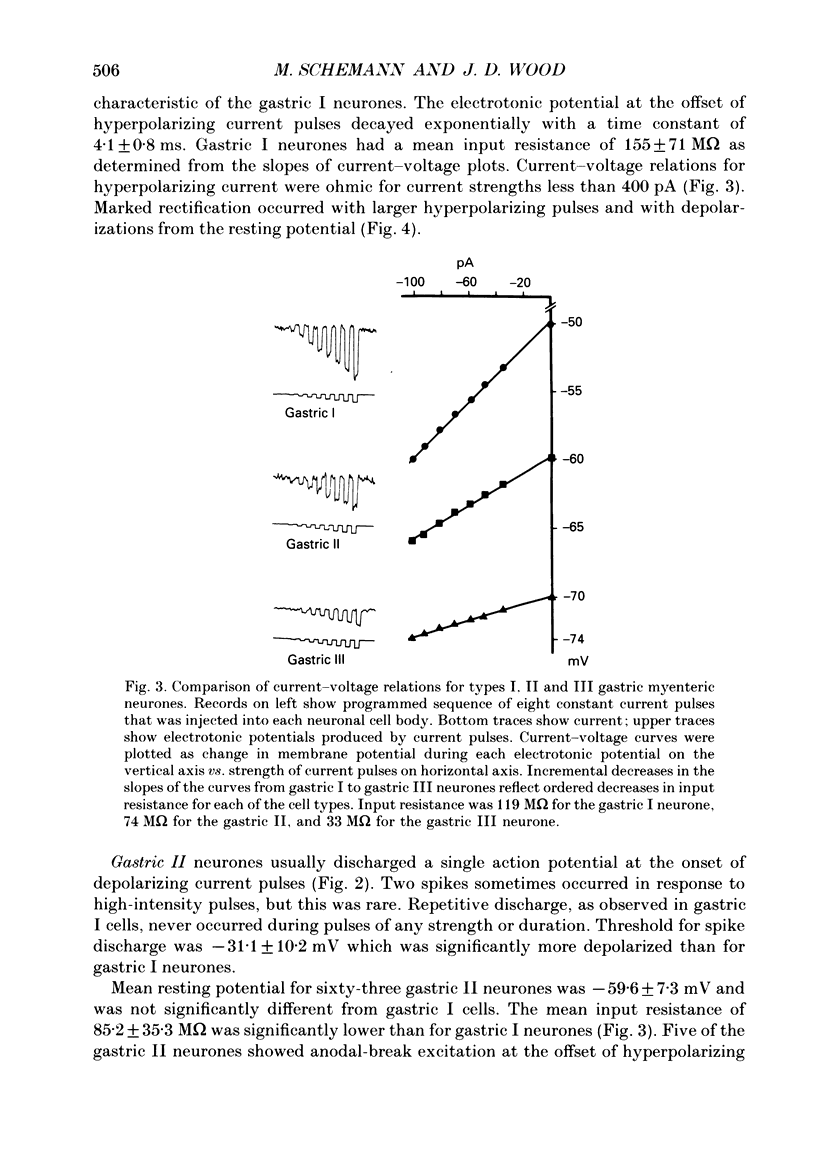
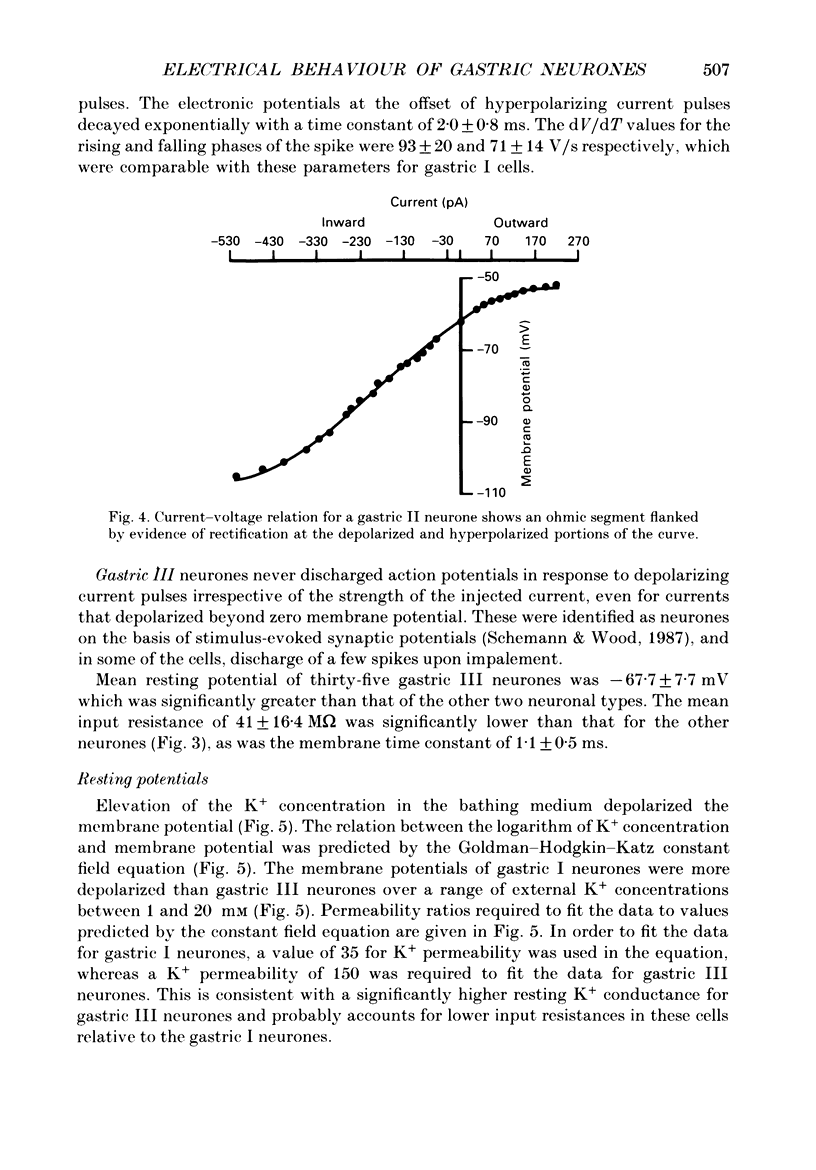
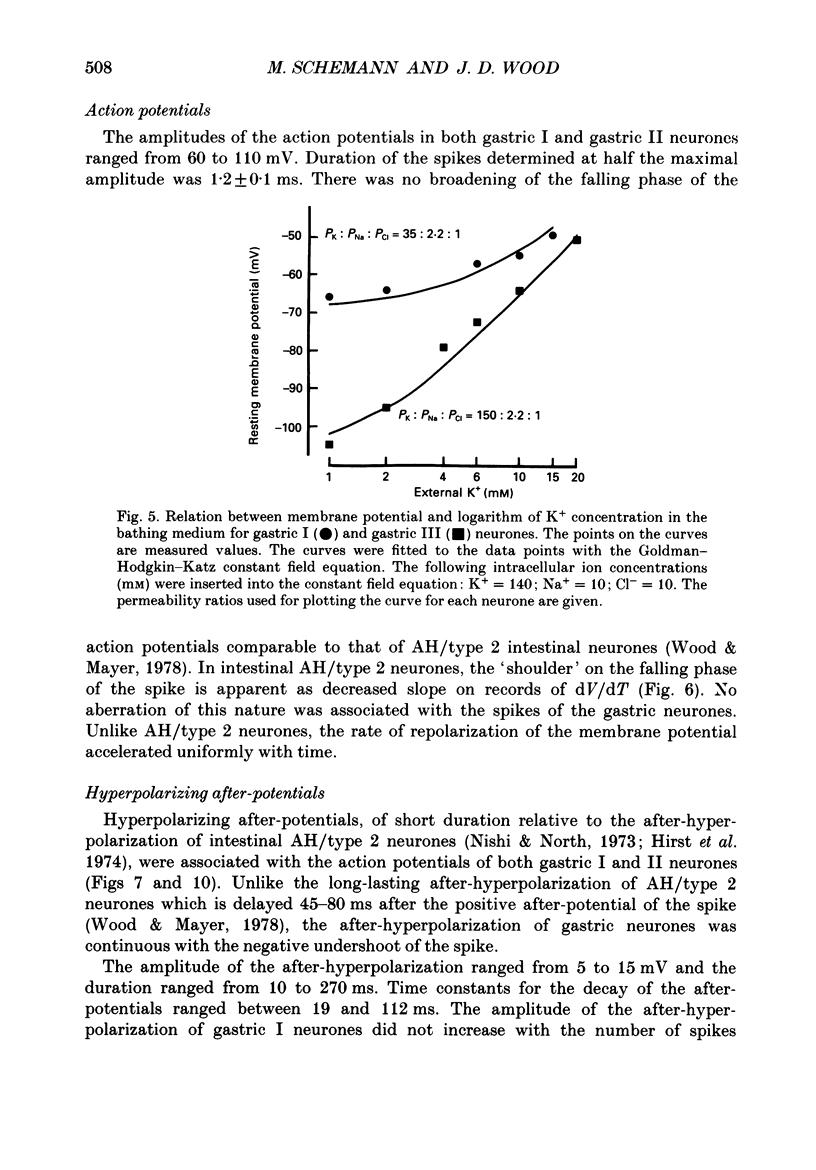
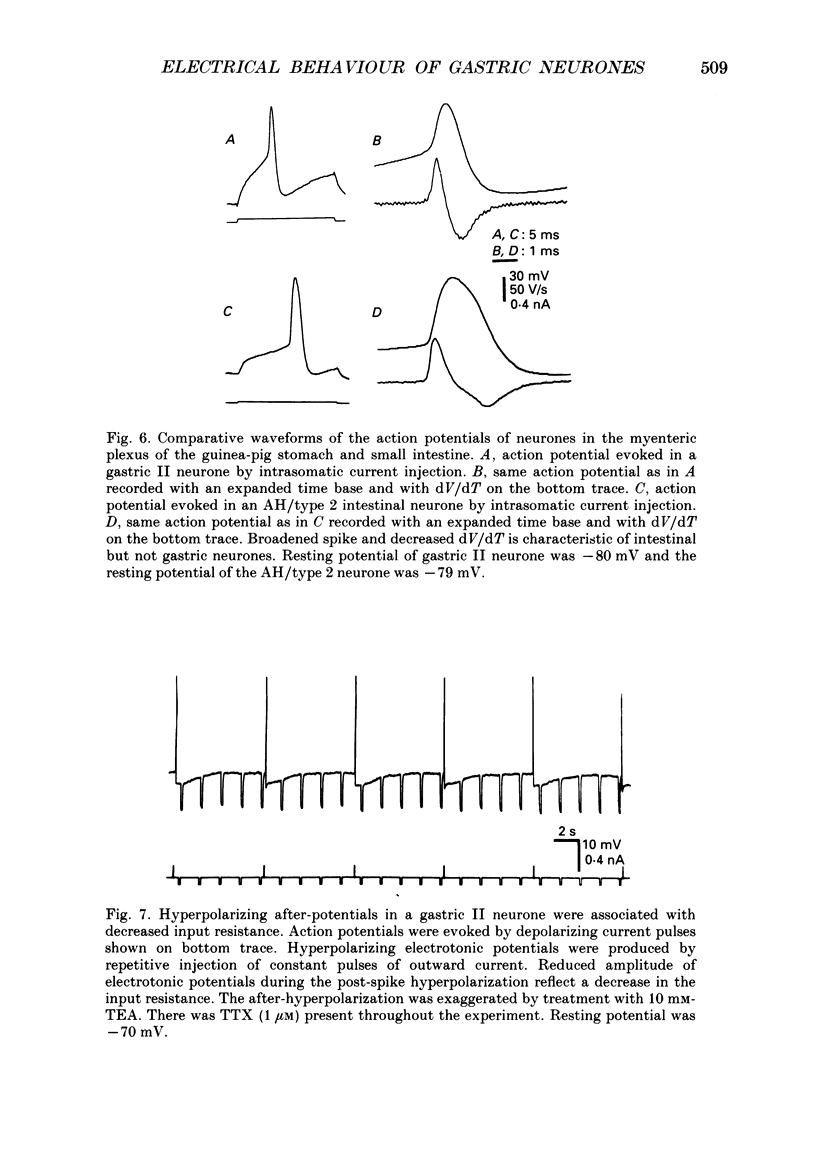
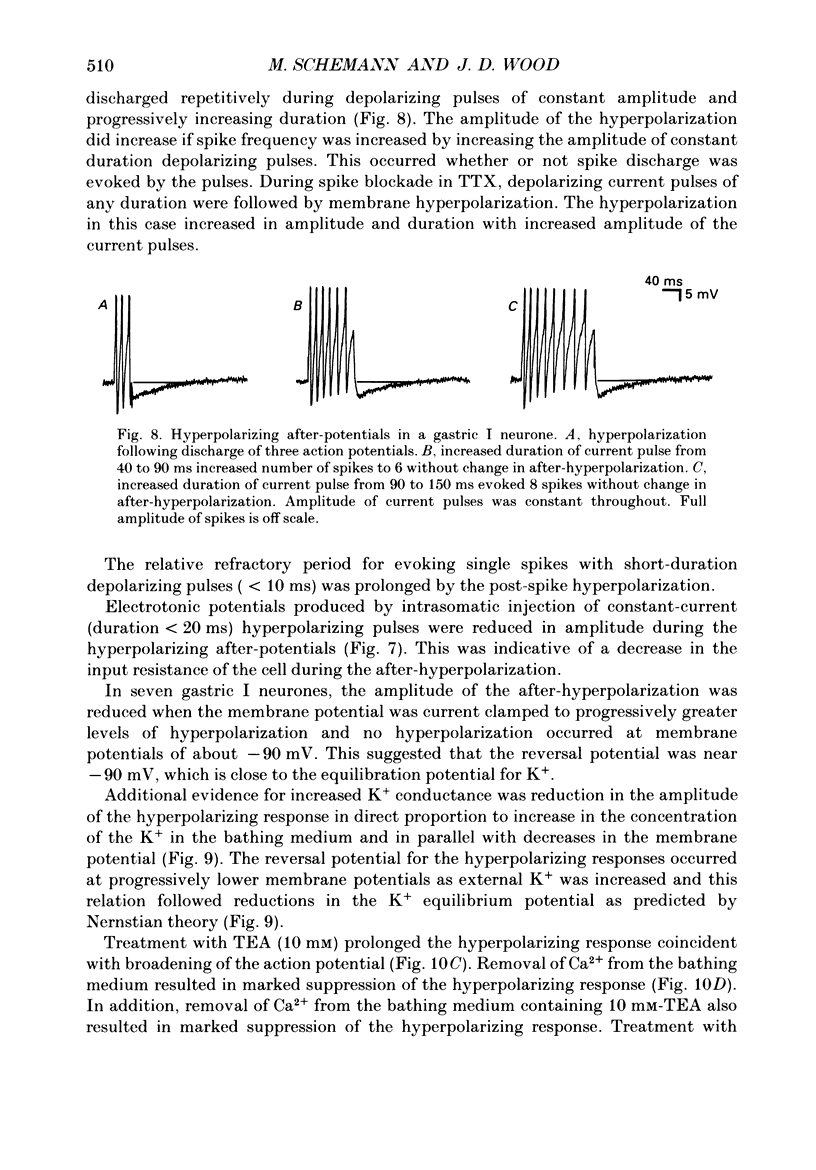
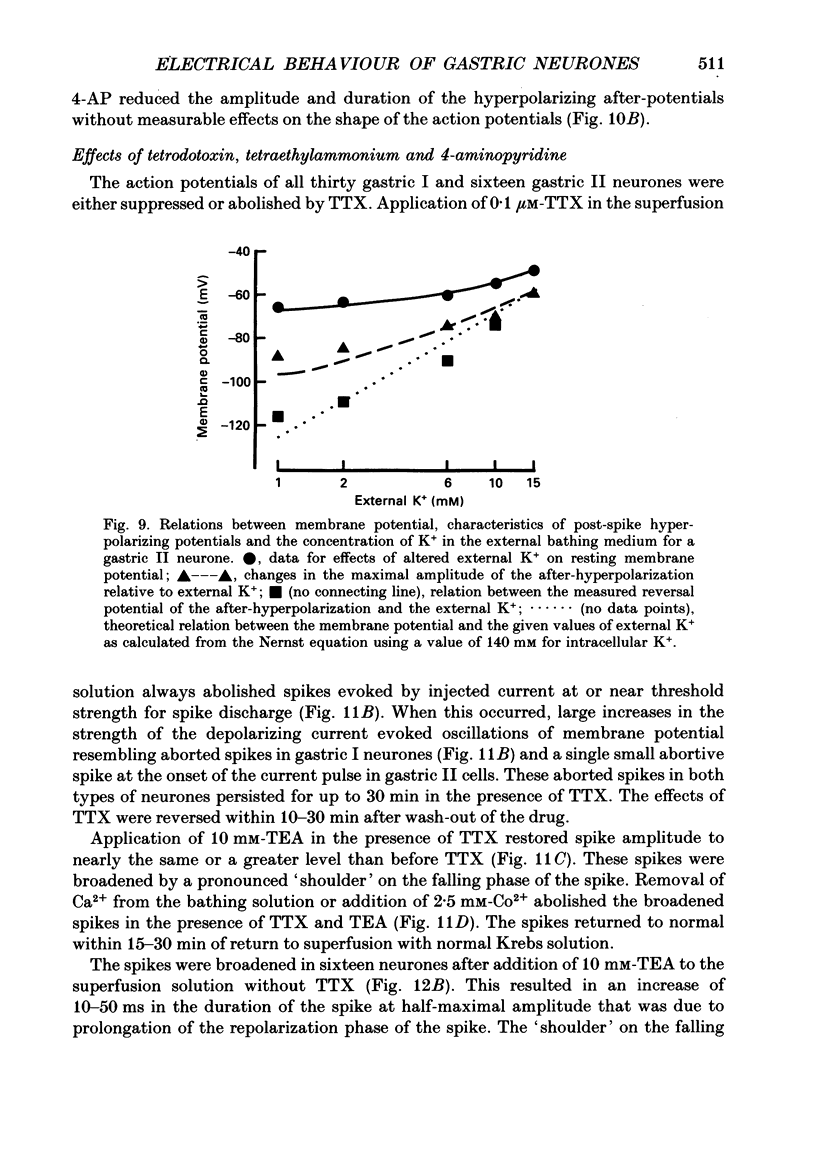
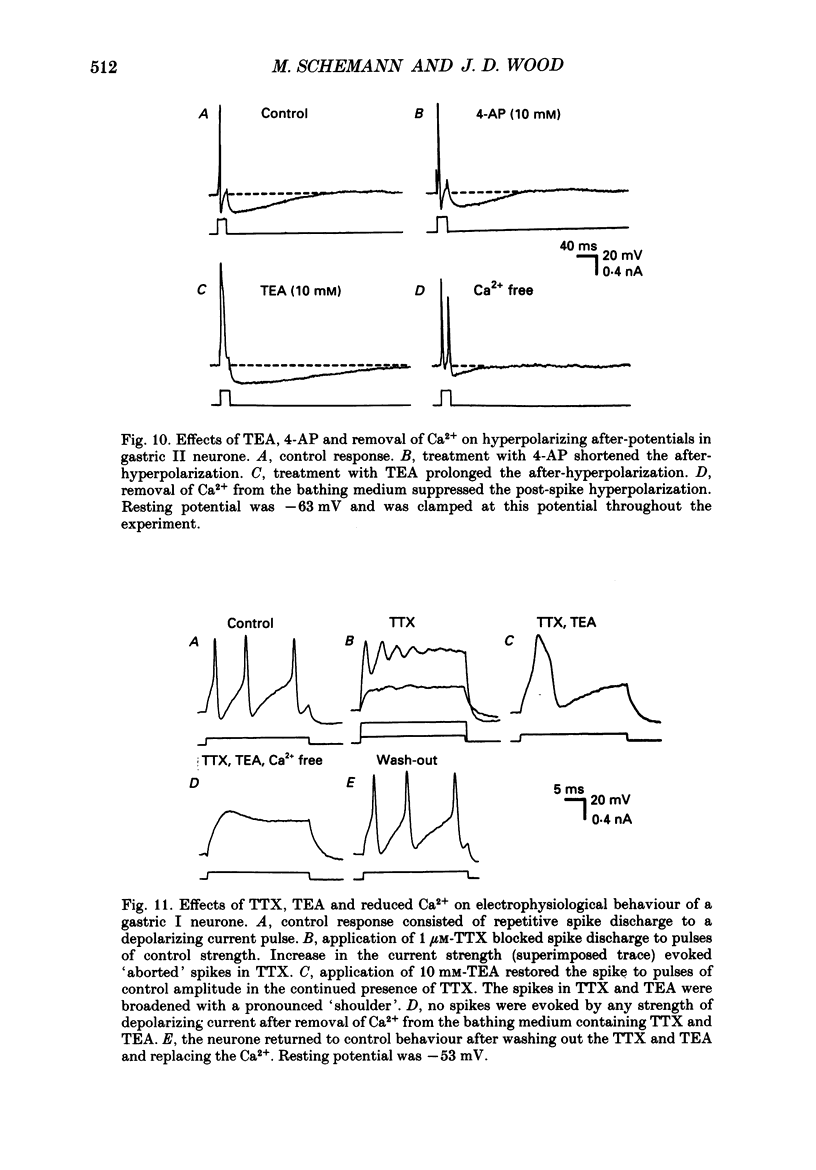
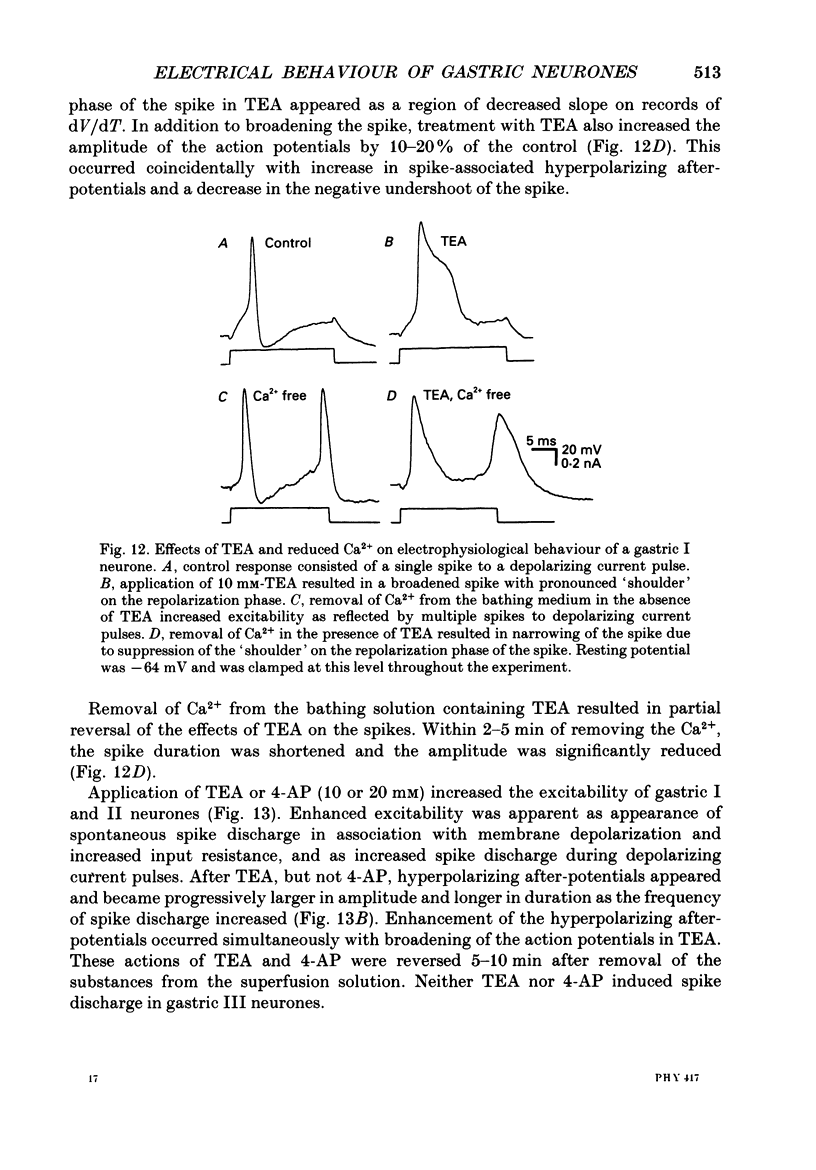
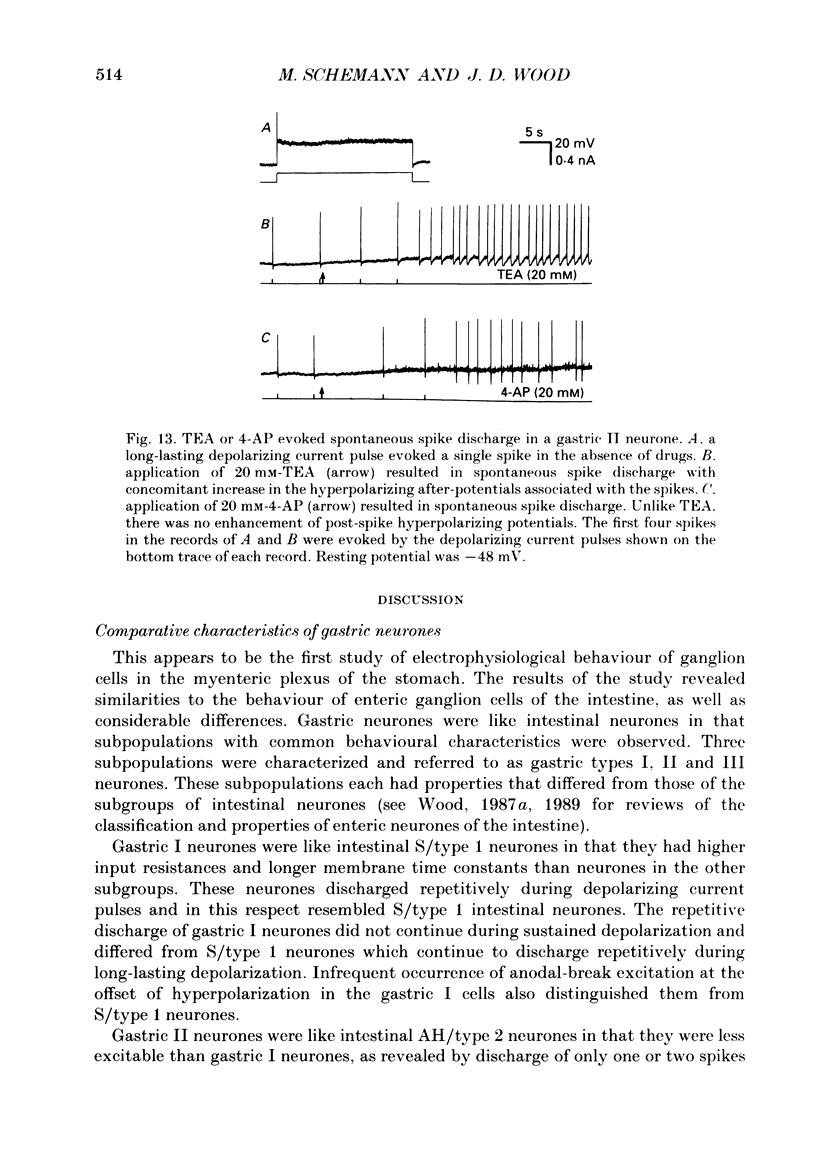
1. Electrical behaviour of ganglion cells in the myenteric plexus of the guinea-pig stomach was investigated using intracellular recording methods. 2. Three subpopulations were identified and classified for convenience of discussion as gastric I, II and III neurones. Gastric I neurones were characterized by repetitive spike discharge during depolarizing current pulses and by higher input resistance than the other types. Gastric II neurones discharged one or two spikes only at the onset of long-lasting depolarizing current pulses. Gastric III neurones did not discharge spikes to depolarizing current pulses and had higher membrane potentials and lower input resistances than the other types. Non-stimulus evoked discharge ('spontaneous' discharge) did not occur in any of the neurones. 3. Resting membrane potentials were generated primarily by resting K+ conductance, but were smaller than the estimated K+ equilibrium potential. Analysis based on the constant field equation predicted lower K+ conductance in gastric I than in gastric III neurones. 4. Action potentials in gastric I and II neurones were suppressed or blocked by tetrodotoxin. Spikes that were broadened by tetraethylammonium appeared to have an inward component of Ca2+ current. 5. Hyperpolarizing after-potentials were associated with the spikes of both kinds of neurones. These after-potentials had much shorter duration (less than 300 ms) than the post-spike hyperpolarization of AH/type 2 intestinal neurones and unlike intestinal neurones there was no latency between the positive after-potential of the spike and the onset of the hyperpolarization. After-hyperpolarization in the gastric neurones was enhanced when the spikes were broadened by tetraethylammonium and was suppressed by removal of Ca2+ from the bathing solution. 6. Treatment with either tetraethylammonium or 4-aminopyridine enhanced excitability and induced 'spontaneously' occurring repetitive spike discharge. 7. The electrophysiological behaviour of gastric myenteric neurones differed significantly from intestinal neurones. This was interpreted as specialization of the neural networks that control and co-ordinate the activity of vastly different effector systems in the two regions of the alimentary canal.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Erde S. M., Sherman D., Gershon M. D. Morphology and serotonergic innervation of physiologically identified cells of the guinea pig's myenteric plexus. J Neurosci. 1985 Mar;5(3):617–633. doi: 10.1523/JNEUROSCI.05-03-00617.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon M. D., Erde S. M. The nervous system of the gut. Gastroenterology. 1981 Jun;80(6):1571–1594. [PubMed] [Google Scholar]
- Hirst G. D., Holman M. E., Spence I. Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. J Physiol. 1974 Jan;236(2):303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Johnson S. M., van Helden D. F. The calcium current in a myenteric neurone of the guinea-pig ileum. J Physiol. 1985 Apr;361:297–314. doi: 10.1113/jphysiol.1985.sp015647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Spence I. Calcium action potentials in mammalian peripheral neurones. Nat New Biol. 1973 May 9;243(123):54–56. [PubMed] [Google Scholar]
- King B. F., Szurszewski J. H. Intracellular recordings from vagally innervated intramural neurons in opossum stomach. Am J Physiol. 1984 Feb;246(2 Pt 1):G209–G212. doi: 10.1152/ajpgi.1984.246.2.G209. [DOI] [PubMed] [Google Scholar]
- Nishi S., North R. A. Intracellular recording from the myenteric plexus of the guinea-pig ileum. J Physiol. 1973 Jun;231(3):471–491. doi: 10.1113/jphysiol.1973.sp010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payette R. F., Tennyson V. M., Pham T. D., Mawe G. M., Pomeranz H. D., Rothman T. P., Gershon M. D. Origin and morphology of nerve fibers in the aganglionic colon of the lethal spotted (ls/ls) mutant mouse. J Comp Neurol. 1987 Mar 8;257(2):237–252. doi: 10.1002/cne.902570209. [DOI] [PubMed] [Google Scholar]
- Schemann M., Wood J. D. Synaptic behaviour of myenteric neurones in the gastric corpus of the guinea-pig. J Physiol. 1989 Oct;417:519–535. doi: 10.1113/jphysiol.1989.sp017816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. W. Lucifer dyes--highly fluorescent dyes for biological tracing. Nature. 1981 Jul 2;292(5818):17–21. doi: 10.1038/292017a0. [DOI] [PubMed] [Google Scholar]
- Wood J. D., Mayer C. J. Intracellular study of electrical activity of Auerbach's plexus in guinea-pig small intestine. Pflugers Arch. 1978 May 31;374(3):265–275. doi: 10.1007/BF00585604. [DOI] [PubMed] [Google Scholar]
- Wood J. D. Neurophysiological theory of intestinal motility. Nihon Heikatsukin Gakkai Zasshi. 1987 Jun;23(3):143–186. [PubMed] [Google Scholar]




