Abstract
Proximal tubular reabsorption of filtered sodium by the sodium/hydrogen exchanger isoform 3 (NHE3), located on the apical membrane, is fundamental to the maintenance of systemic volume and pH homeostasis. NHE3 is finely regulated by a variety of hormones and by changes in ionic composition and volume, likely requiring redistribution of the exchangers. We analyzed the subcellular distribution and dynamics of the exchangers by generating an epithelial line expressing NHE3 tagged with an exofacial epitope, which enabled us to monitor exchanger mobility and traffic in intact cells. Using determinations of fluorescence recovery after photobleaching in combination with dynamic measurements of subcellular distribution, we found that, in renal epithelial cells, NHE3 exists in four distinct subcompartments: a virtually immobile subpopulation that is retained on the apical membrane by interaction with the actin cytoskeleton in a manner that depends on the sustained activity of Rho GTPases; a mobile subpopulation on the apical membrane, which can be readily internalized; and two intracellular compartments that can be differentiated by their rate of exchange with the apical pool of NHE3. We provide evidence that detachment of the immobile fraction from its cytoskeletal anchorage leads to rapid internalization. These observations suggest that modulation of the mobile fraction of NHE3 on the apical membrane can alter the number of functional exchangers on the cell surface and, consequently, the rate of transepithelial ion transport. Regulation of the interaction of NHE3 with the actin cytoskeleton can therefore provide a new mode of regulation of sodium and hydrogen transport.
Keywords: fluorescence recovery after photobleaching, pH regulation, sodium proton exchange, hypertension, Madin-Darby canine kidney cells
The electroneutral exchange of sodium for protons plays a critical role in the maintenance of cytosolic pH and in the control of cellular volume (1). Systemic acid/base homeostasis and net fluid (re)absorption are also dependent on sodium-proton exchange (2). Participation in such a wide variety of physiological roles is made possible by the coexistence of multiple isoforms of the sodium hydrogen exchanger (NHE) with distinct structural and functional properties. Nine members of the NHE family have been identified to date and designated NHE1 through NHE9 (3). Some isoforms, including NHE1 and NHE6-NHE9, are expressed ubiquitously and are thought to play housekeeping roles by exchanging cations across the plasmalemmal and organellar membranes. Others are tissue-specific and are therefore believed to serve specialized functions. Among these, NHE3 has been studied most extensively because of its central role in renal and intestinal ion homeostasis (2, 3). Indeed, ablation of the NHE3 gene in mice causes both hypotension and acidosis (2).
NHE3 is located primarily on the apical membrane of renal and intestinal epithelial cells, with a fraction residing in subapical vesicles (4). The coexistence of multiple NHE isoforms in epithelial cells has complicated the analysis of NHE3, particularly the assessment of the structural and functional consequences of targeted mutations of this isoform. To circumvent this limitation, investigators have taken advantage of mutant cells devoid of plasmalemmal NHE activity, where the activity of heterologously expressed NHE3 can be analyzed in isolation (5, 6). Such antiport-deficient cells have yielded much valuable information regarding NHE3 function and regulation but have been of limited use in analyzing the subcellular distribution and trafficking of this exchanger. Because the commonly used antiport-deficient lines (PS120 and AP-1) are fibroblastic, they lack the unique polarity and targeting determinants of differentiated epithelia. As a result, when expressed in such cells, NHE3 resides largely in endomembranes, and only a minor (≈15%) fraction reaches the plasmalemma, where it is dispersed homogeneously (7). For these reasons, the determinants of apical targeting and retention of NHE3 remain unknown.
Proteins can accumulate in defined membrane domains through targeted delivery from the biosynthetic pathway and/or by selective retention. Because NHE3 expressed in fibroblasts readily reaches the plasmalemma, yet is rapidly internalized to recycling endosomes, we hypothesized that its apical accumulation in epithelial cells results from preferential retention in the brush border membrane. The apical membrane of epithelia has a characteristic lipid composition of unique rigidity that could immobilize NHE3 and is subtended by a specialized cytoskeletal network that could also serve to anchor the exchangers. We therefore analyzed the apical retention of NHE3 and its determinants. To this end, we measured the mobility and the recycling of apical NHE3 in confluent monolayers of renal cells. Our data indicate that apical NHE is largely immobile, retained by a Rho-GTPase-dependent mechanism likely involving anchorage to cytoskeletal elements.
Materials and Methods
Materials and Solutions. Nigericin, the acetoxymethyl ester of 2′,7′-bis(carboxyethyl)-5(6)-carboxyfluorescein (BCECF), 5-(N-ethyl-N-isopropyl)-amiloride (EIPA), Alexa Fluor 488-conjugated goat anti-mouse F(ab′)2 fragment, and Alexa Fluor 488-conjugated goat anti-mouse antibody were obtained from Molecular Probes. Clostridium difficile toxin B (TxB) was from TechLab (Blacksburg, VA). Mouse anti-hemagglutinin (HA) and mouse anti-HA Fab fragment were from Babco (Richmond, CA). Cy3-conjugated secondary antibody, 18-nm gold-conjugated goat anti-mouse antibody, and horseradish peroxidase-conjugated donkey anti-mouse antibody were from Jackson ImmunoResearch. Isotonic Na+ medium contained 140 mM NaCl/3 mM KCl/1 mM MgCl2/20 mM Hepes, pH 7.4 with Tris. Isotonic K+-rich medium had the same composition except that NaCl was replaced by KCl. Hypotonic medium was a 1:1 mixture of RPMI medium 1640 and H2O. K+-free medium contained 100 mM NaCl/1 mM CaCl2/1 mM MgCl2/50 mM Hepes, pH 7.4 with Tris.
Cells and Constructs. Madin-Darby canine kidney (MDCK)-II cells from American Type Culture Collection were stably transfected with NHE3 containing three tandem copies of the influenza virus HA epitope (YPYDVPDYAS) inserted between the first and second membrane-spanning domains, between R38 and F39 ( ), generated as described (8). To select stable lines, cells were cotransfected with the pCMV plasmid (containing the aminoglycoside phosphotransferase gene, which confers resistance to G418), cloned by limiting dilution in the presence of 500 μg/ml G418, and screened by immunofluorescence for expression of HA-tagged NHE3. MDCK cells were maintained in a 1:1 DMEM/nutrient mixture F12 with 5% FBS in an atmosphere containing 5% CO2. Experiments were performed at least 72 h after the monolayer had reached confluence. The cDNA constructs used to express GFP coupled to glycerophosphoinositides (GPIs) or to the FcIIa receptor have been described elsewhere (7, 8).
), generated as described (8). To select stable lines, cells were cotransfected with the pCMV plasmid (containing the aminoglycoside phosphotransferase gene, which confers resistance to G418), cloned by limiting dilution in the presence of 500 μg/ml G418, and screened by immunofluorescence for expression of HA-tagged NHE3. MDCK cells were maintained in a 1:1 DMEM/nutrient mixture F12 with 5% FBS in an atmosphere containing 5% CO2. Experiments were performed at least 72 h after the monolayer had reached confluence. The cDNA constructs used to express GFP coupled to glycerophosphoinositides (GPIs) or to the FcIIa receptor have been described elsewhere (7, 8).
Measurement of Na+/H+ Exchange Activity. NHE3 activity was assessed as the rate of Na+-induced intracellular pH (pHi) recovery in the presence of 5 μM EIPA after an acid load, imposed by prepulsing the cells with NH4Cl. Dual excitation ratio determinations of the fluorescence of BCECF were used to measure pHi as previously detailed (9). Cells were grown to confluence on 25-mm glass coverslips, placed into Attofluor cell chambers, and mounted on the stage of the microscope. Next, they were loaded with 2 μg/ml BCECF acetoxymethyl ester and prepulsed with 40 mM NH4Cl in Hepes-buffered RPMI medium 1640 at 37°C for 10 min for subsequent acid loading (10). Extracellular dye and NH4Cl were then washed away with Na+ free solution, and Na+/H+ exchange was initiated by reintroduction of Na+. pHi was calibrated by equilibrating the cells with K+-rich media titrated to defined pH values and containing 10 μg/ml nigericin (11).
Fluorescence Recovery After Photobleaching (FRAP). Cells stably expressing  were labeled with mouse anti-HA Fab fragment, (1:300 dilution in RPMI medium 1640) and then with secondary Alexa Fluor 488-conjugated goat anti-mouse Fab fragment (1:500 dilution). Samples placed into Attofluor chambers were mounted on the stage of a confocal laser microscope (Zeiss LSM 510). The apical plane was brought into focus, and two equal areas (2-μm diameter) were defined. After acquiring two baseline fluorescence measurements, one of the selected areas was irreversibly photobleached, and then the fluorescence of both areas was measured over time. The fractional fluorescence recovery of the bleached area was determined relative to the average of the two prebleach measurements. The unbleached area was used to estimate possible bleaching incurred during image acquisition.
were labeled with mouse anti-HA Fab fragment, (1:300 dilution in RPMI medium 1640) and then with secondary Alexa Fluor 488-conjugated goat anti-mouse Fab fragment (1:500 dilution). Samples placed into Attofluor chambers were mounted on the stage of a confocal laser microscope (Zeiss LSM 510). The apical plane was brought into focus, and two equal areas (2-μm diameter) were defined. After acquiring two baseline fluorescence measurements, one of the selected areas was irreversibly photobleached, and then the fluorescence of both areas was measured over time. The fractional fluorescence recovery of the bleached area was determined relative to the average of the two prebleach measurements. The unbleached area was used to estimate possible bleaching incurred during image acquisition.
Electron Microscopy. Cells grown to confluence on 25-mm coverslips were fixed at 4°C with 4% paraformaldehyde for 30 min, washed, and then labeled with anti-HA antibody (1:500 dilution) in PBS for 60 min at room temperature. Goat anti-mouse antibody conjugated to 18 nm gold was applied, and then the gold particles were enhanced with a GoldEnhanced EM Kit (Nanoprobes, Yaphank, NY). Samples were dehydrated, mounted on stubs, coated with carbon, and then analyzed by using an XL30 environmental ESM (ESEM) from FEI (Hillsboro, Oregon). Images were generated through the combination of signals from the gaseous secondary electron detector and the backscatter electron detector.
Other Methods. Immunostaining of surface and total cellular NHE3 was performed essentially as in ref. 12. Quantification of surface-exposed and total cellular NHE3 was performed as detailed in ref. 13 and in Supporting Materials and Methods, which is published as supporting information on the PNAS web site. Cellular cholesterol content was determined by using an AmplexRed Kit from Molecular Probes. SDS/PAGE and immunoblotting were performed as described in ref. 7. Where indicated, the cells were pretreated with 4 μg/ml TxB for 2 h at 37°C before analysis.
Results
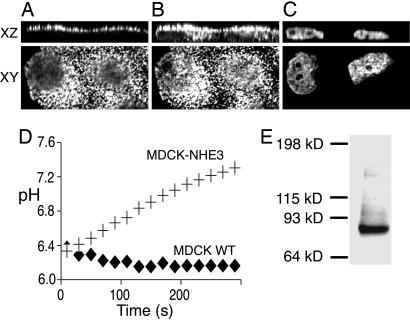
Generation of MDCK- Cells. To measure the mobility and traffic of NHE3 in epithelial cells, we stably transfected MDCK cells with exchangers bearing an exofacial epitope tag (see Fig. 3 for diagram). When probed by immunoblotting with anti-HA antibodies, the resulting cell line, referred to hereafter as MDCK-
Cells. To measure the mobility and traffic of NHE3 in epithelial cells, we stably transfected MDCK cells with exchangers bearing an exofacial epitope tag (see Fig. 3 for diagram). When probed by immunoblotting with anti-HA antibodies, the resulting cell line, referred to hereafter as MDCK- , displayed a single tight band of ≈85 kDa (Fig. 1). This size is consistent with the predicted molecular weight of NHE3, taking into account the contribution of the HA tags and unglycosylated nature of this isoform (14). In intact cells grown to confluence, a sizable fraction of the epitope was readily accessible to extracellular antibodies, implying that NHE3 was present in the apical membrane (Fig. 1 A). The remainder of the exchangers were accessed by the antibodies only after treatment with detergent (Fig. 1B). Although this finding may be attributable in part to unmasking of epitopes of plasmalemmal exchangers, it is clear that a fraction of NHE3 resides intracellularly and can be accessed only after permeabilization, as is evident from optical sections acquired by confocal microscopy (Fig. 1B). Quantification before and after permeabilization was performed by coupling a horseradish peroxidase-conjugated secondary antibody to anti-HA antibodies bound to the the NHE3 epitope tag. Bound peroxidase was quantified by colorimetry, using O-phenylenediamine as a substrate. These determinations (see Materials and Methods) indicated that ≥49 ± 1% (mean ± SE; n = 16) of the exchangers reside on the apical membrane of MDCK-
, displayed a single tight band of ≈85 kDa (Fig. 1). This size is consistent with the predicted molecular weight of NHE3, taking into account the contribution of the HA tags and unglycosylated nature of this isoform (14). In intact cells grown to confluence, a sizable fraction of the epitope was readily accessible to extracellular antibodies, implying that NHE3 was present in the apical membrane (Fig. 1 A). The remainder of the exchangers were accessed by the antibodies only after treatment with detergent (Fig. 1B). Although this finding may be attributable in part to unmasking of epitopes of plasmalemmal exchangers, it is clear that a fraction of NHE3 resides intracellularly and can be accessed only after permeabilization, as is evident from optical sections acquired by confocal microscopy (Fig. 1B). Quantification before and after permeabilization was performed by coupling a horseradish peroxidase-conjugated secondary antibody to anti-HA antibodies bound to the the NHE3 epitope tag. Bound peroxidase was quantified by colorimetry, using O-phenylenediamine as a substrate. These determinations (see Materials and Methods) indicated that ≥49 ± 1% (mean ± SE; n = 16) of the exchangers reside on the apical membrane of MDCK- cells. These exchangers are functional, as shown in Fig. 1D. Wild-type MDCK cells express NHE1, yet little NHE3. Therefore, when tested in the presence of the isoform-selective inhibitor EIPA (15), which at the concentration used inhibits NHE1 but not NHE3, these cells display negligible Na+-induced recovery from an acid load. In contrast, a robust EIPA-resistant recovery was observed in MDCK-
cells. These exchangers are functional, as shown in Fig. 1D. Wild-type MDCK cells express NHE1, yet little NHE3. Therefore, when tested in the presence of the isoform-selective inhibitor EIPA (15), which at the concentration used inhibits NHE1 but not NHE3, these cells display negligible Na+-induced recovery from an acid load. In contrast, a robust EIPA-resistant recovery was observed in MDCK- cells, indicative of the activity of the heterologously expressed NHE3 (Fig. 1D).
cells, indicative of the activity of the heterologously expressed NHE3 (Fig. 1D).
Fig. 3.
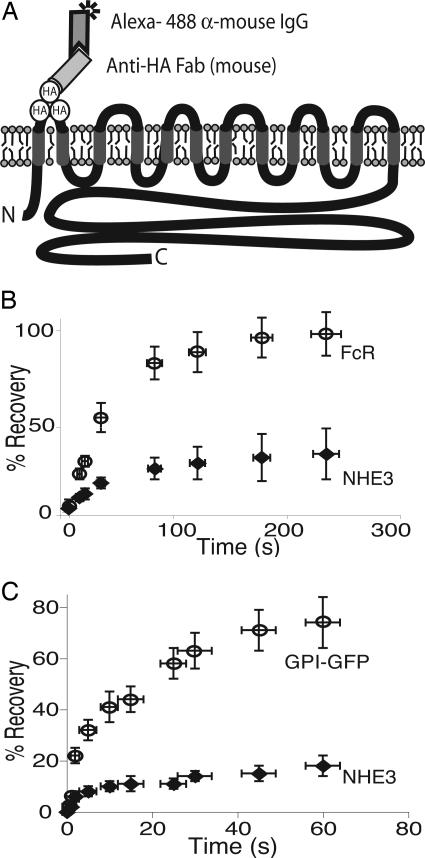
FRAP determinations. (A) Strategy used to measure FRAP of NHE3. The putative transmembrane topology of NHE3 is illustrated, showing the predicted exofacial location of the triple HA tag. The diagram also shows labeling of the tag with monoclonal anti-HA F(ab) fragment and Alexa Fluor 488-conjugated goat anti-mouse F(ab) fragment. (B) Recovery of fluorescence after photobleaching of apical  labeled as described above (♦) and of apical GFP-tagged FcR (○). (C) Recovery after photobleaching of apical
labeled as described above (♦) and of apical GFP-tagged FcR (○). (C) Recovery after photobleaching of apical  labeled as described above (♦) and of apical GPI-GFP (○). Data in B and C were binned over defined time intervals, and the bars are means ± SE of 10 determinations.
labeled as described above (♦) and of apical GPI-GFP (○). Data in B and C were binned over defined time intervals, and the bars are means ± SE of 10 determinations.
Fig. 1.
Characterization of MDCK cells expressing  . (A-C) Immunofluorescence staining. MDCK-
. (A-C) Immunofluorescence staining. MDCK- cells were stained with anti-HA antibodies before (A) and after (B) permeabilization to localize superficial and total NHE3. For reference, the location of the nucleus was revealed by staining with DAPI (C). Representative x vs. y and x vs. z confocal optical sections are illustrated. (D) Na+/H+ exchange activity determinations. The rate of recovery of the cytosolic pH from an imposed acid load was measured in either wild-type MDCK cells (+) or MDCK-
cells were stained with anti-HA antibodies before (A) and after (B) permeabilization to localize superficial and total NHE3. For reference, the location of the nucleus was revealed by staining with DAPI (C). Representative x vs. y and x vs. z confocal optical sections are illustrated. (D) Na+/H+ exchange activity determinations. The rate of recovery of the cytosolic pH from an imposed acid load was measured in either wild-type MDCK cells (+) or MDCK- cells (♦) after addition of extracellular Na+. Data are the mean ± SE of eight experiments. (E) Immunoblot of a lysate of MDCK-
cells (♦) after addition of extracellular Na+. Data are the mean ± SE of eight experiments. (E) Immunoblot of a lysate of MDCK- using anti-HA antibodies. The blot in E is representative of three experiments.
using anti-HA antibodies. The blot in E is representative of three experiments.
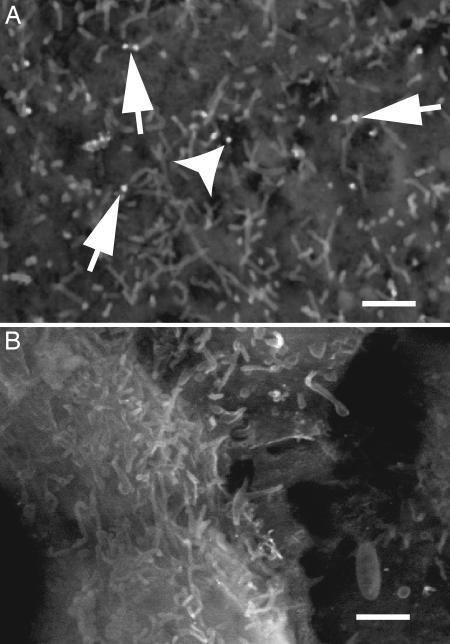
The distribution of NHE3 in the transfectants was studied in more detail by using gold immunolabeling and backscattered scanning electron microscopy. As shown in Fig. 2, the gold particles indicative of NHE3 were found preferentially associated with the microvilli, despite the fact that, unlike native epithelia, the apical surface of MDCK cells is composed predominantly of intervillar membrane expanses. Quantification of the number and location of gold particles in 32 separate fields like that in Fig. 2, obtained from three separate experiments, revealed that 71% of the immunoreactive apical NHE3 are located on microvilli. In the rat kidney, the endogenous NHE3 was similarly reported to reside predominantly on microvilli (4). Jointly, these observations imply that MDCK- cells are a suitable model to study epithelial NHE3, because they recapitulate the subcellular distribution and activity of the exchangers in their native setting.
cells are a suitable model to study epithelial NHE3, because they recapitulate the subcellular distribution and activity of the exchangers in their native setting.
Fig. 2.
SEM of the apical surface of MDCK cells expressing NHE3. MDCK- cells were either left untreated (A) or were treated with TxB for 2 h (B), as described in Materials and Methods. The cells were then immunolabeled with anti-HA antibodies, followed by secondary antibodies conjugated to 18-nm gold particles. Samples were visualized by SEM, and the gold particles were identified by backscattering. Arrows point to gold particles located on microvilli and arrowheads to gold in the intermicrovillar space. Note the absence of gold particles in B. Micrographs are representative of multiple fields from three separate experiments. (Scale bars, 1 μm.)
cells were either left untreated (A) or were treated with TxB for 2 h (B), as described in Materials and Methods. The cells were then immunolabeled with anti-HA antibodies, followed by secondary antibodies conjugated to 18-nm gold particles. Samples were visualized by SEM, and the gold particles were identified by backscattering. Arrows point to gold particles located on microvilli and arrowheads to gold in the intermicrovillar space. Note the absence of gold particles in B. Micrographs are representative of multiple fields from three separate experiments. (Scale bars, 1 μm.)
Assessment of NHE3 Mobility Using FRAP. We took advantage of the extracellular location of the triple-HA epitope to assess the mobility of apical NHE3 by FRAP. To this end, we labeled the antiporters using fluoresceinated antibodies. Fab fragments of anti-HA and of the Alexa Fluor 488-labeled antibodies were used, as illustrated in Fig. 3A, to preclude cross-linking that could alter the mobility of NHE3. Labeling was performed in the cold immediately before the assay, to minimize entry of label into the endomembrane compartments. As shown in Fig. 3 B and C, recovery of NHE3-associated fluorescence after photobleaching was poor over the period analyzed. In 12 experiments, only an average of 33% of the fluorescence reappeared after 320 s, the longest period analyzed. These findings suggest that a major fraction of the apical NHE3 (nearly 70%) is virtually immobile. A smaller fraction (40-60%) of NHE3 was found to be immobile when the fluorescence of transiently expressed NHE3-GFP was used to determine FRAP. The source of this apparent discrepancy is discussed below. To ensure that the apparent immobility represented a characteristic behavior of NHE3 and not a limitation of our experimental system, due perhaps to the convolutions characteristic of apical membranes, we also measured the mobility of Fc receptors, transmembrane proteins that move freely in other membranes (16). When expressed in MDCK cells, FcIIA receptors were found to be very mobile, as indicated by the rapid and nearly complete recovery after photobleaching (Fig. 3B). Similar results were obtained with other membrane markers (see below), including the basolateral NHE1-GFP (not illustrated), implying that the limited mobility displayed by NHE3 is characteristic of this molecule.
Are Lipid Rafts Responsible for the Limited Mobility of NHE3? Apical membranes are rich in glycosphingolipids and cholesterol, the key components of lipid rafts, and NHE3 has been reported to partition into rafts (17). It is therefore conceivable that association with these lipid domains restricts the mobility of the antiporters. Two approaches were used to analyze this possibility. First, we assessed the lateral mobility of lipid rafts in the apical membrane. For this purpose MDCK- cells were transfected with GPI-linked GFP, because GPI-linked proteins are typically located within rafts (18). The fluorescence of the chimeric construct was used for FRAP measurements, and representative results are shown in Fig. 3C. Fluorescence recovery was rapid and extensive, clearly different from the behavior of NHE3. This finding suggests that partitioning within rafts is unlikely to account for the poor mobility of NHE3.
cells were transfected with GPI-linked GFP, because GPI-linked proteins are typically located within rafts (18). The fluorescence of the chimeric construct was used for FRAP measurements, and representative results are shown in Fig. 3C. Fluorescence recovery was rapid and extensive, clearly different from the behavior of NHE3. This finding suggests that partitioning within rafts is unlikely to account for the poor mobility of NHE3.
This conclusion is buttressed by findings using β-methylcyclodextrin (MCD), an agent commonly used to extract cholesterol from membranes, thereby destabilizing rafts (19). Cells were treated with 20 mM MCD for a total of 60 min, and the success of the extraction was verified by direct measurement of the cholesterol content of the cells by using the AmplexRed Kit. Despite removal of 40.4% of the cellular cholesterol, the mobility of NHE3 assessed by FRAP as above was unaltered (fractional recovery = 34%; data not shown). Together with the data in Fig. 3B, these findings strongly suggest that factors other than lipid rafts restrict the mobility of NHE3.
Cytoskeletal Anchorage Accounts for the Limited Mobility of NHE3. We sought to disrupt the microvillar cytoskeleton to analyze its possible role in immobilizing NHE3. Unlike other actin-based structures, microvilli are uniquely refractory to the depolymerizing effects of cytochalasin or latrunculin. On the other hand, agents that impair Rho GTPase function, such as TxB have profound effects on microvillar structure (20). Treatment of MDCK- cells with TxB altered their morphology, depleting the homogeneously sized microvilli seen in control cells and generating instead irregular ruffles and filopodia (Fig. 2B). A similar TxB-induced “flaring” of the plasma membrane was reported earlier (21). Unfortunately, as found in LLC-PK1 cells (12), exposure to TxB resulted in extensive internalization of NHE3 (Figs. 2B and 4 B and E), precluding measurement of apical NHE3 mobility by FRAP.
cells with TxB altered their morphology, depleting the homogeneously sized microvilli seen in control cells and generating instead irregular ruffles and filopodia (Fig. 2B). A similar TxB-induced “flaring” of the plasma membrane was reported earlier (21). Unfortunately, as found in LLC-PK1 cells (12), exposure to TxB resulted in extensive internalization of NHE3 (Figs. 2B and 4 B and E), precluding measurement of apical NHE3 mobility by FRAP.
Fig. 4.
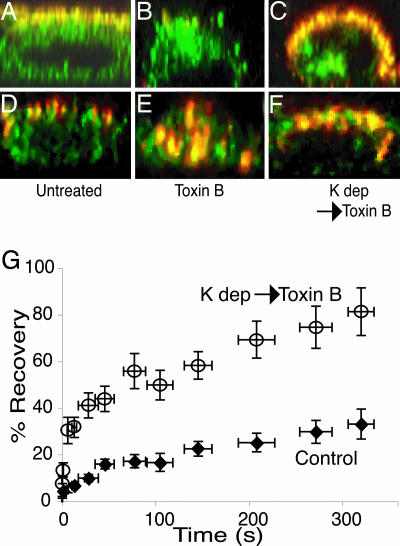
Effect of TxB on NHE3 distribution and mobility. (A-F) x vs. z digital reconstruction of serial optical slices of immunostained MDCK- cells, obtained by using confocal microscopy, surface (red) and total (green) NHE3. In A-C, the cells were fixed after the indicated treatment (see below) and exposed to anti-HA antibody followed by red-labeled secondary antibody. Next, the cells were permeabilized and exposed again to anti-HA and then green-labeled secondary antibodies. In D-F, surface-exposed NHE3 was tagged by addition of anti-HA to live cells at 22°C for 45 min, followed by exposure to red-labeled secondary at 22°C for an additional 45 min. After the indicated treatment, the cells were fixed, permeabilized, and exposed again to anti-HA and then green-labeled secondary antibodies to reveal the total NHE3 population. Shown are untreated cells (A and D), cells treated with TxB as described in Fig. 2 (B and E), and K+-depleted cells treated with TxB (C and F). (G) Recovery of fluorescence after photobleaching of apical
cells, obtained by using confocal microscopy, surface (red) and total (green) NHE3. In A-C, the cells were fixed after the indicated treatment (see below) and exposed to anti-HA antibody followed by red-labeled secondary antibody. Next, the cells were permeabilized and exposed again to anti-HA and then green-labeled secondary antibodies. In D-F, surface-exposed NHE3 was tagged by addition of anti-HA to live cells at 22°C for 45 min, followed by exposure to red-labeled secondary at 22°C for an additional 45 min. After the indicated treatment, the cells were fixed, permeabilized, and exposed again to anti-HA and then green-labeled secondary antibodies to reveal the total NHE3 population. Shown are untreated cells (A and D), cells treated with TxB as described in Fig. 2 (B and E), and K+-depleted cells treated with TxB (C and F). (G) Recovery of fluorescence after photobleaching of apical  labeled as described in Fig. 3. Otherwise untreated cells (♦) are compared with cells that had been K+-depleted and treated with TxB (○). Data were binned as in Fig. 3, and the bars are means ± SE of at least 12 determinations.
labeled as described in Fig. 3. Otherwise untreated cells (♦) are compared with cells that had been K+-depleted and treated with TxB (○). Data were binned as in Fig. 3, and the bars are means ± SE of at least 12 determinations.
We reasoned that mobility measurements would become feasible if we prevented the internalization of NHE3 that accompanies microvillar collapse. Because endocytosis of NHE3 is believed to occur via clathrin-coated vesicles, we depleted the cells of K+. This maneuver has been demonstrated to obliterate clathrin-mediated internalization in a variety of cells (21, 22). When K+-depleted cells were subjected to treatment with TxB, they underwent microvillar resorption, but failed to internalize NHE3 (Fig. 4 C and F), enabling us to assess its mobility by FRAP. The results of these measurements are summarized in Fig. 4G. When compared with control cells (filled symbols), the TxB-treated cells showed markedly increased mobility of NHE3. In 12 experiments, the mobile fraction increased from 32 ± 8% to 81 ± 10%. These observations suggest that cytoskeletal anchorage is responsible for the limited mobility of NHE3. The residual immobile fraction may reflect incomplete disassembly of the actin skeleton in TxB-treated cells, or the contribution of an additional anchorage process.
Effect of Cytoskeletal Association on NHE3 Traffic. When expressed in fibroblastic cells, virtually all of the plasmalemmal NHE3 becomes rapidly internalized (t1/2 ≈ 15 min; Fig. 6A, which is published as supporting information on the PNAS web site) and is replaced by endomembrane NHE3 to maintain a steady surface density (8). This behavior would seem to be incompatible with the firm anchorage of the exchangers to the cytoskeleton observed in epithelial cells. We therefore assessed the rate of recycling of surface NHE3 in MDCK- cells. These experiments revealed that only a small fraction (<20%) of the NHE3 became internalized in 80 min (see Fig. 6A). These findings not only highlight the differences between nonpolar and polarized epithelial cells, but also imply that the latter have at least three distinct subpopulations: one that is not measurably mobile and likely internalizes poorly if at all, and a second superficial subpopulation that is more mobile and may be in dynamic equilibrium with a third, endomembrane pool. To ascertain whether these three components account for the entire complement of epithelial NHE3, or whether additional pools exist that do not exchange measurably with surface NHE3, cells were incubated at 37°C in the presence of anti-HA antibody. Just under 50% of the exchangers ligated the antibody rapidly (Fig. 6B), suggesting that they are located apically, consistent with the measurements above. A second subpopulation (≈25% of the total) became labeled within the next 30-60 min. We attribute this slower increase to the appearance at the cell surface of a population of NHE3 from an intracellular recycling compartment. The increase is not likely due to the appearance at the surface of recently synthesized NHE3 for two reasons: (i) the existence of such a rapid metabolic turnover would result in a progressive, sustained increase in antibody uptake, which was not observed and (ii) NHE3 in these epithelial cells seems to turn over extremely slowly. This finding is demonstrated in Fig. 6C, where the half-life of NHE3 (as estimated by immunoblotting after addition of cycloheximide) exceeds 8 h. It is noteworthy in Fig. 6 that ≈25% of the total NHE3 was not accessible to the antibody even after 3 h. This result is not due to slow association of the antibody with its epitope, because even at 4°C the reaction seemed to plateau within a much shorter period (Fig. 6B Inset). Instead, the results point to the existence of a distinct, very slowly or nonrecycling endomembrane pool.
cells. These experiments revealed that only a small fraction (<20%) of the NHE3 became internalized in 80 min (see Fig. 6A). These findings not only highlight the differences between nonpolar and polarized epithelial cells, but also imply that the latter have at least three distinct subpopulations: one that is not measurably mobile and likely internalizes poorly if at all, and a second superficial subpopulation that is more mobile and may be in dynamic equilibrium with a third, endomembrane pool. To ascertain whether these three components account for the entire complement of epithelial NHE3, or whether additional pools exist that do not exchange measurably with surface NHE3, cells were incubated at 37°C in the presence of anti-HA antibody. Just under 50% of the exchangers ligated the antibody rapidly (Fig. 6B), suggesting that they are located apically, consistent with the measurements above. A second subpopulation (≈25% of the total) became labeled within the next 30-60 min. We attribute this slower increase to the appearance at the cell surface of a population of NHE3 from an intracellular recycling compartment. The increase is not likely due to the appearance at the surface of recently synthesized NHE3 for two reasons: (i) the existence of such a rapid metabolic turnover would result in a progressive, sustained increase in antibody uptake, which was not observed and (ii) NHE3 in these epithelial cells seems to turn over extremely slowly. This finding is demonstrated in Fig. 6C, where the half-life of NHE3 (as estimated by immunoblotting after addition of cycloheximide) exceeds 8 h. It is noteworthy in Fig. 6 that ≈25% of the total NHE3 was not accessible to the antibody even after 3 h. This result is not due to slow association of the antibody with its epitope, because even at 4°C the reaction seemed to plateau within a much shorter period (Fig. 6B Inset). Instead, the results point to the existence of a distinct, very slowly or nonrecycling endomembrane pool.
Discussion
The salient finding of these studies was the observation that a majority of the apical NHE3 is immobile at the apical surface. Due to the fact that rapid lateral mobility was restored by treatment with TxB, we concluded that Rho-family GTPases were responsible for the retention of NHE3, in all likelihood by means of anchorage to the cytoskeletal scaffold. This conclusion is consistent with the earlier finding that a considerable fraction of NHE3 remains insoluble after extraction with nondenaturing detergents, under conditions where the actin cytoskeleton is largely preserved (23). A substantially smaller fraction of NHE3 was recently reported to be immobile in OK cells (24), in apparent disagreement with our findings. Whereas the use of a less differentiated cell type may account for these differences, we believe that two other factors contributed more importantly. Firstly, Cha et al. (24) used transient transfection of NHE3 tagged with GFP at its C terminus. Not only does transient transfection yield higher and less reproducible levels of expression, which may have saturated the retention sites, but the inclusion of a GFP moiety at the cytosolic tail of the exchangers may have impaired proper anchorage. Secondly, and most importantly, when using GFP-tagged molecules, neither the photobleaching nor the fluorescence acquisition systems can distinguish between apical and subapical signals. As a result, the measurements of Cha et al. (24) must have included a subapical component, possibly encompassing molecules trapped in the endoplasmic reticulum as a consequence of overexpression. Accordingly, we similarly found a much smaller immobile fraction in cells transfected with NHE3-GFP (see above). We therefore feel that results of FRAP measurements obtained by tagging an exofacial epitope with Fab fragments are more reliable than the use of transient transfection with GFP fusion constructs.
The conclusion that NHE3 is largely immobile at the apical membrane, together with the finding that the exchangers were preferentially associated with the microvilli, are consistent with the notion that apical accumulation of NHE3 results, at least in part, from a retention process that involves Rho GTPases and the actin cytoskeleton. In accordance with this interpretation, not only does the mobility of NHE3 increase upon impairment of the GTPases, but the exchangers rapidly relocalize to an endomembrane compartment, recapitulating the distribution seen earlier in nonepithelial cells. We propose that, when freely mobile on the (apical) surface, NHE3 associates readily with coated pits and becomes internalized into a recycling compartment. The degree of plasmalemmal (apical) accumulation will therefore depend on the efficiency of retention by the microvillar skeleton. In accordance with this model, we find that a greater fraction of NHE3 is retained on the membrane of epithelial lines that are richer in microvilli (e.g., MDCK), than in lines with less profuse microvillar structure (e.g., LLC-PK1 or OK).
Previous studies suggest that tethering of NHE3 to the cytoskeleton is mediated by NHE regulatory factor 1 (NHERF1/EBP50), a bridging molecule that also associates with ezrin, which, in turn, binds directly to actin (25). Not only do these proteins colocalize apically with NHE3 in epithelia, but they have been shown to interact in vitro and to coimmunoprecipitate when coexpressed in model cells systems (23, 25, 26). In the MDCK cells used in our experiments, the vast majority of NHE3 and of ezrin, as well as a fraction of NHERF1/EBP50, remain in the insoluble (cytoskeletal) fraction after extraction with nonionic detergents (Fig. 7, which is published as supporting information on the PNAS web site). This insolubility prevented attempts to coimmunoprecipitate the putative complex to verify the tripartite interaction, but the intimate colocalization of NHE3 and ezrin in the extracted material, despite solubilization of the majority of the cellular proteins and lipids, supports the earlier notion that NHE3 is attached to the cytoskeleton by means of NHERF1/EBP50 and ezrin.
The small fraction of mobile NHE3 (20-30%) is of a similar magnitude to the component of the apical exchangers found to undergo endocytosis. The internalized exchangers must reach a recycling compartment, because the net amount of NHE3 remains at steady state, implying the existence of compensatory exocytosis. The latter does not originate directly from the biosynthetic pathway, because the half-life of the exchangers was found to be much longer than the residence time of the mobile fraction on the apical membrane. We also detect a component of latent NHE3 that is not readily accessible to externally added antibodies, even after prolonged incubation at 37°C. We interpret this fraction to represent a nonexchangeable or at best slowly exchangeable intracellular compartment comprising ≈25% of the total NHE3. This amount may be overestimated to the extent that the detergent used to reveal this compartment may increase the exposure of epitopes masked in exchangers that belong to other compartments.
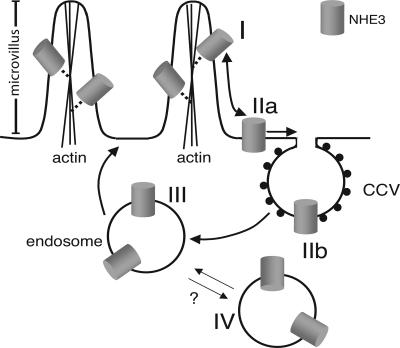
These findings are summarized in Fig. 5. We propose the existence of four distinct compartments of NHE3 in epithelial cells. The largest component consists of cytoskeleton-associated apical transporters. These transporters are presumably located on microvilli (Fig. 2) and likely correspond to the fraction of exchangers that Biemesderfer et al. (27) found not to associate with megalin. As such, these exchangers are most likely to function actively in ion transport. The balance of the apical exchangers are more mobile and may correspond to the less active, megalin-bound fraction described by Biemesderfer et al. (28). We propose that this subpopulation is more readily internalized via clathrin-coated pits, as would be expected for the megalin-associated pool. The intracellular complement of NHE3 seems to consist of at least two kinetically distinguishable components. A rapidly exchangeable compartment that we propose is in equilibrium with the mobile fraction at the cell surface and likely represents apical recycling endosomes, and a second pool that exchanges poorly, at least in unstimulated cells. The latter may represent the juxtanuclear sorting endosome, where apical and basolateral cargo has been found to coexist.
Fig. 5.
Proposed model depicting the subpopulations of NHE3 in epithelia. I, Immobile fraction of NHE3 at the apical membrane. The mobility of this subpopulation is restricted by anchorage to the actin cytoskeleton. II, Mobile fraction of NHE3 at the apical membrane. This subpopulation is likely to enter coated pits more readily than the immobile fraction. III, Rapidly recycling endomembrane fraction. IV, Endomembrane fraction of NHE3 that is not readily accessible from the external milieu. This fraction may exchange with pool III slowly, if at all.
The coexistence of these four separate compartments adds new dimensions to the regulation of NHE3 activity. Transport activity could be modulated up or downward by altering the balance between the mobile and immobile fractions of the plasmalemma, which may in turn result in changes in the rate and extent of internalization. Lastly, the balance between the rapidly and slowly exchanging internal compartments may similarly vary, potentially altering the abundance and transport activity of the exchangers at the surface.
Supplementary Material
Acknowledgments
This work was supported by an operational grant from the Canadian Institutes of Health Research (CIHR). R.T.A. is a CIHR Strategic Training Fellow of the Canadian Child Health Clinician Scientist Program. K.S. is a recipient of a CIHR Senior Research fellowship. S.G. is the current holder of the Pitblado Chair in Cell Biology.
Author contributions: R.T.A., J.O., and S.G. designed research; R.T.A., W.F., and K.S. performed research; R.T.A. and S.G. analyzed data; J.O. contributed new reagents/analytic tools; and R.T.A., K.S., and S.G. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: NHE, sodium/hydrogen exchanger; BCECF, 2′,7′-bis(carboxyethyl)-5(6)-carboxyfluorescein; EIPA, 5-(N-ethyl-N-isopropyl)-amiloride; TxB, Clostridium difficile toxin B; HA, hemagglutinin; MDCK, Madin-Darby canine kidney; FRAP, fluorescence recovery after photobleaching; GPI, glycerophosphoinositide.
References
- 1.Slepkov, E. & Fliegel, L. (2002) Biochem. Cell Biol. 80, 499-508. [DOI] [PubMed] [Google Scholar]
- 2.Schultheis, P. J., Clarke, L. L., Meneton, P., Miller, M. L., Soleimani, M., Gawenis, L. R., Riddle, T. M., Duffy, J. J., Doetschman, T., Wang, T., et al. (1998) Nat. Genet. 19, 282-285. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi, H., Szaszi, K. & Grinstein, S. (2002) Ann. N.Y. Acad. Sci. 976, 248-258. [DOI] [PubMed] [Google Scholar]
- 4.Biemesderfer, D., Rutherford, P. A., Nagy, T., Pizzonia, J. H., Abu-Alfa, A. K. & Aronson, P. S. (1997) Am. J. Physiol. 273, F289-F299. [DOI] [PubMed] [Google Scholar]
- 5.Pouyssegur, J., Sardet, C., Franchi, A., L'Allemain, G. & Paris, S. (1984) Proc. Natl. Acad. Sci. USA 81, 4833-4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rotin, D. & Grinstein, S. (1989) Am. J. Physiol. 257, C1158-C1165. [DOI] [PubMed] [Google Scholar]
- 7.D'Souza, S., Garcia-Cabado, A., Yu, F., Teter, K., Lukacs, G., Skorecki, K., Moore, H. P., Orlowski, J. & Grinstein, S. (1998) J. Biol. Chem. 273, 2035-2043. [DOI] [PubMed] [Google Scholar]
- 8.Kurashima, K., Szabo, E. Z., Lukacs, G., Orlowski, J. & Grinstein, S. (1998) J. Biol. Chem. 273, 20828-20836. [DOI] [PubMed] [Google Scholar]
- 9.Kapus, A., Grinstein, S., Wasan, S., Kandasamy, R. & Orlowski, J. (1994) J. Biol. Chem. 269, 23544-23552. [PubMed] [Google Scholar]
- 10.Roos, A. & Boron, W. F. (1981) Physiol. Rev. 61, 296-434. [DOI] [PubMed] [Google Scholar]
- 11.Thomas, J. A., Buchsbaum, R. N., Zimniak, A. & Racker, E. (1979) Biochemistry 18, 2210-2218. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi, H., Szaszi, K., Coady-Osberg, N., Furuya, W., Bretscher, A. P., Orlowski, J. & Grinstein, S. (2004) J. Gen. Physiol. 123, 491-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Touret, N., Furuya, W., Forbes, J., Gros, P. & Grinstein, S. (2003) J. Biol. Chem. 278, 25548-25557. [DOI] [PubMed] [Google Scholar]
- 14.Counillon, L., Pouyssegur, J. & Reithmeier, R. A. (1994) Biochemistry 33, 10463-10469.] [DOI] [PubMed] [Google Scholar]
- 15.Noel, J., Roux, D. & Pouyssegur, J. (1996) J. Cell Sci. 109, 929-939. [DOI] [PubMed] [Google Scholar]
- 16.Zhang, F., Yang, B., Odin, J. A., Shen, Z., Lin, C. T., Unkeless, J. C. & Jacobson, K. (1995) FEBS Lett. 376, 77-80. [DOI] [PubMed] [Google Scholar]
- 17.Li, X., Galli, T., Leu, S., Wade, J. B., Weinman, E. J., Leung, G., Cheong, A., Louvard, D. & Donowitz, M. (2001) J. Physiol. (London) 537, 537-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varma, R. & Mayor, S. (1998) Nature 394, 798-801. [DOI] [PubMed] [Google Scholar]
- 19.Manes, S., del Real, G., Lacalle, R. A., Lucas, P., Gomez-Mouton, C., Sanchez-Palomino, S., Delgado, R., Alcami, J., Mira, E. & Martinez, A. C. (2000) EMBO Rep. 1, 190-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riegler, M., Sedivy, R., Pothoulakis, C., Hamilton, G., Zacherl, J., Bischof, G., Cosentini, E., Feil, W., Schiessel, R., LaMont, J. T., et al. (1995) J. Clin. Invest. 95, 2004-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carpentier, J. L., Sawano, F., Geiger, D., Gorden, P., Perrelet, A. & Orci, L. (1989) J. Cell. Physiol. 138, 519-526. [DOI] [PubMed] [Google Scholar]
- 22.Madshus, I. H., Sandvig, K., Olsnes, S. & van Deurs, B. (1987) J. Cell. Physiol. 131, 14-22. [DOI] [PubMed] [Google Scholar]
- 23.Lamprecht, G., Weinman, E. J. & Yun, C. H. (1998) J. Biol. Chem. 273, 29972-29978. [DOI] [PubMed] [Google Scholar]
- 24.Cha, B., Kenworthy, A., Murtazina, R. & Donowitz, M. (2004) J. Cell Sci. 117, 3353-3365.] [DOI] [PubMed] [Google Scholar]
- 25.Weinman, E. J., Cunningham, R. & Shenolikar, S. (2005) Pflugers Arch. 450, 137-144. [DOI] [PubMed] [Google Scholar]
- 26.Reczek, D., Berryman, M. & Bretscher, A. (1997) J. Cell Biol. 139, 169-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biemesderfer, D., Nagy, T., DeGray, B. & Aronson, P. S. (1999) J. Biol. Chem. 274, 17518-17524. [DOI] [PubMed] [Google Scholar]
- 28.Biemesderfer, D., DeGray, B. & Aronson, P. S. (2001) J. Biol. Chem. 276, 10161-10167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.