Abstract
BACKGROUND:
Patients with heart failure with preserved ejection fraction and obesity have significant disability and frequent exacerbations of heart failure. We hypothesized that tirzepatide, a long-acting agonist of glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptors, would improve a comprehensive suite of clinical end points, including measures of health status, functional capacity, quality of life, exercise tolerance, patient well-being, and medication burden, in these patients.
METHODS:
We randomized (double-blind) 731 patients with class II to IV heart failure, ejection fraction ≥50%, and body mass index ≥30 kg/m2 to tirzepatide (titrated up to 15 mg SC weekly; n=364) or placebo (n=367) added to background therapy for a median of 104 weeks (quartile 1, 66; quartile 3, 126 weeks). The primary end points were whether tirzepatide reduced the combined risk of cardiovascular death or worsening heart failure and improved Kansas City Cardiomyopathy Questionnaire Clinical Summary Score. The current expanded analysis included sensitivity analyses of the primary end points, 6-minute walk distance, EQ-5D-5L health state index, Patient Global Impression of Severity Overall Health score, New York Heart Association class, use of heart failure medications, and a hierarchical composite based on all-cause death, worsening heart failure, and 52-week changes in Kansas City Cardiomyopathy Questionnaire Clinical Summary Score and 6-minute walk distance.
RESULTS:
Patients were 65.2±10.7 years of age; 53.8% (n=393) were female; body mass index was 38.2±6.7 kg/m2; Kansas City Cardiomyopathy Questionnaire Clinical Summary Score was 53.5±18.5; 6-minute walk distance was 302.8±81.7 m; and 53% (n=388) had a worsening heart failure event in the previous 12 months. Compared with placebo, tirzepatide produced a consistent beneficial effect across all composites of death and worsening heart failure events, analyzed as time to first event (hazard ratios, 0.41–0.67). At 52 weeks, tirzepatide increased the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score by 6.9 points (95% CI, 3.3–10.6; P<0.001), 6-minute walk distance 18.3 meters (95% CI, 9.9–26.7; P<0.001), and EQ-5D-5L 0.06 (95% CI, 0.03–0.09; P<0.001). The tirzepatide group shifted to a more favorable Patient Global Impression of Severity Overall Health score (proportional odds ratio, 1.99 [95% CI, 1.44–2.76]) and New York Heart Association class (proportional odds ratio, 2.26 [95% CI, 1.54–3.31]; both P<0.001) and required fewer heart failure medications (P=0.015). The broad spectrum of effects was reflected in benefits on the hierarchical composite (win ratio, 1.63 [95% CI, 1.17–2.28]; P=0.004).
CONCLUSIONS:
Tirzepatide produced a comprehensive, meaningful improvement in heart failure across multiple complementary domains; enhanced health status, quality of life, functional capacity, exercise tolerance, and well-being; and reduced symptoms and medication burden in patients with heart failure with preserved ejection fraction and obesity.
REGISTRATION:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT04847557.
Keywords: heart failure, preserved ejection fraction; obesity; tirzepatide
Clinical Perspective.
What Is New?
SUMMIT (Study of Tirzepatide in Participants with Heart Failure With Preserved Ejection Fraction and Obesity) is the first trial on patients with heart failure with preserved ejection fraction and obesity in which heart failure outcomes were examined as the primary prespecified end point.
Tirzepatide reduced the risk of worsening heart failure events requiring hospitalization or the use of intravenous medications in an urgent care setting; the hazard ratio for this analysis was 0.41 (95% CI, 0.22–0.75; P=0.004).
Tirzepatide also improved a broad range of measures of heart failure severity, which included health status, functional capacity, global well-being, quality of life, exercise tolerance, and medication burden.
What Are Clinical Implications?
The SUMMIT trial demonstrated that in patients with heart failure with preserved ejection fraction and obesity, tirzepatide treatment produces a consistent and highly significant beneficial effect on multiple orthogonal metrics of disease severity at all measured time points.
These results may be applicable to a wide range of patients with heart failure with preserved ejection fraction with obesity, including patients without meaningfully increased levels of NT-proBNP (N-terminal prohormone B-type natriuretic peptide) before treatment.
The SUMMIT trial data may have important implications in the development of guidelines for the treatment of patients with heart failure with preserved ejection fraction and obesity.
Editorial, see p 669
Patients with heart failure (HF) with preserved ejection fraction (HFpEF) have severe functional disability and an unfavorable clinical disease trajectory, characterized by frequent exacerbations of HF.1,2 Over the past decade, the use of medications that have been demonstrated to be effective in patients with HF with reduced ejection fraction (angiotensin receptor neprilysin inhibitors, mineralocorticoid receptor antagonists, and sodium-glucose cotransporter 2 [SGLT2] inhibitors) has been evaluated in large-scale trials in patients with HF and mildly reduced ejection fraction and patients with HFpEF.3–6 These trials have reported favorable effects in patients with HFpEF7,8; however, taken collectively, the magnitude of the benefit of these medications on worsening HF events, health status, and exercise capacity has generally been modest.9–12 As a result, patients with HFpEF continue to have substantial symptoms, functional disability, and an unfavorable clinical course.
None of the medications developed for HFpEF target obesity, one of the primary mechanisms that drive the development of HFpEF.13–15 The evolution and progression of HFpEF are enhanced as body mass index (BMI) increases,14,16 and weight-loss interventions (eg, gastric bypass surgery and glucagon-like peptide-1 receptor agonists) can reduce the risk of incident HF and improve symptoms in patients with established HFpEF.16–20
Tirzepatide, a long-acting agonist of glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptors,21 has been shown to produce ≈12% to 21% weight loss in patients with obesity.22 In the SUMMIT trial (Study of Tirzepatide in Participants With Heart Failure With Preserved Ejection Fraction and Obesity), tirzepatide reduced the risk of the composite of cardiovascular death and worsening HF events.23 In this report, we asked whether tirzepatide would improve a comprehensive suite of clinical end points across multiple domains of HF, including diverse definitions of worsening HF events and measures of health status, functional capacity, quality of life, exercise tolerance, and medication burden, in patients with HFpEF and obesity.
Methods
The primary results, trial protocol, and statistical analysis plan for the SUMMIT trial have been published.23 The ethics committee at each investigative site approved the trial, and all patients provided written informed consent. The ClinicalTrials.gov identifier is NCT04847557. The sponsor was Eli Lilly and Company. In collaboration with the sponsor, the academic members of the steering committee developed and amended the protocol, oversaw the recruitment of patients and the quality of follow-up, supervised the analysis of data, and provided an independent interpretation of the results.
Eli Lilly and Company provided access to all individual participant data collected during the trial after anonymization, except for pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set after data have been made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data-sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data-sharing environment. For details on submitting a request, instructions are provided online (https://vivli.org).
Study Patients
Participants with HFpEF who were ≥40 years of age with chronic New York Heart Association (NYHA) class II to IV HF, left ventricular ejection fraction ≥50%, and BMI ≥30 kg/m2 were enrolled. Patients were required to have substantial symptoms and functional limitation attributable to HFpEF, as reflected by a 6-minute walk distance (6MWD) of 100 to 425 m and a Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) ≤80 at baseline, along with objective evidence of HF, as demonstrated by ≥1 of the following: (1) elevated NT-proBNP (N-terminal prohormone of brain natriuretic peptide; ≥200 pg/mL in sinus rhythm or ≥600 pg/mL in atrial fibrillation), (2) structural heart disease, defined by left atrial enlargement by 2-dimensional echocardiography, or (3) elevated filling pressures at rest or with exercise (by invasive hemodynamic measurement or echocardiography). To enrich the study for greater risk of HF events, patients were also required to have either HF decompensation in the preceding 12 months or an estimated glomerular filtration rate <70 mL·min−1·1.73 m−2. Full inclusion and exclusion criteria have been published.23
Study Procedures
After screening, eligible patients were randomized double-blind (1:1) to receive placebo or 2.5 mg of tirzepatide subcutaneously per week in addition to usual therapy. Randomization was stratified by HF decompensation within 12 months, type 2 diabetes, and BMI ≥35 kg/m2. The dose of the double-blind study medication was increased by 2.5 mg every 4 weeks as tolerated until a dose of 15 mg per week of tirzepatide or matching placebo could be achieved after 20 weeks, which was maintained until the end of the trial. Double-blind treatment was to be continued until the last randomized patient was followed up for 52 weeks.
Study End Points
Patients were evaluated at prespecified intervals to assess a broad range of measures of HF status, severity, and impact, including ongoing ascertainment of clinical events (death and worsening HF events), with a median follow-up of 104 weeks (quartile 1, 66 weeks; quartile 3, 126 weeks; maximum follow-up, 3.0 years). In addition, the following were assessed at 24 and 52 weeks: KCCQ-CSS, 6MWD, EQ-5D-5L score, the Patient Global Impression of Severity (PGIS) Overall Health,24 and NYHA class. The use of HF medications was evaluated throughout follow-up.
The 2 primary end points of the SUMMIT trial were the time to first worsening HF event or cardiovascular death (α=0.04) and the change in KCCS-CSS at 52 weeks (α=0.01). A worsening HF event was defined as an exacerbation of symptoms requiring hospitalization, intravenous drugs for HF in an urgent care setting, or intensification of oral diuretics. Oral diuretic intensification in the absence of worsening symptoms was not designated as a worsening HF event. All events were blindly adjudicated by a clinical events committee. Secondary end points included change in 6MWD, body weight, and high-sensitivity C-reactive protein. Type 1 error rate was controlled for the evaluation of these end points with a prespecified graphical testing scheme, which allowed α=0.01 to be recycled to the primary events outcome (yielding α=0.05).
In the present study, we prospectively evaluated a comprehensive suite of additional prespecified clinical end points, including sensitivity analyses of the primary and secondary end points and changes in the EQ-5D-5L health state index, PGIS Overall Health, NYHA class, and use of HF medications. In addition, a composite hierarchical end point (analyzed as a win ratio) was based on time to all-cause mortality throughout the study, timing and number of worsening HF events throughout the study, threshold changes in KCCQ-CSS at 52 weeks, and threshold changes in 6MWD at 52 weeks.
Statistical Methods
Analyses were performed according to the intention-to-treat principle based on data from all randomized participants, regardless of adherence to study medication, and with multiple imputation methods for missing data (conducted when specified); sensitivity analyses were based on the on-treatment estimands. Time-to-first-event analysis of composite outcome end points was conducted with Cox proportional hazards model with treatment as a fixed effect with adjustment for the prespecified covariates of: (1) history of type 2 diabetes (yes or no), (2) baseline HFpEF-ABA score25 (clinical model using age, body mass index, and history of atrial fibrillation; <0.8 or ≥0.8), and (3) baseline NT-proBNP (<200 or ≥200 ng/L).25 Sensitivity analyses examined alternative definitions of mortality and worsening HF events.
For the intention-to-treat analyses, changes in KCCQ-CSS and 6MWD at week 52 from baseline were evaluated with the stratified Wilcoxon test, and missing data at 52 weeks were imputed according to prespecified procedures. In addition, for the intention-to-treat analysis, a mixed-effects model repeated-measures analysis was conducted to analyze the change in KCCQ-CSS, 6MWD, NT-proBNP, and EQ-5D-5L at 12, 24, and 52 weeks. NT-proBNP was log-transformed before modeling and presented as percent change. The mixed-effects repeated-measures model included treatment, time, treatment-by-time interaction, stratification factors (HF decompensation within 12 months of screening [yes or no], history of type 2 diabetes [yes or no], and baseline BMI [<35, or ≥35 kg/m2]) as fixed effects and baseline value as a covariate. Restricted maximum likelihood was used to obtain model parameter estimates, and the Kenward-Roger option was used to estimate the denominator degrees of freedom. An unstructured covariance structure was used to model the within-participant errors. Mixed-effects repeated-measures sensitivity analysis was performed using data during the on-treatment period, defined as a measurement within 7 days of the last dose of study medication.
Logistic regression was used to analyze the proportion of subjects who reached different thresholds of change from baseline in KCCQ-CSS and 6MWD. The logistic regression analysis included baseline value, treatment, and stratification factors (HF decompensation within 12 months of screening [yes or no], diagnosed type 2 diabetes [yes or no], and baseline BMI [<35 or ≥35 kg/m2]) as covariates.
The categorical change in NYHA class and PGIS Overall Health (improved, no change, or worsened) from baseline was analyzed with a proportional odds model. The response variable of the analysis model was the change in class from baseline. The independent variables of the model were the categorical effect of treatment, stratification factors, and baseline class.
The meaningful within-patient change thresholds of improvement for the KCCQ-CSS (20 points) and 6MWD (25 m) were determined with anchor-based methods based on blinded data from the first two-thirds of randomized participants in the SUMMIT study. A win ratio, calculated as the number of pairs of tirzepatide-treated participants wins divided by number of pairs of placebo participants wins, was obtained using a nonparametric generalized pairwise comparison within each stratum based on the following hierarchy: time to all-cause mortality, number and timing of HF events, change from baseline in KCCQ-CSS category at 52 weeks (≥10-point worsening, ≥5- but <10-point worsening or no change [<5-point change]; ≥5- but <10-point improvement, ≥10- but <15-point improvement, and ≥15-point improvement), and change from baseline in the 6MWD category at 52 weeks (≥30% worsening, ≥20% and <30% worsening, ≥10% and <20% worsening, no change [<10% change], ≥10% and <20% improvement, ≥20% and <30% improvement, and ≥30% improvement). Variance and P values were calculated with the asymptotic normal U statistics approach. The Wilcoxon rank-sum test was used to analyze differences in medications. Statistical software used for analysis was SAS Enterprise Guide 7.15 and 8.2.
Results
Patient Characteristics
A total of 731 patients with HFpEF and obesity were randomly assigned to receive tirzepatide (n=364) or placebo (n=367) at 129 centers in 9 countries (Figure S1). The study population displayed typical characteristics of HFpEF: 65.2±10.7 years of age, 53.8% (n=393) female, BMI of 38.2±6.7 kg/m2, and substantial HF symptom burden and high prevalence of comorbidities, including diabetes, hypertension, atrial fibrillation, and chronic kidney disease. Patient-reported health status and exercise capacity were severely impaired (mean KCCQ-CSS, 53.5±18.5; 6MWD, 302.8±81.7 m), and 53% of the participants (n=388) had experienced a hospitalization or urgent care visit for worsening HF in the preceding 12 months. At baseline, 466 patients (63.7%) were taking loop diuretics, and 121 (16.6%) were taking thiazide diuretics. There were no baseline differences in those randomized to tirzepatide and those randomized to placebo (Table). At 52 weeks, by intention to treat, compared with placebo, tirzepatide decreased body weight by 11.6% (95% CI, 12.9–10.4; P<0.001).
Table.
Characteristics of the Patients at Baseline

Effect of Tirzepatide on Outcomes
As reported separately,23 tirzepatide reduced the risk of the composite of cardiovascular death or worsening HF events (hazard ratio, 0.62 [95% CI, 0.41–0.95]; P=0.026). Favorable effects of tirzepatide were consistently demonstrated in all sensitivity analyses (Figures 1 and 2). When all-cause mortality was analyzed instead of cardiovascular death as a component of the composite, the hazard ratio for the primary events end point was 0.67 (95% CI, 0.46–0.99; P=0.045; Figure 1A). The effect of tirzepatide was driven by an effect on worsening HF events requiring hospitalization or the use of intravenous medications in an urgent care setting; the hazard ratio for this analysis was 0.41 (95% CI, 0.22–0.75; P=0.004; Figure 1B). The hazard ratios for all sensitivity analyses of the primary composite events end point ranged from 0.41 to 0.67 (Figure 2).
Figure 1.
Effects of tirzepatide on fatal and HF events. A, Effect of tirzepatide on time to first all-cause death or worsening heart failure (HF) event. In an intention-to-treat analysis, tirzepatide significantly reduced this end point by 33% compared with placebo (hazard ratio [HR], 0.67 [95% CI, 0.45–0.99]; P=0.045). Worsening HF event was defined as worsening symptoms of HF requiring hospitalization, intravenous therapy in an urgent care setting, or oral diuretic intensification. B, Effect of tirzepatide on time to first HF event (excluding oral diuretic intensification). In an intention-to-treat analysis, tirzepatide significantly reduced this end point by 59% compared with placebo (HR, 0.41 [95% CI, 0.22–0.75]; P=0.004).
Figure 2.
Forest plot examining effects of tirzepatide vs placebo on end-point event rates. In intention-to-treat analysis, 9 composite and individual event combinations were examined; compared with placebo, tirzepatide produced a consistent beneficial effect across all composite and individual event combinations (hazard ratios, 0.41–0.67).
Key Secondary End Points of Health Status and Exercise Function
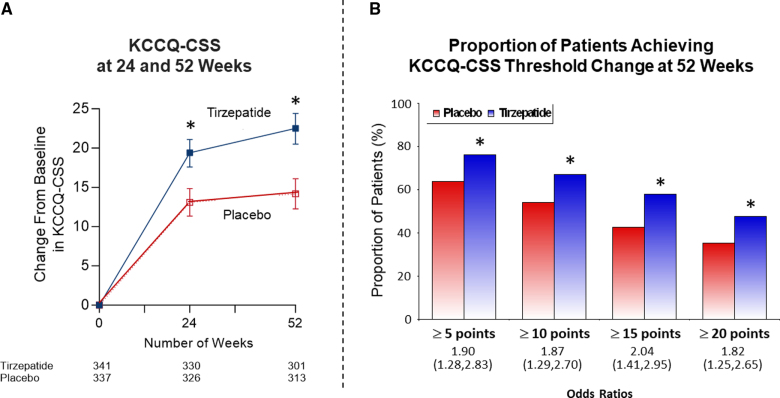
At 52 weeks, compared with placebo, tirzepatide increased KCCQ-CSS (between-group median difference, 6.9 points [95% CI, 3.3–10.6]; P<0.001 by intention-to-treat23; Figure 3A) and a between-group mean difference of 9.8 points (95% CI, 7.1–12.5; P<0.001) for the on-treatment estimand (Figure S2). The treatment difference was evident at 24 weeks. At 52 weeks, the proportions of subjects who reached threshold changes of ≥5-, ≥10-, ≥15-, and ≥20-point increase in KCCQ-CSS according to intention-to-treat are displayed in Figure 3B. Tirzepatide increased the proportion of patients reaching each threshold compared with placebo, with odds ratios ranging from 1.8 to 2.0. At 52 weeks, a significantly greater proportion of participants treated with tirzepatide met the minimum clinically meaningful improvement threshold of 20 points compared with the placebo group (47.6% versus 35.2%; odds ratio, 1.82 [95% CI, 1.25–2.65]; P=0.002).
Figure 3.
Effects of tirzepatide on Kansas City Cardiomyopathy Questionnaire. A, Effect of tirzepatide on change from baseline in Kansas City Cardiomyopathy Questionnaire-Clinical Summary Score (KCCQ-CSS) at 24 and 52 weeks of treatment. In an intention-to-treat analysis, compared with placebo, tirzepatide increased KCCQ-CSS with a between-group difference of 6.9 points (95% CI, 3.3–10.6; P<0.001). B, Effect of tirzepatide on the proportion of participants achieving KCCQ-CSS thresholds of ≥5, 10, 15, and 20 points. In intention-to-treat analysis and with prespecified imputation procedures for missing data, tirzepatide increased the proportion of subjects reaching each threshold compared with placebo, with odds ratios ranging from 1.8 to 2.0. *P<0.01, tirzepatide vs placebo.
At 52 weeks, by intention to treat, compared with placebo, the tirzepatide group showed a greater increase in 6MWD (between-group median difference, 18.3 m [95% CI, 9.9–26.7]; P<0.00123; Figure 4A); the on-treatment estimand for the mean effect of tirzepatide was 30.3 m (95% CI, 20.3–40.3; P<0.001; Figure S2). The difference between tirzepatide and placebo was evident at 24 weeks of treatment. The proportion of subjects who reached threshold changes of ≥10-, ≥20-, and ≥30-m increase in 6MWD at 52 weeks using intention to treat is displayed in Figure 4B, showing odds ratios ranging from 2.0 to 2.2. The anchor-based clinically meaningful threshold (meaningful within-patient change) for the 6MWD was 25 m. At 52 weeks, a significantly greater proportion of participants treated with tirzepatide met the minimum clinically meaningful improvement threshold of 25 m compared with the placebo group (51.7% versus 34.0%; odds ratio, 2.1 [95% CI, 1.5–2.9]; P<0.001).
Figure 4.
Effect of tirzepatide on exercise tolerance and health status. A, Effect of tirzepatide on change from baseline in 6-minute walk distance (6MWD). In an intention-to-treat analysis, compared with placebo, tirzepatide increased 6MWD with a between-group difference of 18.3 m (95% CI, 9.9, 26.7; P<0.001) at 52 weeks. B, Effect of tirzepatide on the proportion of subjects who reached threshold changes of ≥10-, ≥20-, and ≥30-m increase in 6MWD. In intention-to-treat analysis and with prespecified imputation procedures for missing data, tirzepatide increased the proportion of subjects reaching each threshold compared with placebo, with odds ratios ranging from 2.2 to 2.0. *P<0.001, tirzepatide vs placebo.
Additional Patient-Centered Measures of Health Status
At 52 weeks, by intention to treat, the EQ-5D-5L health state index increased by 0.12 (95% CI, 0.10–0.14) in the tirzepatide group and by 0.06 (95% CI, 0.04–0.08) in the placebo group (between-group difference, 0.06 [95% CI, 0.03–0.09]; P<0.001; Figure 5A). The difference between tirzepatide and placebo was also evident at 24 weeks of treatment.
Figure 5.
Effect of tirzepatide on change from baseline in EQ-5D-5L health state index and PGIS Overall Health. A, Effect of tirzepatide on change from baseline in EQ-5D-5L health state index. At 52 weeks, in an intention-to-treat analysis, tirzepatide increased EQ-5D-5L health state index with a between-group difference of 0.06 (95% CI, 0.033–0.087; P<0.001). B, Effect of tirzepatide on change in Patient Global Impression of Severity (PGIS) Overall Health. At 24 weeks, the tirzepatide group by intention to treat was more likely to show improvement in PGIS Overall Health (55.2% vs 40.4%) and less likely to report worsening in PGIS Overall Health (9.1% vs 11.5%). Proportional odds ratio (OR) for improvement in PGIS Overall Health with tirzepatide vs placebo was 1.68 (95% CI, 1.22–2.29; P=0.001). At 52 weeks, tirzepatide group by intention to treat was more likely to show improvement in PGIS Overall Health (55.2% vs 38.5%) and less likely to report worsening in PGIS Overall Health (7.4% vs 11.3%). Proportional OR for improvement in PGIS Overall Health with tirzepatide vs placebo was 1.99 (95% CI, 1.44–2.76; P<0.001). *P<0.001, tirzepatide vs placebo.
In addition, compared with placebo, more patients treated with tirzepatide reported improvement in the PGIS Overall Health (Figure 5B). At 52 weeks, by intention to treat, compared with the placebo group, patients in the tirzepatide group were more likely to show PGIS Overall Health improvement (55.2% versus 38.5%) and were less likely to report PGIS Overall Health worsening (7.4% versus 11.3%); the proportional odds ratio for a favorable effect was 1.99 (95% CI, 1.44–2.76; P<0.001; Figure 5B). The difference between tirzepatide and placebo was also evident at 24 weeks of treatment.
Physician-Based Measures of Clinical Status and Changes in HF Therapy
At 52 weeks, by intention to treat, participants in the tirzepatide group were significantly more likely to show improvement and less likely to show worsening NYHA functional class (Figure 6A). The proportional odds ratio for a favorable shift in NYHA class with tirzepatide versus placebo was 2.26 (95% CI, 1.54–3.31; P<0.001). Differences between tirzepatide and placebo in NYHA class were also evident at 24 weeks (Figure 6A).
Figure 6.
Effect of tirzepatide on change in NYHA class and medication use. A, Effect of tirzepatide on change in New York Heart Association (NYHA) class. Tirzepatide improved NYHA class at 52 weeks with a proportional odds ratio for improvement in ≥1 NYHA classes of 2.26 (95% CI, 1.54–3.31). B, Effect of tirzepatide on change in medications from baseline to end of follow-up by intention-to-treat analysis. Intensification of heart failure (HF) medications (inclusive of the use of inhibitors of the renin-angiotensin system and neprilysin, beta-blockers, mineralocorticoid receptor antagonists, and diuretics) occurred less frequently in the tirzepatide group; use of these HF medications was reduced more frequently in the tirzepatide group (P=0.015; left). Similar results occurred in changes in diuretics (P<0.001; right).
At 52 weeks, intensification of HF medications in the aggregate (inclusive of the use of inhibitors of the renin-angiotensin system and neprilysin, beta-blockers, mineralocorticoid receptor antagonists, SGLT2 inhibitors, and diuretics) occurred less frequently in the tirzepatide group, whereas the use of these HF medications was reduced more frequently in the tirzepatide group (Wilcoxon rank-sum test, P=0.015; Figure 6B). The differences in changes in diuretics (as a single category) was especially notable, with ≈50% fewer diuretic dose increases and 50% more frequent diuretic dose decreases in the tirzepatide group compared with the placebo group (P<0.001).
Other Measures of Tirzepatide Efficacy
Compared with placebo, patients treated with tirzepatide were more likely to show clinical benefit according to the hierarchical composite end point (win ratio, 1.63 [95% CI, 1.17–2.28]; P=0.004). The beneficial effect was consistent across each of the individual components of the hierarchical end point (Figure S3).
Compared with placebo, there was an ≈10% decrease in NT-proBNP levels from baseline at each of 3 time points in the tirzepatide group (P=0.024 at 12 weeks; P=0.063 at 24 weeks; P=0.072 at 52 weeks; Figure S4).
Discussion
The present report provides a comprehensive analysis of the effect of tirzepatide on a broad range of measures of HF severity, which included worsening HF events, health status, functional capacity, global well-being, quality of life, exercise tolerance, and medication burden. In patients with HFpEF and obesity, tirzepatide demonstrated a consistent and highly significant beneficial effect on multiple orthogonal metrics of disease severity at all measured time points. All sensitivity analyses of worsening HF events demonstrated a major reduction in exacerbations of HF, with hazard ratios of 0.41 to 0.67. In addition, every prespecified measurement of patient- and physician-reported health status, functional capacity, quality of life, patient global impression of severity overall health, exercise tolerance, and medication burden was substantially improved by tirzepatide compared with placebo.
Our findings are broadly similar to those reported for semaglutide in patients with HFpEF and obesity.19,20 In STEP-HFpEF (Research Study to Investigate How Well Semaglutide Works in People Living With Heart Failure and Obesity) and STEP-HFpEF-DM (Research Study to Look at How Well Semaglutide Works in People Living With Heart Failure, Obesity and Type 2 Diabetes), glucagon-like peptide-1 receptor agonism alone improved KCCQ-CSS, 6MWD, and NYHA class and reduced the need for diuretics,19,20,26,27 but these trials did not report effects on other patient-centered measures of quality of life and well-being (ie, EQ-5D-5L and PGIS Overall Health). In the SUMMIT trial, we showed an improvement in numerous measures of the clinical trajectory of HF, although patients in the tirzepatide group underwent less intensification of background therapy for HF and were more likely to have a reduction in the doses of HF treatments. The STEP-HFpEF program reported a decrease in worsening HF events with semaglutide in exploratory analyses28; in contrast, cardiovascular death and worsening HF events made up the primary end point of the SUMMIT trial. In the SUMMIT trial, the reduced risk of HF events was related primarily to substantially fewer exacerbations of HF requiring hospitalization or the use of intravenous medications in an urgent care setting.
The substantial effects of tirzepatide in the SUMMIT trial should be contrasted with the modest effects seen with neurohormonal antagonists and SGLT2 inhibitors in large-scale trials of patients with HFpEF. In the PARAGON-HF trial (Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction), neprilysin inhibition reduced the risk of cardiovascular death and total hospitalizations by 13%, improved KCCQ by 1.0 points, and had no overall effect on the EuroQol Visual Analog Scale.3,9 In the EMPEROR-Preserved trial (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction) and DELIVER trial (Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure), SGLT2 inhibition reduced the risk of cardiovascular death and worsening HF events by 18% to 21%4,5 and improved KCCQ-CSS by 1.5 to 2.3 points, with odds ratios for threshold improvements in KCCQ-CSS of 1.1 to 1.2,10,11 and SGLT2 inhibition did not improve 6MWD in patients with HFpEF in 2 trials.29,30 Somewhat more meaningful effects of SGLT2 inhibitors on KCCQ and 6MWD have been reported in patients with HFpEF and obesity.31 In FINEARTS-HF (Study to Evaluate the Efficacy [Effect on Disease] and Safety of Finerenone on Morbidity [Events Indicating Disease Worsening] & Mortality [Death Rate] in Participants With Heart Failure and Left Ventricular Ejection Fraction [Proportion of Blood Expelled Per Heart Stroke] Greater or Equal to 40%), finerenone reduced the risk of cardiovascular death and worsening HF events by 16%6 and improved KCCQ-CSS by 1.1 points, with odds ratios for threshold improvements in KCCQ-CSS of ≈1.1.12 In many of these trials,11,12 patients with a KCCQ-CCS >80 to 85 at baseline who maintained this score after randomization were considered to have improved 15 to 20 points, even if their KCCQ scores had not actually changed; we did not use such imputations in the SUMMIT trial. In the SUMMIT trial, tirzepatide reduced the risk of cardiovascular death or worsening HF events by 38% and improved KCCQ-CSS by 6.9 points, as well as 6MWD, EQ-5D-5L health state index, and PGIS Overall Health; the odds ratios for threshold changes in all these metrics were 1.8 to 2.2. The magnitude of the tirzepatide-mediated improvement in the win ratio was large and similar to the effect of transcatheter mitral valve repair in patients with HF and secondary mitral regurgitation.32
A large proportion of patients with HFpEF and obesity have circulating levels of NT-proBNP in the normal range33–35 or lower than those that have been used to qualify patients for participation in large-scale HFpEF trials. However, these patients were allowed to participate in the SUMMIT trial; thus, the median NT-proBNP in our trial was <200 pg/mL. This level was significantly lower than that in the STEP-HFpEF program (median, ≈470 pg/mL)36 and substantially lower than in other large-scale outcome trials in HFpEF (median 900–1400 pg/mL).3–6 Despite the lower NT-proBNP levels, patients enrolled in SUMMIT had a significantly increased rate of cardiovascular death or worsening HF events because patients were required to have a KCCQ-CSS <80 and impaired renal function or a recent worsening HF event.23 It should be noted that marked weight loss in patients with obesity produced by bariatric surgery or intense calorie restriction leads to 2- to 5-fold increases in NT-proBNP,37,38 potentially related to greater ventricular distention as a result of relief of adiposity-related chamber constraint.39 This increase in NT-proBNP may contribute to the natriuresis observed after marked weight loss.40 However, these expected increases in NT-proBNP may not be observed in HFpEF if the treatment produces a meaningful improvement in HF. It is therefore noteworthy that despite between-group weight loss of 11.6% and a reduced use of diuretics, tirzepatide decreased NT-proBNP by ≈10% in the SUMMIT trial. The magnitude of the decline in NT-proBNP was less marked than the 18% decrease observed in the STEP-HFpEF trials,36 potentially because of our much lower baseline value and because of the smaller decrease in body weight produced by semaglutide (ie, ≈8%–9%) compared with tirzepatide.
The favorable effects of tirzepatide on HF may be related to an amelioration of the blood volume expansion that characterizes obesity-related HF,41 and the decrease in adipocyte mass would be expected to minimize the antinatriuretic effects of secreted adipocytokines (particularly leptin and aldosterone).42–44 In addition, shrinkage of fat depots would diminish the proinflammatory phenotype of hypertrophied fat cells; this inflammatory response is manifest both systemically and locally by virtue of deleterious paracrine influence of epicardial adipose tissue.45,46 The lessened secretion of inflammatory adipocytokines might act to alleviate coronary endothelial inflammation with secondary beneficial effects on microvascular rarefaction and myocardial fibrosis, 2 important mechanisms in the pathogenesis of HFpEF.46–48 It is noteworthy that glucose-dependent insulinotropic polypeptide receptors are abundant in epicardial adipocytes,49 and the action of tirzepatide on glucose-dependent insulinotropic polypeptide receptors may produce incremental anti-inflammatory effects.44,50
The results of the present study should be considered in light of its strengths and limitations. The trial was prospectively designed to evaluate the long-term effects of tirzepatide on the risk of major adverse HF outcomes, but the effects on KCCQ-CSS, 6MWD, EQ-5D-5L health state index, PGIS Overall Health, and NYHA functional class were designed to be assessed only at 24 and 52 weeks. It is noteworthy that the effects of tirzepatide on these measures of symptom burden, exercise capacity, and quality of life were more pronounced at 52 weeks than at 24 weeks, but further study of the durability of these benefits beyond 1 year is warranted.
Conclusions
The treatment of patients with HFpEF and obesity with tirzepatide produced a broad spectrum of large and clinically meaningful benefits on the trajectory of HF, evident across multiple domains and including improved health status and well-being, enhanced functional capacity and exercise tolerance, reduced symptom and medication burden, and prevention of worsening HF events.
Article Information
Sources of Funding
The SUMMIT trial was funded by Eli Lilly and Company.
Disclosures
Dr Zile receives research support from the Department of Veterans Affairs (projects BX005943 and BX005848) and serves as a consultant for Abbott, Adona Medical, Aria CV, Avery Therapeutics, Inc, Boehringer-Ingelheim, Boston Scientific, Cardiovascular Research Foundation Clinical Trials Center, CVRx, DIASTOL Therapeutics, LLC, EBR, Edwards, Lilly, GenKardia, Innoventric, KestraMedical, Medtronic, Merck, Morphic Therapeutics, Novartis, Pulnova, Salubris Biotherapeutics, Sonata, SRNALYTICS, INC, V-WAVE, and Vectorious. Dr Borlaug receives research support from the National Institutes of Health (R01 HL128526, R01 HL162828, and U01 HL160226 from the National Heart, Lung, and Blood Institute) and the US Department of Defense, as well as research grant funding from AstraZeneca, Axon, Corvia, Novo Nordisk, and Tenax Therapeutics. Dr Borlaug has served as a consultant for Actelion, Amgen, Aria, Axon Therapies, BD, Boehringer Ingelheim, Cytokinetics, Edwards Lifesciences, Lilly, Imbria, Janssen, Merck, Novo Nordisk, NGM, NXT, and VADovations. Dr Borlaug is named inventor (US Patent No. 10 307 179) for the tools and approach for a minimally invasive pericardial modification procedure to treat heart failure. Dr Kramer has served as a consultant for and received research grants from Lilly and has received research grants from Cytokinetics and Bristol Meyers Squibb. Dr Baum has served as consultant for Altimmune, Amgen, Beren Therapeutics, Boehringer Ingelheim, Lilly, Esperion, Ionis Pharmaceuticals, Madrigal Pharmaceuticals, Merck, Novartis, and Regeneron. Dr Litwin reports being on the patient selection committee for Corvia and Axon and being a consultant for Novo Nordisk and Lilly. Dr Menon reports no conflicts. Drs Ou, Weerakkody, Hurt, Kanu, and Murakami are employed by Eli Lilly and Company. Dr Packer has served as a consultant for 89bio, Abbvie, Actavis, Altimmune, Alnylam, Amarin, Amgen, Ardelyx, ARMGO, AstraZeneca, Attralus, Biopeutics, Boehringer Ingelheim, Caladrius, Casana, CSL Behring, Cytokinetics, Lilly, Imara, Medtronic, Moderna, Novartis, Pharmacocosmos, Reata, Regeneron, Roche, and Salamandra.
Supplemental Material
Figures S1–S4
Nonstandard Abbreviations and Acronyms
- 6MWD
- 6-minute walk distance
- BMI
- body mass index
- HF
- heart failure
- HFpEF
- heart failure with preserved ejection fraction
- KCCQ-CSS
- Kansas City Cardiomyopathy Questionnaire Clinical Summary Score
- NT-proBNP
- N-terminal prohormone of brain natriuretic peptide
- NYHA
- New York Heart Association
- PGIS
- Patient Global Impression of Severity
- SGLT2
- sodium-glucose cotransporter 2
- STEP-HFpEF
- Research Study to Investigate How Well Semaglutide Works in People Living With Heart Failure and Obesity
- SUMMIT
- Study of Tirzepatide in Participants With Heart Failure With Preserved Ejection Fraction and Obesity
Supplemental Material, the podcast, and transcript are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.124.072679.
This work was presented as an abstract at AHA Scientific Sessions, Chicago, IL, November 16–18, 2024.
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
For Sources of Funding and Disclosures, see page 666.
Circulation is available at www.ahajournals.org/journal/circ
Contributor Information
Barry A. Borlaug, Email: borlaug.barry@mayo.edu.
Christopher M. Kramer, Email: cmk2n@uvahealth.org.
Sheldon E. Litwin, Email: litwins@musc.edu.
Venu Menon, Email: menonv@ccf.org.
Yang Ou, Email: yang.ou@lilly.com.
Govinda J. Weerakkody, Email: weerakkody_govinda@lilly.com.
Karla C. Hurt, Email: hurt_karla_c@lilly.com.
Masahiro Murakami, Email: murakami_masahiro@lilly.com.
Milton Packer, Email: milton.packer@baylorhealth.edu.
References
- 1.Redfield MM, Borlaug BA. Heart failure with preserved ejection fraction: a review. JAMA. 2023;329:827–838. doi: 10.1001/jama.2023.2020 [DOI] [PubMed] [Google Scholar]
- 2.Borlaug BA, Sharma K, Shah SJ, Ho JE. Heart failure with preserved ejection fraction: JACC scientific statement. J Am Coll Cardiol. 2023;81:1810–1834. doi: 10.1016/j.jacc.2023.01.049 [DOI] [PubMed] [Google Scholar]
- 3.Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, et al. ; PARAGON-HF Investigators and Committees. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655 [DOI] [PubMed] [Google Scholar]
- 4.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, et al. ; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 5.Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, et al. ; DELIVER Trial Committees and Investigators. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–1098. doi: 10.1056/NEJMoa2206286 [DOI] [PubMed] [Google Scholar]
- 6.Solomon SD, McMurray JJV, Vaduganathan M, Claggett B, Jhund PS, Desai AS, Henderson AD, Lam CSP, Pitt B, Senni M, et al. Finerenone in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2024;391:1475–1485. doi: 10.1056/NEJMoa2407107 [DOI] [PubMed] [Google Scholar]
- 7.Solomon SD, Vaduganathan M, Claggett BL, Packer M, Zile M, Swedberg K, Rouleau J, Pfeffer MA, Desai A, Lund LH, et al. Sacubitril/valsartan across the spectrum of ejection fraction in heart failure. Circulation. 2020;141:352–361. doi: 10.1161/CIRCULATIONAHA.119.044586 [DOI] [PubMed] [Google Scholar]
- 8.Butler J, Packer M, Filippatos G, Ferreira JP, Zeller C, Schnee J, Brueckmann M, Pocock SJ, Zannad F, Anker SD. Effect of empagliflozin in patients with heart failure across the spectrum of left ventricular ejection fraction. Eur Heart J. 2022;43:416–426. doi: 10.1093/eurheartj/ehab798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandra A, Polanczyk CA, Claggett BL, Vaduganathan M, Packer M, Lefkowitz MP, Rouleau JL, Liu J, Shi VC, Schwende H, et al. Health-related quality of life outcomes in PARAGON-HF. Eur J Heart Fail. 2022;24:2264–2274. doi: 10.1002/ejhf.2738 [DOI] [PubMed] [Google Scholar]
- 10.Kosiborod MN, Bhatt AS, Claggett BL, Vaduganathan M, Kulac IJ, Lam CSP, Hernandez AF, Martinez FA, Inzucchi SE, Shah SJ, et al. Effect of dapagliflozin on health status in patients with preserved or mildly reduced ejection fraction. J Am Coll Cardiol. 2023;81:460–473. doi: 10.1016/j.jacc.2022.11.006 [DOI] [PubMed] [Google Scholar]
- 11.Butler J, Filippatos G, Jamal Siddiqi T, Brueckmann M, Böhm M, Chopra VK, Pedro Ferreira J, Januzzi JL, Kaul S, Piña IL, et al. Empagliflozin, health status, and quality of life in patients with heart failure and preserved ejection fraction: the EMPEROR-Preserved trial. Circulation. 2022;145:184–193. doi: 10.1161/CIRCULATIONAHA.121.057812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang M, Hendreson AD, Talebi A, Atherton JJ, Chiang C-E, Chopra V, Comin-Colet J, Kosiborod MN, Kerr Saraiva JF, Claggett BL, et al. Effect of finerenone on the KCCQ in patients with HFmrEF/HFpEF: a prespecified analysis of FINEARTS-HF [published online September 29, 2024]. J Am Coll Cardiol. doi: 10.1016/j.jacc.2024.09.023. https://sciencedirect.com/science/article/pii/S0735109724085322?via%3Dihub [Google Scholar]
- 13.Borlaug BA, Jensen MD, Kitzman DW, Lam CSP, Obokata M, Rider OJ. Obesity and heart failure with preserved ejection fraction: new insights and pathophysiological targets. Cardiovasc Res. 2023;118:3434–3450. doi: 10.1093/cvr/cvac120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oguntade AS, Taylor H, Lacey B, Lewington S. Adiposity, fat-free mass and incident heart failure in 500 000 individuals. Open Heart. 2024;11:e002711. doi: 10.1136/openhrt-2024-002711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Packer M, Kitzman DW. Obesity-related heart failure with a preserved ejection fraction: the mechanistic rationale for combining inhibitors of aldosterone, neprilysin, and sodium-glucose cotransporter-2. JACC Heart Fail. 2018;6:633–639. doi: 10.1016/j.jchf.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 16.Savji N, Meijers WC, Bartz TM, Bhambhani V, Cushman M, Nayor M, Kizer JR, Sarma A, Blaha MJ, Gansevoort RT, et al. The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Heart Fail. 2018;6:701–709. doi: 10.1016/j.jchf.2018.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Höskuldsdóttir G, Sattar N, Miftaraj M, Näslund I, Ottosson J, Franzén S, Svensson AM, Eliasson B. Potential effects of bariatric surgery on the incidence of heart failure and atrial fibrillation in patients with type 2 diabetes mellitus and obesity and on mortality in patients with preexisting heart failure: a nationwide, matched, observational cohort study. J Am Heart Assoc. 2021;10:e019323. doi: 10.1161/JAHA.120.019323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lincoff AM, Brown-Frandsen K, Colhoun HM, Deanfield J, Emerson SS, Esbjerg S, Hardt-Lindberg S, Hovingh GK, Kahn SE, Kushner RF, et al. ; SELECT Trial Investigators. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389:2221–2232. doi: 10.1056/NEJMoa2307563 [DOI] [PubMed] [Google Scholar]
- 19.Kosiborod MN, Abildstrøm SZ, Borlaug BA, Butler J, Rasmussen S, Davies M, Hovingh GK, Kitzman DW, Lindegaard ML, Møller DV, et al. ; STEP-HFpEF Trial Committees and Investigators. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med. 2023;389:1069–1084. doi: 10.1056/NEJMoa2306963 [DOI] [PubMed] [Google Scholar]
- 20.Kosiborod MN, Petrie MC, Borlaug BA, Butler J, Davies MJ, Hovingh GK, Kitzman DW, Møller DV, Treppendahl MB, Verma S, et al. ; STEP-HFpEF DM Trial Committees and Investigators. Semaglutide in patients with obesity-related heart failure and type 2 diabetes. N Engl J Med. 2024;390:1394–1407. doi: 10.1056/NEJMoa2313917 [DOI] [PubMed] [Google Scholar]
- 21.Willard FS, Douros JD, Gabe MB, Showalter AD, Wainscott DB, Suter TM, Capozzi ME, van der Velden WJ, Stutsman C, Cardona GR, et al. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight. 2020;5:e140532. doi: 10.1172/jci.insight.140532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin W, Yang J, Ni Y, Deng C, Ruan Q, Ruan J, Zhou P, Duan K. Efficacy and safety of once-weekly tirzepatide for weight management compared to placebo: an updated systematic review and meta-analysis including the latest SURMOUNT-2 trial. Endocrine. 2024;86:70–84. doi: 10.1007/s12020-024-03896-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Packer M, Zile MR, Kramer CM, Baum SJ, Litwin SE, Menon V, Ge J, Weerakkody GJ, Ou Y, Bunck MC, et al. Tirzepatide for heart failure with preserved ejection fraction and obesity [published online November 26, 2024]. N Engl J Med. doi: 10.1056/NEJMoa2410027. https://nejm.org/doi/full/10.1056/NEJMoa2410027 [DOI] [PubMed] [Google Scholar]
- 24.Butler J, Shahzeb Khan M, Lindenfeld J, Abraham WT, Savarese G, Salsali A, Zeller C, Peil B, Filippatos G, Ponikowski P, et al. Minimally clinically important difference in health status scores in patients with HFrEF vs HFpEF. JACC Heart Fail. 2022;10:651–661. doi: 10.1016/j.jchf.2022.03.003 [DOI] [PubMed] [Google Scholar]
- 25.Reddy YNV, Carter RE, Sundaram V, Kaye DM, Handoko ML, Tedford RJ, Andersen MJ, Sharma K, Obokata M, Verbrugge FH, et al. An evidence-based screening tool for heart failure with preserved ejection fraction: the HFpEF-ABA score. Nat Med. 2024;30:2258–2264. doi: 10.1038/s41591-024-03140-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schou M, Petrie MC, Borlaug BA, Butler J, Davies MJ, Kitzman DW, Shah SJ, Verma S, Patel S, Chinnakondepalli KM, et al. ; STEP-HFpEF Trial Committees and Investigators. Semaglutide and NYHA functional class in obesity-related heart failure with preserved ejection fraction: the STEP-HFpEF program. J Am Coll Cardiol. 2024;84:247–257. doi: 10.1016/j.jacc.2024.04.038 [DOI] [PubMed] [Google Scholar]
- 27.Shah SJ, Sharma K, Borlaug BA, Butler J, Davies M, Kitzman DW, Petrie MC, Verma S, Patel S, Chinnakondepalli KM, et al. Semaglutide and diuretic use in obesity-related heart failure with preserved ejection fraction: a pooled analysis of the STEP-HFpEF and STEP-HFpEF-DM trials. Eur Heart J. 2024;45:3254–3269. doi: 10.1093/eurheartj/ehae322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butler J, Shah SJ, Petrie MC, Borlaug BA, Abildstrøm SZ, Davies MJ, Hovingh GK, Kitzman DW, Møller DV, Verma S, et al. ; STEP-HFpEF Trial Committees and Investigators. Semaglutide versus placebo in people with obesity-related heart failure with preserved ejection fraction: a pooled analysis of the STEP-HFpEF and STEP-HFpEF DM randomised trials. Lancet. 2024;403:1635–1648. doi: 10.1016/S0140-6736(24)00469-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMurray JJV, Docherty KF, de Boer RA, Hammarstedt A, Kitzman DW, Kosiborod MN, Maria Langkilde A, Reicher B, Senni M, Shah SJ, et al. Effect of dapagliflozin versus placebo on symptoms and 6-minute walk distance in patients with heart failure: the DETERMINE randomized clinical trials. Circulation. 2024;149:825–838. doi: 10.1161/CIRCULATIONAHA.123.065061 [DOI] [PubMed] [Google Scholar]
- 30.Abraham WT, Lindenfeld J, Ponikowski P, Agostoni P, Butler J, Desai AS, Filippatos G, Gniot J, Fu M, Gullestad L, et al. Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes. Eur Heart J. 2021;42:700–710. doi: 10.1093/eurheartj/ehaa943 [DOI] [PubMed] [Google Scholar]
- 31.Nassif ME, Windsor SL, Borlaug BA, Kitzman DW, Shah SJ, Tang F, Khariton Y, Malik AO, Khumri T, Umpierrez G, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. 2021;27:1954–1960. doi: 10.1038/s41591-021-01536-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, et al. ; COAPT Investigators. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–2318. doi: 10.1056/NEJMoa1806640 [DOI] [PubMed] [Google Scholar]
- 33.Kosyakovsky LB, Liu EE, Wang JK, Myers L, Parekh JK, Knauss H, Lewis GD, Malhotra R, Nayor M, Robbins JM, et al. Uncovering unrecognized heart failure with preserved ejection fraction among individuals with obesity and dyspnea. Circ Heart Fail. 2024;17:e011366. doi: 10.1161/CIRCHEARTFAILURE.123.011366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reddy YNV, Rikhi A, Obokata M, Shah SJ, Lewis GD, AbouEzzedine OF, Dunlay S, McNulty S, Chakraborty H, Stevenson LW, et al. Quality of life in heart failure with preserved ejection fraction: importance of obesity, functional capacity, and physical inactivity. Eur J Heart Fail. 2020;22:1009–1018. doi: 10.1002/ejhf.1788 [DOI] [PubMed] [Google Scholar]
- 35.Verbrugge FH, Omote K, Reddy YNV, Sorimachi H, Obokata M, Borlaug BA. Heart failure with preserved ejection fraction in patients with normal natriuretic peptide levels is associated with increased morbidity and mortality. Eur Heart J. 2022;43:1941–1951. doi: 10.1093/eurheartj/ehab911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrie MC, Borlaug BA, Butler J, Davies MJ, Kitzman DW, Shah SJ, Verma S, Jensen TJ, Einfeldt MN, Liisberg K, et al. ; STEP-HFpEF Trial Committees and Investigators. Semaglutide and NT-proBNP in obesity-related HFpEF: insights from the STEP-HFpEF Program. J Am Coll Cardiol. 2024;84:27–40. doi: 10.1016/j.jacc.2024.04.022 [DOI] [PubMed] [Google Scholar]
- 37.Abrahamsson N, Engström BE, Sundbom M, Karlsson FA. Gastric bypass surgery elevates NT-ProBNP levels. Obes Surg. 2013;23:1421–1426. doi: 10.1007/s11695-013-0889-z [DOI] [PubMed] [Google Scholar]
- 38.Kistorp C, Bliddal H, Goetze JP, Christensen R, Faber J. Cardiac natriuretic peptides in plasma increase after dietary induced weight loss in obesity. BMC Obes. 2014;1:24. doi: 10.1186/s40608-014-0024-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Obokata M, Reddy YNV, Melenovsky V, Sorimachi H, Jarolim P, Borlaug BA. Uncoupling between intravascular and distending pressures leads to underestimation of circulatory congestion in obesity. Eur J Heart Fail. 2022;24:353–361. doi: 10.1002/ejhf.2377 [DOI] [PubMed] [Google Scholar]
- 40.Docherty NG, Fändriks L, le Roux CW, Hallersund P, Werling M. Urinary sodium excretion after gastric bypass surgery. Surg Obes Relat Dis. 2017;13:1506–1514. doi: 10.1016/j.soard.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 41.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19. doi: 10.1161/CIRCULATIONAHA.116.026807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Packer M. Leptin-aldosterone-neprilysin axis: identification of its distinctive role in the pathogenesis of the three phenotypes of heart failure in people with obesity. Circulation. 2018;137:1614–1631. doi: 10.1161/CIRCULATIONAHA.117.032474 [DOI] [PubMed] [Google Scholar]
- 43.Hartman ML, Sanyal AJ, Loomba R, Wilson JM, Nikooienejad A, Bray R, Karanikas CA, Duffin KL, Robins DA, Haupt A. Effects of novel dual GIP and GLP-1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. Diabetes Care. 2020;43:1352–1355. doi: 10.2337/dc19-1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson JM, Lin Y, Luo MJ, Considine G, Cox AL, Bowsman LM, Robins DA, Haupt A, Duffin KL, Ruotolo G. The dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist tirzepatide improves cardiovascular risk biomarkers in patients with type 2 diabetes: a post hoc analysis. Diabetes Obes Metab. 2022;24:148–153. doi: 10.1111/dom.14553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol. 2018;71:2360–2372. doi: 10.1016/j.jacc.2018.03.509 [DOI] [PubMed] [Google Scholar]
- 46.Packer M, Lam CSP, Lund LH, Maurer MS, Borlaug BA. Characterization of the inflammatory-metabolic phenotype of heart failure with a preserved ejection fraction: a hypothesis to explain influence of sex on the evolution and potential treatment of the disease. Eur J Heart Fail. 2020;22:1551–1567. doi: 10.1002/ejhf.1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131:550–559. doi: 10.1161/CIRCULATIONAHA.114.009625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092 [DOI] [PubMed] [Google Scholar]
- 49.Malavazos AE, Iacobellis G, Dozio E, Basilico S, Di Vincenzo A, Dubini C, Menicanti L, Vianello E, Meregalli C, Ruocco C, et al. Human epicardial adipose tissue expresses glucose-dependent insulinotropic polypeptide, glucagon, and glucagon-like peptide-1 receptors as potential targets of pleiotropic therapies. Eur J Prev Cardiol. 2023;30:680–693. doi: 10.1093/eurjpc/zwad050 [DOI] [PubMed] [Google Scholar]
- 50.Varol C, Zvibel I, Spektor L, Mantelmacher FD, Vugman M, Thurm T, Khatib M, Elmaliah E, Halpern Z, Fishman S. Long-acting glucose-dependent insulinotropic polypeptide ameliorates obesity-induced adipose tissue inflammation. J Immunol. 2014;193:4002–4009. doi: 10.4049/jimmunol.1401149 [DOI] [PubMed] [Google Scholar]