Abstract
Angiogenesis restores blood flow to healing tissues, a process that is inhibited by high doses of glucocorticoids. However, the role of endogenous glucocorticoids and the potential for antiglucocorticoid therapy to enhance angiogenesis is unknown. Using in vitro and in vivo models of angiogenesis in mice, we examined effects of (i) endogenous glucocorticoids, (ii) blocking endogenous glucocorticoid action with the glucocorticoid receptor antagonist RU38486, and (iii) abolishing local regeneration of glucocorticoids by the enzyme 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1). Glucocorticoids, administered at physiological concentrations, inhibited angiogenesis in an in vitro aortic ring model and in vivo in polyurethane sponges implanted s.c. RU38486-enhanced angiogenesis in s.c. sponges, in healing surgical wounds, and in the myocardium of mice 7 days after myocardial infarction induced by coronary artery ligation. 11βHSD1 knockout mice showed enhanced angiogenesis in vitro and in vivo within sponges, wounds, and infarcted myocardium. Endogenous glucocorticoids, including those generated locally by 11βHSD1, exert tonic inhibition of angiogenesis. Inhibition of 11βHSD1 in liver and adipose has been advocated to reduce cardiovascular risk in the metabolic syndrome: these data suggest that 11βHSD1 inhibition offers a previously uncharacterized therapeutic approach to improve healing of ischemic or injured tissue.
Keywords: myocardial infarction, wound healing
Angiogenesis, the formation of new vessels from existing ones, is a key factor in many common diseases (1-4), and manipulation of angiogenesis is an important therapeutic target (5, 6). Supraphysiological concentrations of glucocorticoids have been used in vitro and in vivo to inhibit angiogenesis (7-11). It is unknown, however, whether physiological concentrations of endogenous glucocorticoids (principally cortisol in humans and corticosterone in mice) regulate angiogenesis.
The influence of glucocorticoids on their target tissues is regulated in a tissue-specific manner by the isozymes of 11β-hydroxysteroid dehydrogenase (11βHSD) (12). 11βHSD type 1 functions predominantly as an 11-oxidoreductase converting inactive 11-keto metabolites (cortisone in humans; 11-dehydrocorticosterone in mice) into active 11-hydroxy glucocorticoids (cortisol and corticosterone) (13). 11βHSD-1 is highly expressed in liver, adipose tissue, and regions of the central nervous system, where it amplifies intracellular glucocorticoid concentrations and thereby maintains glucocorticoid receptor activation (13). 11βHSD type 2 is an exclusive 11β-dehydrogenase, inactivating cortisol or corticosterone in distal nephron, colon, and sweat glands, thus preventing inappropriate access of glucocorticoids to mineralocorticoid receptors (12). Both 11βHSD isozymes are expressed in the blood vessel wall (14-18). In mouse and rat aorta, 11βHSD-2 is localized in endothelial cells and 11βHSD-1 in vascular smooth muscle (15, 16).
Glucocorticoids have diverse effects on vascular function, altering vasoconstrictor responses (19), impairing endothelium-dependent vasodilatation (19), and inhibiting inflammation and cell proliferation (20, 21). We recently reported studies of vascular function in knockout mice deficient in either 11βHSD isozyme (22). In aortae from 11βHSD-2 -/- mice, endothelium-dependent vasodilatation was impaired, suggesting that 11βHSD-2 protects endothelial cell receptors from glucocorticoids. However, there was no abnormality of vascular tone in 11βHSD-1 -/- mice, so the role of the type 1 isozyme in the vessel wall remained unclear. At that time, Cai et al. (23) demonstrated that 11βHSD1 expression in vascular smooth muscle is up-regulated in response to proinflammatory cytokines, raising the possibility that increased local generation of glucocorticoids contributes to feedback regulation of vascular inflammation.
Given that inflammatory cytokines can promote angiogenesis (24) and pharmacological doses of glucocorticoids have antiangiogenic activity, we hypothesized that 11βHSD-1 modulates angiogenesis by determining the local regeneration of active glucocorticoid in the vessel wall. If so, then manipulation of 11βHSD-1 may provide a novel therapeutic tool to alter angiogenesis. Here, we have tested this hypothesis by using in vitro, in vivo, and pathological models of angiogenesis in mice.
Methods
Mice. Male, C57Bl6J wild-type and 11βHSD-1 homozygous null (-/-) mice aged 8-10 weeks were used (Charles River Laboratories). Genetic inactivation of 11βHSD-1 has been described in MF-1/129 mice (25); for the current experiments, mice were backcrossed over >10 generations onto a C57Bl6J background (26).
Aortic Ring Preparations. Mice were killed, and thoracic aortae were removed, washed in serum-free MCDB 131 medium (Invitrogen), cleaned of periadventitial tissue, and divided into 1- to 3-mm rings.
11βHSD activities were measured by incubating wild-type aortic rings for 24 h at 37°C in 1 ml of DMEM-F12 medium (Invitrogen) containing 3H-steroid supplemented with FBS (1%), streptomycin (100 μg/ml), penicillin (100 units/ml), and amphotericin (0.25 μg/ml) (27). 11β-Reductase activity was determined by adding 10 pmol [3H4]-11-dehydrocorticosterone [synthesized in-house from 1,2,6,7-[3H4]-corticosterone (Amersham Pharmacia Biosciences) by using rat placental homogenate]. Mouse liver (28 ± 5 mg) and medium alone were used as positive and negative controls, respectively. 11β-Dehydrogenase activity was determined by adding 10 pmol 1,2,6,7-[3H4]-corticosterone. Mouse kidney (13 ± 3 mg) and medium alone served as positive and negative controls. After incubation, steroids were extracted from media by using Sep-Pak C18 columns (Waters Millipore). Aortic rings, which contain only 2-3% of the added radioactivity, were not included in the extraction (27). [3H4]-Corticosterone and [3H4]-11-dehydrocorticosterone were separated by HPLC and quantified by on-line liquid scintillation counting (16). Enzyme activity was expressed as conversion after subtraction of apparent conversion in negative control wells. Both 11β-reductase (0.65 ± 0.24 pmol/mg) and 11β-dehydrogenase (0.66 ± 0.28 pmol/mg) activities were detected in aortic rings with similar conversion rates as in positive controls: liver for 11βHSD-1 (0.18 ± 0.03 pmol/mg) and kidney for 11βHSD-2 (2.13 ± 1.65 pmol/mg).
To quantify angiogenesis, aortic rings were embedded in 200 μl of steroid-free Matrigel (Becton Dickinson) (Fig. 1) and incubated at 37°C in serum-free MCDB 131, with heparin, ascorbic acid, and GA1000 (Cambrex Biosciences) in the presence and absence of corticosterone (3, 30, 300, and 600 nM), 11-dehydrocorticosterone (300 and 600 nM), the glucocorticoid receptor antagonist RU38486 (10-6 M), the mineralocorticoid receptor antagonist spironolactone (10-6 M), and/or the nonselective 11βHSD inhibitor carbenoxolone (10-6 M). All drugs (Sigma-Aldrich) were dissolved in ethanol and diluted in aqueous solution; final ethanol concentration 1-3% vol/vol. Media were changed every 48 h. Experiments were performed in triplicate. In initial experiments, new vessels were counted daily by using light microscopy (ref. 8 and Fig. 1). From these studies, day 7 was selected as the appropriate time point to examine the effects of glucocorticoids (Fig. 1B).
Fig. 1.
Angiogenesis in aortic rings in vitro. (A) Light microscopy of new vessels shown sprouting from aortic rings. (i) Aortic ring incubated for 7 days without glucocorticoid. (ii) Aortic ring incubated for 7 days in the presence of glucocorticoid. Thick white arrows indicate the aortic ring; thin white arrows indicate new vessels. (Scale bar: 0.2 mm.) (iii) Uptake of low-density lipoprotein (LDL) is shown by fluorescence microscopy. This ring was incubated for 7 days without steroids. Thick white arrows indicate the aortic ring; thin white arrows indicate uptake of fluorescent labeled LDL in endothelial cells in new vessels; black arrows indicate uptake in endothelial cells of the aortic ring. (Scale bar: 0.2 mm.) (iv) High power view of new vessels; thick white arrows indicate the aortic ring and thin white arrows indicate uptake of fluorescent labeled low density lipoprotein in endothelial cells (Scale bar: 0.02 mm.) (Bi) Time course and effect of corticosterone on angiogenesis. ○, results from vessels incubated without steroids; ♦, results from vessels incubated with corticosterone (600 nM). Results are mean ± SEM for n = 4 per group. Comparison was by repeated measures ANOVA; *, P < 0.02. (Bii) Effects of corticosterone and 11-dehydrocorticosterone. Vessels were counted after 7-day incubation with steroids. Results are mean ± SEM. #, P < 0.01 versus vehicle by 2-way ANOVA and least squares difference post hoc test. (C) Influence of receptor antagonism. (i) Effects of the mineralocorticoid receptor antagonist spironolactone. Aortic rings from C57Bl6J mice were incubated with (filled bars) and without (open bars) spironolactone (10-6 M) and glucocorticoids (600 nM). Results are mean ± SEM for n = 6 experiments. #, P < 0.02 versus corresponding vehicle. Spironolactone alone had no effect. (ii) Effects of the glucocorticoid receptor antagonist RU38486. Aortic rings from C57Bl6 mice were incubated with (filled bars) and without (open bars) RU38486 (10-6 M) and glucocorticoids (600 nM). Results are mean ± SEM for n = 4-6 experiments. # P < 0.01 versus corresponding vehicle. ***, P < 0.001 for the effect of RU38486 in the presence of glucocorticoid. RU38486 alone had no effect. (D) Effects of 11βHSD inhibition (i) Pharmacological inhibitor carbenoxolone. Aortic rings from C57Bl6J mice were incubated with (filled bars) and without (open bars) carbenoxolone (10-6 M) and glucocorticoids (600 nM). Results are mean ± SEM for n = 5 experiments. #, P < 0.01 versus corresponding vehicle. *, P < 0.04 for the effect of carbenoxolone in the presence of 11-dehydrocorticosterone. Carbenoxolone had no effect in the presence of corticosterone or vehicle alone. (ii) Transgenic deletion of 11βHSD1. Effects of corticosterone and 11-dehydrocorticosterone on angiogenesis in vessels from 11βHSD1 -/- mice. Aortic rings from C57Bl6J wild-type (open bars) or 11βHSD1 -/- (filled bars) mice were incubated with and without glucocorticoids (600 nM). Results are mean ± SEM for n = 7 experiments. #, P < 0.01 versus corresponding vehicle. **, P < 0.01 for differences in angiogenesis between wild-type and 11βHSD1 -/- mice. Angiogenesis was not different between strains in the presence of vehicle or corticosterone but was inhibited by 11-dehydrocorticosterone in wild-type but not 11βHSD1 -/- mice.
To confirm the nature of apparent new vessels, endothelial cells were identified by uptake of fluorescent-labeled acetylated low-density lipoprotein (Biogenesis, Poole, U.K.) (Fig. 1 A).
s.c. Sponge Implant Assay. Mice were anesthetized with halothane, and a sterilized sponge cylinder (0.5 cm × 1 cm) (Caligen Foam, Accrington, Lancashire, U.K.) was implanted s.c. on each flank. Sponges contained a silastic insert (Silastic 20 medical grade, Dow Corning) impregnated with vehicle, 2 mg of cortisol or cortisone, or 5.25 mg of RU38486. Each animal had an intervention-impregnated sponge (steroid or RU38486) on one side and a placebo-impregnated sponge (silastic only) on the other. Such inserts release their impregnated compounds in vivo at a constant rate for 3 weeks (28). Human steroids (cortisol and cortisone, equivalent to corticosterone and 11-dehydrocorticosterone) were used to allow distinction from endogenous steroids. In separate experiments (data not shown), angiogenesis in placebo-impregnated sponges was not altered by the presence or absence of a contralateral steroid-treated sponge.
A further cohort of wild-type mice underwent adrenalectomy or sham surgery as described (29) at the time of implantation of untreated sponges. These mice were then maintained on 0.9% saline in place of drinking water.
Twenty days after implantation (10), mice were killed, sponges were excised, and inserts were removed. Sponges were bisected; one half was fixed in 10% formalin and embedded in paraffin wax. Sections (8 μm) were stained with hematoxylin/eosin for identification of blood vessels, as described in ref. 30. The second half of the sponge was weighed, homogenized in 2 ml of sterile PBS at 4°C, and centrifuged (2,000 × g for 30 min). Steroids were extracted from the supernatant by using ethyl acetate and cortisol quantified by using a specific RIA (Amersham Pharmacia Biotech). Sponge vessel density was determined by using the mean of triplicate Chalkley counts on two sections per sponge (31, 32).
Chronic Coronary Artery Ligation. Wild-type and 11βHSD-1 -/- mice were anesthetized with an i.p. injection of xylazine (0.018 mg/kg), ketamine (100 mg/kg), and atropine (600 mcg/kg) (33). Surgery was performed as described in ref. 34. Briefly, after endotracheal intubation and mechanical ventilation (MiniVent, Harvard Apparatus, Holliston, MA), superficial tissues were dissected, an incision was made in the fourth intercostal space, the pericardium was divided, and the left main descending artery was ligated with 6.0 prolene suture (Ethicon). In sham operated animals, the suture was not ligated. The thoracic wall was closed by layered suturing; the skin was stitched with a continuous suture by using 5-0 Mersilk with a 10-mm 3/8c round-bodied needle (Ethicon). At the time of surgery completion, animals received i.p. atipamazole (5 mg/kg) and s.c. buprenorphine (0.05 mg/kg).
A further cohort of wild-type mice received a s.c. 10 mg implant (28) containing either vehicle or 5.25 mg of RU38486 1 week before coronary artery surgery.
In preliminary experiments, mice were killed on days 1, 3, 5, 7, and 14 after surgery by cervical dislocation. The angiogenic response was well established 7 days after infarction (see Fig. 3B), so this interval was selected for comparisons between the groups above. Excised hearts and surgical thoracotomy wounds were fixed in 10% formalin, paraffin embedded, and sectioned at 8 μm. Sections were stained with an anti-von Willebrand factor antibody (DakoCytomation, Cambridgeshire, U.K.) to label endothelial cells and quantify angiogenesis. Hematoxylin and eosin was used to stain sections from hearts collected at day 7 after coronary ligation to measure the area of the left ventricle affected by infarction.
Fig. 3.
Effect of injury on angiogenesis in mouse myocardium and skin. (A) Light microscopy (×50) of anti-von Willebrand factor immunostaining with fast red chromogen substrate in day 7 wild-type sham (i) and infarcted (ii) hearts. Scattered medium and large vessels were detected in sham hearts. In contrast, many more vessels were observed in the healing myocardium after infarction. Black arrows, vessels; lv, left ventricle, es, endocardial surface. (Scale bar: 100 μm.) (iii) Medium power (×100) light microscopy of hematoxylin/eosin staining in day-7 wild-type infarcted heart. (Scale bar: 100 μm.) (iv) High power (×400) light microscopy of anti-von Willebrand immunostaining in day-7 wild-type infarcted heart; (Scale bar: 100 μm.) The vascularity of the infarcted myocardium was increased and multiple vessels containing erythrocytes were observed (black arrows indicate vessels). (Bi) Vascularity of myocardium of wild-type mouse hearts after ligation (filled bars, n = 3-11) or sham surgery (open bars, n = 1-6). Sham-operated animals show a constant vascularity in contrast to CCL animals in which vessel counts increase with time, achieving a maximum at day 7. (Bii) Day-7 hearts from wild-type and 11βHSD1 -/- mice. Ligation (filled bars) in wild-type and 11βHSD1 -/- increased angiogenesis in comparison to sham (open bars) (wild type, n = 6 sham and n = 11 ligations; 11βHSD1-/-, n = 5 sham and n = 10 ligations). Ligations in mice that received RU38486 (dark gray bars, n = 6) induced greater myocardial angiogenesis in comparison to vehicle-treated ligated controls (light gray bars, n = 3). Results are mean ± SEM. #, P < 0.001 versus corresponding sham. ***, P < 0.001 for differences between wild-type and 11HSD1 -/-.*, P < 0.02 for differences between coronary artery ligated wild-type mice treated with RU38486 or vehicle. (C) Identification of blood vessels in 7-day-old cutaneous wounds from wild-type mice stained with hematoxylin/eosin (i) or an antibody against von Willebrand factor (ii). (Magnification: ×400; scale bars: 100 μm.)
Quantification of vessels within the myocardium was achieved by counting large- and medium-sized vessels as described in ref. 35. Vessels were identified at ×400 magnification (Zeiss) in vWF (36-38) stained sections. Counting (39) was performed in the four most vascular fields (two endocardial and two epicardial) by using a 0.0625-mm2 reticule; the borders of the reticule were within the infarct. The area of left ventricle affected by infarction was determined as a percentage of left ventricular wall area (34) and measured at direct light microscopy; images were captured by using a Research Systems (Imaging Research, St. Catherine's, ON, Canada) photometric camera and analyzed by using in-house scripts.
Wound vessel density was determined in the dermis of vWF-stained sections at ×250 light microscopy by using the mean of triplicate Chalkley counts on two sections per wound (31).
Statistics. Data are mean ± SEM. Comparisons were made by ANOVA with least squares difference post hoc tests. Vessel quantification was performed by investigators “blinded” to the origin of the sections. Interassay and intraassay coefficients of variation in wild-type mice were 17% (n = 32) and 22% (n = 18), respectively, for vessel number in aortic rings after 7 days in culture; 12% (n = 6) and 12% (n = 6) for vessel density in sponge implants; 19% (n = 11) and 10% (n = 11) in day-7 infarcts; and 7% (n = 4) and 12% (n = 4) for day-7 wounds.
Results
Effects of Glucocorticoids and 11βHSD-1 on Angiogenesis in Vitro in Aortic Rings. Both corticosterone and 11-dehydrocorticosterone inhibited angiogenesis in wild-type mouse vessels across a range of physiological concentrations (Fig. 1B). The angiostatic effect is mediated by glucocorticoid receptors because it was prevented by the antagonist RU38486 (which has no effect in the absence of steroid) but not by the mineralocorticoid receptor antagonist spironolactone (Fig. 1C).
Measurement of relevant product generation confirmed both 11β-reductase (0.65 ± 0.24 pmol/mg) and 11β-dehydrogenase (0.66 ± 0.28 pmol/mg) activities in aortic rings with similar conversion rates as in positive controls, liver for 11βHSD-1 (0.18 ± 0.03 pmol/mg), and kidney for 11βHSD-2 (2.13 ± 1.65 pmol/mg). Pharmacological inhibition of 11βHSDs in aortic rings was achieved with the nonselective inhibitor carbenoxolone (10-6M), which had no direct effect and prevented the antiangiogenic effect of 11-dehydrocorticosterone but not corticosterone (Fig. 1Di).
To confirm the role of 11βHSD-1, aortic rings were obtained from homozygous 11βHSD-1 null (-/-) mice congenic on a C57Bl6J genetic background (26) and C57Bl6J controls. Angiogenesis in aortic rings from 11βHSD-1 -/- mice was similar to that in wild-type controls in the absence of steroid and inhibited to a similar degree by corticosterone. However, 11-dehydrocorticosterone did not inhibit angiogenesis in vessels from 11βHSD-1 -/- mice (Fig. 1Dii).
Effect of Endogenous Glucocorticoids and 11βHSD-1 on Angiogenesis in s.c. Sponge Implants in Vivo. Placebo-impregnated sponges excised after 20 days (10) were red on gross inspection with a lace-like covering of blood vessels. At histology, there was an inflammatory infiltrate and an abundance of blood vessels (Fig. 2Ai). Sponges from adrenalectomized animals and sponges impregnated with the glucocorticoid receptor antagonist RU38486 both exhibited enhanced angiogenesis (Fig. 2Bi) in wild-type mice.
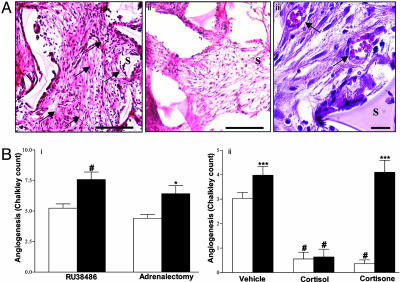
Fig. 2.
Angiogenesis in s.c. implanted sponges. (A) Light microscopy of hematoxylin/eosin stained sponge 8-μm sections from wild-type mice: vehicle (i) and cortisol-treated (ii) sponge (Scale bar: 400 μm) and vehicle-treated sponge at high power (iii) (Scale bar: 50 μm.) Sponges were covered with a fibroblast-rich fibrous coat and were infiltrated with inflammatory neutrophils and lymphocytes. Placebo-treated sponges alone were also infiltrated with an organized matrix and an abundance of blood vessels (black arrows). S denotes sponge matrix. (Bi) Sponges from C57Bl6J wild-type (n = 6) mice. Exposure to RU38486 or adrenalectomy (filled bars) compared with placebo or sham surgery (open bars). Results are mean ± SEM. #, P < 0.01 versus vehicle; *, P < 0.02 versus sham. New vessel formation was greater in RU38486-impregnated sponges or sponges from adrenalectomized mice versus their relevant controls. (Bii) Sponges from C57Bl6J wild-type (open bars, n = 12) or 11βHSD1 -/- (filled bars, n = 6) mice with and without glucocorticoids. Results are mean ± SEM. #, P < 0.001 versus corresponding vehicle. ***, P < 0.001 for differences between wild type and 11βHSD1 -/-. Placebo-impregnated sponges exhibited an increased angiogenic response in 11βHSD1 -/- compared to wild-type mice. Cortisol inhibited angiogenesis in both strains, but cortisone inhibited angiogenesis only in wild-type mice.
To test the effects of 11-hydroxy and 11-keto-glucocorticoids we used the “human” steroids cortisol and cortisone, which allowed measurement of steroid concentrations within the sponge independently of endogenous murine corticosterone and 11-dehydrocorticosterone (Table 1). In wild-type C57Bl6J mice, both cortisol and cortisone inhibited angiogenesis in vivo (Fig. 2 Aii and Bii). In 11βHSD-1 null mice, angiogenesis was increased in placebo-impregnated sponges. Impregnation with cortisol produced similar cortisol concentrations in wild-type and 11βHSD-1 null mice (Table 1) and inhibited angiogenesis to a similar degree (Fig. 2Bii). However, impregnation with cortisone, in contrast with its effects in wild-type controls, did not elevate sponge cortisol concentrations in 11βHSD-1 null mice (Table 1) and did not inhibit angiogenesis (Fig. 2Bii).
Table 1. Cortisol concentration in sponge homogenates from wild-type and 11 βHSD1 −/− homozygous null mice.
| Cortisol level (ng/g sponge)
|
|||
|---|---|---|---|
| Strain | Steroid impregnated | Ipsilateral steroid-treated sponge | Contralateral placebo-treated sponge |
| Wild type | Cortisol | 4,271 ± 186# | 161 ± 18 |
| Cortisone | 295 ± 25#** | 98 ± 19 | |
| 11 βHSD-1 −/− | Cortisol | 3,775 ± 1,703# | 135 ± 46 |
| Cortisone | 87 ± 11 | 90 ± 30 | |
Results are mean ± SEM for n = 3-6 experiments, #, P < 0.01 versus contralateral placebo. **, P < 0.01 for comparison of wild type and 11 βHSD1 −/−.
Effect of Endogenous Glucocorticoids and 11βHSD-1 on Myocardial Revascularization After Coronary Artery Ligation. At day 7, the proportional area of the left ventricular myocardium affected by coronary artery ligation was similar in all treatment groups (41.8 ± 6.2% in vehicle versus 45.5 ± 4.8% in RU38486 and 44.2 ± 3.4% in wild types versus 44.2 ± 2.6% in 11βHSD-1-/-). RU38486 increased angiogenesis in the left ventricle after infarction in wild-type mice (Fig. 3Bii).
There was no difference in myocardial vascularity between sham-operated wild-type and 11βHSD-1 null mice. In contrast, 7 days after coronary artery ligation, 11βHSD-1 null mice exhibited enhanced revascularization in the infarcted myocardium (Fig. 3Bii),
Effect of Endogenous Glucocorticoids and 11βHSD-1 on Angiogenesis in Cutaneous Surgical Wounds. New vessel formation was examined in cutaneous surgical wounds in mice that underwent thoracotomy for the coronary artery ligation studies (Fig. 3c). The dermal angiogenic response was greater in RU38486-treated mice (4.8 ± 0.29 Chalkley count versus vehicle 3.5 ± 0.21; P < 0.01) and in 11βHSD-1 null mice (5.1 ± 0.27 Chalkley count; P < 0.01) in comparison to wild-type controls (3.5 ± 0.25 Chalkley count).
Discussion
Folkman et al. described the angiostatic effects of pharmacological glucocorticoids in vitro >20 years ago (11), and these effects have been confirmed in vivo (3, 10). Here, we show that the angiostatic effect occurs at physiological concentrations of glucocorticoids and is mediated by glucocorticoid receptors, and that endogenous glucocorticoids tonically repress angiogenic responses. Moreover, we show that 11βHSD1, by regenerating active glucocorticoids locally, amplifies the angiostatic effect of glucocorticoids and, thereby, constrains the angiogenic response after ischemia and injury.
These observations raise the intriguing possibility that local variations in cortisol levels or in tissue sensitivity to cortisol are key determinants of angiogenesis in disease. It is well recognized that, in Cushing's syndrome, glucocorticoid excess is associated with impaired wound healing (40). More recently, we showed that exogenous glucocorticoid therapy is associated not only with increased incidence of myocardial infarction but also with an unexpected increase in prevalence of heart failure (41, 42), suggesting an impact on the outcome and the incidence of cardiovascular disease. More subtle variations in cortisol secretion and action, including variations in responses to stress, have been described in many populations and have been related to risk factors for occlusive vascular disease, mood, development in early life, gender, age, etc. (43-45). We now suggest that effects of cortisol on angiogenesis could explain the links between these quantitative traits in the population and the health outcomes from vascular disease and, perhaps, from other diseases involving angiogenesis, including neoplasia. If so, then therapies, which reduce glucocorticoid action within ischemic tissue, may be valuable in improving collateral perfusion. This result cannot be achieved safely with systemic antiglucocorticoid therapy that is likely to lead to Addisonian crisis after a severe stressor such as myocardial infarction. The role of 11βHSD-1 described here offers an opportunity for tissue-specific targeting of therapy.
We described the presence of 11βHSD-1 in the vessel wall >10 years ago (16), but its importance has remained obscure. The observations that nonselective 11βHSD inhibitors influence vascular tone (27, 46) can be attributed to effects on the 11βHSD-2 isozyme that catalyses inactivation of glucocorticoids within endothelial cells (15, 22). Here, we show that regeneration of glucocorticoids by 11βHSD-1 in isolated aortae amplifies their angiostatic effect. We found no evidence that dehydrogenase 11βHSD-2 influences angiogenesis in vitro because the nonselective 11βHSD inhibitor carbenoxolone did not potentiate the angiostatic effect of corticosterone.
In vivo 11βHSD-1 null mice have no obvious difference in vascular structure in healthy tissues. Normal vascular development occurs in other models of altered angiogenesis in which the abnormality is apparent only in adult pathology (47), thus reflecting the distinct pathways underlying vasculogenesis and adult angiogenesis. However, when angiogenesis is stimulated in adult mice, we found that 11βHSD-1 amplifies the angiostatic effect of endogenous glucocorticoids. In s.c. sponge implants, this effect is local, rather than systemic, because angiogenesis in contralateral sponges was unaffected. Moreover, cortisol concentrations in the sponges were lower after impregnation with cortisone than with cortisol, suggesting that it is the generation of cortisol locally within the cells that express 11β-HSD1, rather than levels of cortisol in the interstitial fluid of the sponge, which determines the angiostatic effect. Finally, the relevance of 11βHSD-1 was confirmed by the demonstration that 11βHSD-1 null mice exhibit greater angiogenic responses in wounds and infarcted myocardium. In these studies, immunohistochemical localization of vWF enabled quantification of large- and medium-sized vessels (35) but not the entire population of endothelial cells in a section. Thus, all of the vessels included in the quantification are likely to be functional.
It is possible that these observations reflect 11βHSD-1 activity either within the vessel wall or in the inflammatory infiltrate that accompanies angiogenesis in all of these in vivo models. 11βHSD-1 is expressed in macrophages (48), and regeneration of glucocorticoids enhances phagocytosis of apoptotic neutrophils (49), hence absence of 11βHSD-1 may confer a prolonged and enhanced acute inflammatory response that, in turn, might stimulate angiogenesis. However, 11βHSD-1 in the inflammatory infiltrate cannot explain the influence of 11βHSD-1 in isolated aortic rings. The findings in the isolated aortic ring model confirm that vessel wall 11βHSD-1 moderates the angiostatic influence of glucocorticoids and confirms that regeneration of active glucocorticoids within vascular smooth muscle cell can inhibit angiogenic processes. Although the in vivo models validated the isolated aortic ring findings, it is apparent nonetheless that inflammatory cytokines induce 11βHSD-1 expression in a variety of cell types (13), including in vascular smooth muscle cells (23), so that the contribution of 11βHSD-1 within the vessel wall may be intimately related with the extent of the inflammatory response.
Angiogenesis is crucially dependent on endothelial cells producing key factors such as vascular endothelial growth factor (VEGF) and forming a de novo collagen basement membrane to allow structured cell proliferation (24). In the chick chorioallantoic membrane, glucocorticoids alter endothelial cell morphology and collagen production (7, 9). It has also been proposed that glucocorticoid effects are mediated by inhibition of endothelial VEGF transcription and endothelial nitric oxide production (19, 50). However, in keeping with a role for 11βHSD-1, the effect of glucocorticoids may be mediated within vascular smooth muscle, where inhibition of matrix metalloproteinase production (51) may alter the efficacy of endothelium-dependent new vessel formation, and antiproliferative effects (20) may attenuate formation of vessel walls around endothelial cell buds.
In contrast to the established effects of supraphysiological concentrations of glucocorticoids, the influence of endogenous glucocorticoids on angiogenesis has until now remained unclear. The current findings in vitro and in vivo confirm a physiological role for endogenous glucocorticoids to regulate angiogenesis and highlight the significance of vascular 11βHSD-1 in modulating this effect. The wider relevance of these findings to pathology is illustrated in the models of wound healing and myocardial infarction. These findings may lead to therapeutic approaches to enhance angiogenesis by preventing glucocorticoid action. Although systemic glucocorticoid receptor blockade is unlikely to be successful as a treatment in the short term (because of adverse effects of preventing the normal cortisol-dependent stress response) or in the long term (because of compensatory activation of the hypothalamic pituitary adrenal axis), the current data suggest that manipulation of local 11βHSD-1 offers a more targeted approach to the blood vessel wall. 11βHSD-1 inhibitors are already being developed for reducing risk factors for cardiovascular disease (45), including in type 2 diabetes mellitus and obesity. These results suggest that pharmacological inhibition of 11βHSD-1 may also be valuable in ischemic heart disease and impaired wound healing.
Acknowledgments
We thank Profs. John Mullins and Jonathan Seckl (University of Edinburgh) for their guidance and kind provision of 11βHSD-1 -/- mice and Jill Harrison, Dr. Janice Paterson, Dr. Paul Perry, Dr. Roy Bicknell, Sandra Peake, and Dr. Kairbaan Hodivala-Dilke for assistance and advice. This work was supported by the Wellcome Trust, British Heart Foundation, and Medical Research Council.
Author contributions: G.R.S., P.W.F.H., A.R.D., D.A., C.J.K., G.A.G., and B.R.W. designed research; G.R.S., P.W.F.H., I.S., A.R.D., D.A., and C.J.K. performed research; I.S. and G.A.G. contributed new reagents/analytic tools; G.R.S., P.W.F.H., A.R.D., D.A., C.J.K., G.A.G., and B.R.W. analyzed data; and G.R.S., P.W.F.H., and B.R.W. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: 11βHSD1, 11β-hydroxysteroid dehydrogenase type 1.
References
- 1.Kurotobi, T., Sato, H., Kinjo, K., Nakatani, D., Mizuno, H., Shimizu, M., Imai, K., Hirayama, A., Kodama, K. & Hori, M. (2004) J. Am. Coll. Cardiol. 44, 28-34. [DOI] [PubMed] [Google Scholar]
- 2.Vernieri, F., Pasqualetti, P., Matteis, M., Passarelli, F., Troisi, E., Rossini, P. M., Caltagirone, C. & Silvestrini, M. (2001) Stroke 32, 1552-1558. [DOI] [PubMed] [Google Scholar]
- 3.Hasan, Q., Tan, S. T., Gush, J., Peters, S. G. & Davis, P. F. (2000) Pediatrics 105, 117-120. [DOI] [PubMed] [Google Scholar]
- 4.Aiello, L. P. & Wong, J. S. (2000) Kidney Int. Suppl. 77, S113-S119. [DOI] [PubMed] [Google Scholar]
- 5.Siemann, D. W., Chaplin, D. J. & Horsman, M. R. (2004) Cancer 100, 2491-2499. [DOI] [PubMed] [Google Scholar]
- 6.Kinnaird, T., Stabile, E., Epstein, S. E. & Fuchs, S. (2003) J. Interv. Cardiol. 16, 289-297. [DOI] [PubMed] [Google Scholar]
- 7.Maragoudakis, M. E., Sarmonika, M. & Panoutsacopoulou, M. (1989) J. Pharmacol. Exp. Ther. 251, 679-682. [PubMed] [Google Scholar]
- 8.Nicosia, R. F. & Ottinetti, A. (1990) Lab. Invest. 63, 115-122. [PubMed] [Google Scholar]
- 9.Folkman, J. & Ingber, D. E. (1987) Ann. Surg. 206, 374-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hori, Y., Hu, D. E., Yasui, K., Smither, R. L., Gresham, G. A. & Fan, T. P. (1996) Br. J. Pharmacol. 118, 1584-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folkman, J., Langer, R., Linhardt, R. J., Haudenschild, C. & Taylor, S. (1983) Science 221, 719-725. [DOI] [PubMed] [Google Scholar]
- 12.Stewart, P. M. & Krozowski, Z. S. (1999) Vitam. Horm. 57, 249-324. [PubMed] [Google Scholar]
- 13.Seckl, J. R. & Walker, B. R. (2001) Endocrinology 142, 1371-1376. [DOI] [PubMed] [Google Scholar]
- 14.Smith, R. E., Little, P. J., Maguire, J. A., Stein-Oakley, A. N. & Krozowski, Z. S. (1996) Clin. Exp. Pharmacol. Physiol. 23, 549-551. [DOI] [PubMed] [Google Scholar]
- 15.Christy, C., Hadoke, P. W. F., Paterson, J. M., Mullins, J. J., Seckl, J. R. & Walker, B. R. (2003) Hypertension 42, 580-587. [DOI] [PubMed] [Google Scholar]
- 16.Walker, B. R., Yau, J. L., Brett, L. P., Seckl, J. R., Monder, C., Williams, B. C. & Edwards, C. R. W. (1991) Endocrinology 129, 3305-3312. [DOI] [PubMed] [Google Scholar]
- 17.Takeda, Y., Miyamori, I., Yoneda, T., Ito, Y. & Takeda, R. (1994) Life Sci. 54, 281-285. [DOI] [PubMed] [Google Scholar]
- 18.Brem, A. S., Bina, R. B., King, T. & Morris, D. J. (1995) Steroids 60, 406-410. [DOI] [PubMed] [Google Scholar]
- 19.Ullian, M. E. (1999) Cardiovasc. Res. 41, 55-64. [DOI] [PubMed] [Google Scholar]
- 20.Longenecker, J. P., Kilty, L. A. & Johnson, L. K. (1984) J. Cell Biol. 98, 534-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Versaci, F., Gaspardone, A., Tomai, F., Ribichini, F., Russo, P., Proietti, I., Ghini, A. S., Ferrero, V., Chiariello, L., Gioffre, P. A., et al. (2002) J. Am. Coll. Cardiol. 40, 1935-1942. [DOI] [PubMed] [Google Scholar]
- 22.Hadoke, P. W. F., Christy, C., Kotelevtsev, Y. V., Williams, B. C., Kenyon, C. J., Seckl, J. R., Mullins, J. J. & Walker, B. R. (2001) Circulation 104, 2832-2837. [DOI] [PubMed] [Google Scholar]
- 23.Cai, T. Q., Wong, B. M., Mundt, S. S., Thieringer, R., Wright, S. D. & Hermanowski-Vosatka, A. (2001) J. Steroid Biochem. 77, 117-122. [DOI] [PubMed] [Google Scholar]
- 24.Conway, E. M., Collen, D. & Carmeliet, P. (2001) Cardiovasc. Res. 49, 507-521. [DOI] [PubMed] [Google Scholar]
- 25.Kotelevtsev, Y. V., Holmes, M. C., Burchell, A., Houston, P. M., Scholl, D., Jamieson, P., Best, R., Brown, R. W., Edwards, C. R. W., Seckl, J. R. & Mullins, J. J. (1997) Proc. Natl. Acad. Sci. USA 94, 14924-14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morton, N. M., Paterson, J. M., Masuzaki, H., Holmes, M. C., Staels, B., Fievet, C., Walker, B. R., Flier, J. S., Mullins, J. J. & Seckl, J. R. (2004) Diabetes 53, 931-938. [DOI] [PubMed] [Google Scholar]
- 27.Souness, G. W., Brem, A. S. & Morris, D. J. (2002) Steroids 67, 195-201. [DOI] [PubMed] [Google Scholar]
- 28.Soro, A., Panarelli, M., Holloway, C. D., Fraser, R. & Kenyon, C. J. (1997) Steroids 62, 388-394. [DOI] [PubMed] [Google Scholar]
- 29.Livingstone, D. E. W., Kenyon, C. J. & Walker, B. R. (2000) J. Endocrinol. 167, 533-539. [DOI] [PubMed] [Google Scholar]
- 30.Andrade, S. P., Fan, T. P. & Lewis, G. P. (1987) Br. J. Exp. Pathol. 68, 755-766. [PMC free article] [PubMed] [Google Scholar]
- 31.Fox, S. B., Leek, R. D., Weekes, M. P., Whitehouse, R. M., Gatter, K. C. & Harris, A. L. (1995) J. Pathol. 177, 275-283. [DOI] [PubMed] [Google Scholar]
- 32.Hague, S., MacKenzie, I. Z., Bicknell, R. & Rees, M. C. (2002) Hum. Reprod. 17, 786-793. [DOI] [PubMed] [Google Scholar]
- 33.Mora, A., Davies, A. M., Bertrand, L., Sharif, I., Budas, G. R., Jovanovic, S., Mouton, V., Kahn, C. R., Lucocq, J. M., Gray, G. A., et al. (2003) EMBO J. 22, 4666-4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lutgens, E., Daemen, M. J., de Muinck, E. D., Debets, J., Leenders, P. & Smits, J. F. (1999) Cardiovasc. Res. 41, 586-593. [DOI] [PubMed] [Google Scholar]
- 35.Heymans, S., Luttun, A., Nuyens, D., Theilmeier, G., Creemers, E., Moons, L., Dyspersin, G. D., Cleutjens, J. P., Shipley, M., Angellilo, A., et al. (1999) Nat. Med. 5, 1135-1142. [DOI] [PubMed] [Google Scholar]
- 36.Jayasankar, V., Woo, Y. J., Bish, L. T., Pirolli, T. J., Chatterjee, S., Berry, M. F., Burdick, J., Gardner, T. J. & Sweeney, H. L. (2003) Circulation 108, 230-236. [DOI] [PubMed] [Google Scholar]
- 37.Schwarz, E. R., Meven, D. A., Sulemanjee, N. Z., Kersting, P. H., Tussing, T., Skobel, E. C., Hanrath, P. & Uretsky, B. F. (2004) J. Cardiovasc. Pharmacol. Ther. 9, 279-289. [DOI] [PubMed] [Google Scholar]
- 38.Li, W., Tanaka, K., Ihaya, A., Fujibayashi, Y., Takamatsu, S., Morioka, K., Sasaki, M., Uesaka, T., Kimura, T., Yamada, N., et al. (2005) Am. J. Physiol. 288, H408-H415. [DOI] [PubMed] [Google Scholar]
- 39.Virag, J. I. & Murry, C. E. (2003) Am. J. Pathol. 163, 2433-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gordon, C. B., Li, D. G., Stagg, C. A., Manson, P. & Udelsman, R. (1994) Surgery 116, 1082-1087. [PubMed] [Google Scholar]
- 41.Souverain, P. C., Berard, A., van Staa, T. P., Cooper, C., Leufkens, H. G. M. & Walker, B. R. (2004) Heart 90, 859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei, L., MacDonald, T. M. & Walker, B. R. (2004) Ann. Intern. Med. 141, 764-770. [DOI] [PubMed] [Google Scholar]
- 43.Phillips, D. I. W., Barker, D. J. P., Fall, C. H. D., Whorwood, C. B., Seckl, J. R., Wood, P. J. & Walker, B. R. (1998) J. Clin. Endocrinol. Metab. 83, 757-760. [DOI] [PubMed] [Google Scholar]
- 44.McEwen, B. S. (1999) Front. Neuroendocrinol. 20, 49-70. [DOI] [PubMed] [Google Scholar]
- 45.Walker, B. R. & Seckl, J. R. (2004) Expert Opin. Ther. Targets 7, 771-783. [DOI] [PubMed] [Google Scholar]
- 46.Teelucksingh, S., Mackie, A. D. R., Burt, D., McIntyre, M. A., Brett, L. & Edwards, C. R. W. (1990) Lancet 335, 1060-1063. [DOI] [PubMed] [Google Scholar]
- 47.Carmeliet, P., Moons, L., Luttun, A., Vincenti, V., Compernolle, V., De Mol, M., Wu, Y., Bono, F., Devy, L., Beck, H., et al. (2001) Nat. Med. 7, 575-583. [DOI] [PubMed] [Google Scholar]
- 48.Thieringer, R., Le Grand, C. B., Carbin, L., Cai, T.-Q., Wong, B. & Wright, S. D. (2001) J. Immunol. 167, 30-35. [DOI] [PubMed] [Google Scholar]
- 49.Rossi, A., Liu, Y., Cousin, J. M., Dransfield, I., Seckl, J. R., Haslett, C. & Savill, J. (1999) J. Immunol. 162, 3639-3646. [PubMed] [Google Scholar]
- 50.Nauck, M., Karakiulakis, G., Perruchoud, A. P., Papakonstantinou, E. & Roth, M. (1998) Eur. J. Pharmacol. 341, 309-315. [DOI] [PubMed] [Google Scholar]
- 51.Pross, C., Farooq, M. M., Lane, J. S., Angle, N., Tomono, C. K., Xavier, A. E., Freischlag, J. A., Collins, A. E., Law, R. E. & Gelabert, H. A. (2002) J. Vasc. Surg. 35, 1253-1259. [DOI] [PubMed] [Google Scholar]