Abstract
Cryptochromes (CRY) are blue light photoreceptors that mediate various light-induced responses in plants and animals. Arabidopsis CRY (CRY1 and CRY2) functions through negatively regulating constitutive photomorphogenic (COP) 1, a repressor of photomorphogenesis. Water evaporation and photosynthesis are regulated by the stomatal pores in plants, which are closed in darkness but open in response to blue light. There is evidence only for the phototropin blue light receptors (PHOT1 and PHOT2) in mediating blue light regulation of stomatal opening. Here, we report a previously uncharacterized role for Arabidopsis CRY and COP1 in the regulation of stomatal opening. Stomata of the cry1 cry2 double mutant showed reduced blue light response, whereas those of the CRY1-overexpressing plants showed hypersensitive response to blue light. In addition, stomata of the phot1 phot2 double mutant responded to blue light, but those of the cry1 cry2 phot1 phot2 quadruple mutant hardly responded. Strikingly, stomata of the cop1 mutant were constitutively open in darkness and stomata of the cry1 cry2 cop1 and phot1 phot2 cop1 triple mutants were open as wide as those of the cop1 single mutant under blue light. These results indicate that CRY functions additively with PHOT in mediating blue light-induced stomatal opening and that COP1 is a repressor of stomatal opening and likely acts downstream of CRY and PHOT signaling pathways.
Keywords: blue light photoreceptor, phototropin, water evaporation, photosynthesis
The stomatal pores of higher plants act as ports that tightly regulate the uptake of CO2 for photosynthesis and the evaporation of water for transpiration. Situated in the epidermis, they are surrounded by a pair of guard cells, which regulate their opening in response to environmental and internal signals, including light, humidity, CO2, phytohormones, calcium, and reactive oxygen species (1-5). Stomata are closed in darkness but open in response to blue light.
Blue light responses are primarily mediated by four blue light photoreceptors in Arabidopsis: cryptochrome (CRY)1, CRY2, phototropin (PHOT)1, and PHOT2. Major blue light responses mediated by CRY1 and CRY2 include inhibition of hypocotyl elongation (6-8), enhancement of cotyledon expansion (9), anthocyanin accumulation (8, 10, 11), and regulation of flowering time (12-15). CRY1 and CRY2, together with the red/far-red light receptor phytochromes, also serve to entrain the circadian clock (16). There is now evidence for a third CRY (CRY3) in Arabidopsis, the role of which is presently unknown (17). PHOT1 and PHOT2 work together to mediate phototropism, blue light-induced chloroplast migration, and blue light-dependent regulation of stomatal opening (18-22). Recent studies have shown that CRY and PHOT perform overlapping roles. For examples, PHOT functions at early stages to regulate photomorphogenic development, including rapid inhibition of hypocotyl elongation (23) and enhancement of cotyledon expansion (24), and CRY and PHOT function together to enhance phototropism under low fluence rate blue light (25).
Insight into the signaling mechanism of Arabidopsis CRY was obtained through the demonstration that transgenic plants expressing the C-terminal domain of either CRY1 (CCT1) or CRY2 (CCT2) fused to β-glucuronidase (GUS) display a constitutive photomorphogenic (COP) phenotype (11), which is similar to that of mutants of both COP1 and COP9 signalosome, the negative regulators of photomorphogenesis (26, 27). Both CCT1 and CCT2 were shown to bind to COP1 (28, 29), indicating that the signaling mechanism of Arabidopsis CRY1 and CRY2 is mediated through negative regulation of COP1 by direct CRY-COP1 interaction. It is now demonstrated that Arabidopsis CRY1 N-terminal domain mediates homodimerization, which is required for light activation of CCT1 (30).
The purpose of the present study was to determine the role of the Arabidopsis CRY and COP1 signaling system in the regulation of stomatal opening. Through molecular, genetic, and physiological analyses, we demonstrate that CRY acts additively with PHOT to mediate blue light-induced stomatal opening and that COP1 is a repressor of stomatal opening and likely acts downstream of CRY and PHOT signaling pathways.
Materials and Methods
Experimental procedures of construction of expression cassettes and transformation, antibody production, PCR, Western blot, and construction of the various double, triple, and quadruple mutants can be found in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Drought Tolerance and Water Loss Studies. Plants were irrigated for 3 weeks and then drought-stressed by terminating irrigation, as described in ref. 31. Leaves were detached from 21-day-old plants, and water loss was measured and expressed as the percentage of initial fresh weight, as described in ref. 32. In all of the drought tolerance and water loss studies, plants or detached leaves were put under continuous 160 μmol·m-2·s-1 fluorescent cool white light at 24°C. The relative humidity was maintained at 45%.
Stomatal Aperture Measurements. Mature stomata of epidermal strips from 3- to 4-week-old plants were used for stomatal aperture measurements. After dark adaptation for 24 h, stomata of the CRY1-ovx plants were found significantly open. Only after 72 h of dark adaptation were they closed. Thus, all of the plants were initially kept in the indicated dark/light conditions (see Figs. 2, 3, 4, 5) for 72 h, and then leaves were collected in the early morning and the epidermal strips were peeled off from the abaxial side of the leaf under dim red light. The strips were floated on 2 ml of basal reaction mixture (5 mM Mes, pH 6.5/50 mM KCl/0.1 mM CaCl2) in 12-well cell culture plates and put back to the same indicated dark/light conditions for 3 h. Stomatal apertures were measured from images obtained by using Nikon ECLIPSE TS100 with imagej software, and confocal images of stomata were obtained by using a Zeiss LSM-510 META scanning microscope. Stomatal apertures in Figs. 2F,2G, 3B,4B,5B, and 5C are expressed as a mean of 40 measurements with standard deviations. The stomatal aperture data for the WT in Figs. 2F, 3B, 4B, and 5B, the cry1 cry2 mutant in Figs. 2F and 5B, and the cop1 mutant in Figs. 3B, 4B, and 5B were obtained from different sets of 40 measurements. The data for the WT, cry1 cry2, phot1 phot2, and cry1 cry2 phot1 phot2 mutants in Fig. 5C is generated from one of the three independent trials with 40 measurements each. All measurements were made between 0900 and 1500 hours.
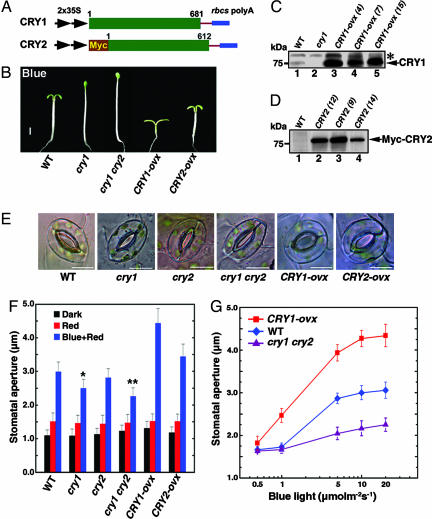
Fig. 2.
Stomatal opening under blue light. (A) Schematic diagrams displaying constructs expressing CRY1 and Myc-tagged CRY2. (B) Six-day-old Arabidopsis seedlings of the WT, cry1, cry1 cry2, CRY1-ovx, and CRY2-ovx plants grown under 5 μmol·m-2·s-1 blue light. (Scale bar, 1 mm.) (C) Immunoblot showing expression of CRY1 by using α-CCT1 antibody. Line 4 [CRY1-ovx (4), lane 3] is shown in B and used for all of the phenotypic analysis throughout this study. *, a band nonspecifically recognized by the antibody. (D) Western blot showing expression of Myc-CRY2 by using α-Myc antibody. Line 9 [CRY2-ovx (9), lane 3] is shown in B and used to generate the data shown in E and F. (E) Confocal images of stomata of the WT, cry1, cry2, cry1 cry2, CRY1-ovx, and CRY2-ovx plants. Epidermal strips were illuminated with 5 μmol·m-2·s-1 blue light under background 50 μmol·m-2·s-1 red light for 3 h. (Scale bars in this and other confocal images represent 10 μm.) (F) Stomatal apertures under different light conditions in the WT, cry1, cry2, cry1 cry2, CRY1-ovx, and CRY2-ovx plants. Stomatal opening was induced by 50 μmol·m-2·s-1 red light and 20 μmol·m-2·s-1 blue light plus 50 μmol·m-2·s-1 red light. Stomata of the WT plants open significantly wider than those of the cry1 single and cry1 cry2 double mutant at *, P ≤ 0.05 and **, P ≤ 0.01 under 20 μmol·m-2·s-1 blue light plus 50 μmol·m-2·s-1 red light (Student's t test), respectively. (G) Fluence rate dependency of stomatal opening in response to blue light. The measurements represent stomatal apertures obtained at different fluence rates of blue light under background 50 μmol·m-2·s-1 red light.
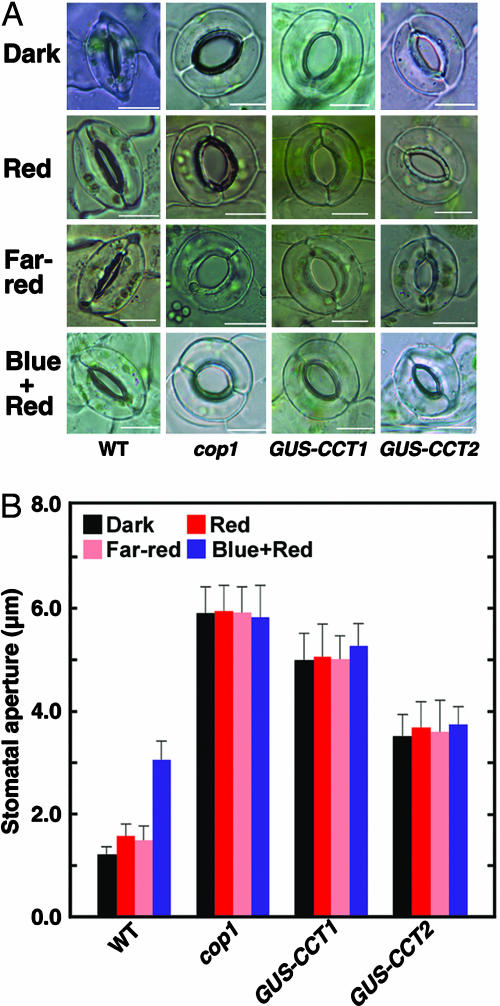
Fig. 3.
Stomata opening of the cop1 mutant, GUS-CCT1, and GUS-CCT2 plants in darkness and under different light conditions. (A) Confocal images showing that stomata of the cop1-4 mutant, GUS-CCT1, and GUS-CCT2 plants are constitutively open in darkness, and open wider than those of the WT under 50 μmol·m-2·s-1 red, 50 μmol·m-2·s-1 far-red, and 5 μmol·m-2·s-1 blue light plus 50 μmol·m-2·s-1 red light. (B) Stomatal apertures in the cop1 mutant, GUS-CCT1, and GUS-CCT2 plants under the same conditions in A.
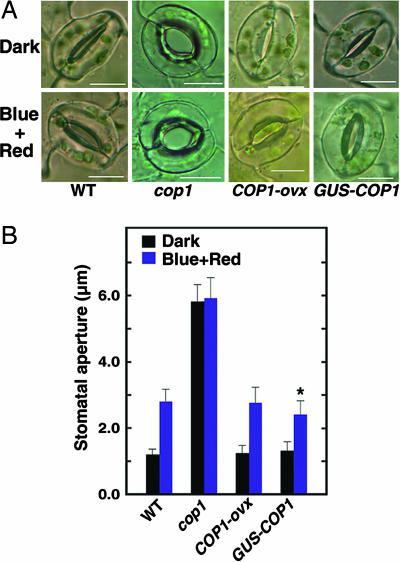
Fig. 4.
Expression of full-length COP1 in the cop1 mutant complements the constitutive stomatal opening phenotype. (A) Confocal images of stomata in the WT, cop1 mutant, transgenic plants expressing COP1 in the cop1 mutant background (COP1-ovx), and plants expressing GUS-COP1 in the WT background in the dark and under 20 μmol·m-2·s-1 blue light plus 50 μmol·m-2·s-1 red light. (B) Stomatal apertures of the WT, cop1 mutant, COP1-ovx, and GUS-COP1 plants under the same conditions in A. Stomata of the WT plants open significantly wider than those of the GUS-COP1 plants (*, P ≤ 0.05, Student's t test).
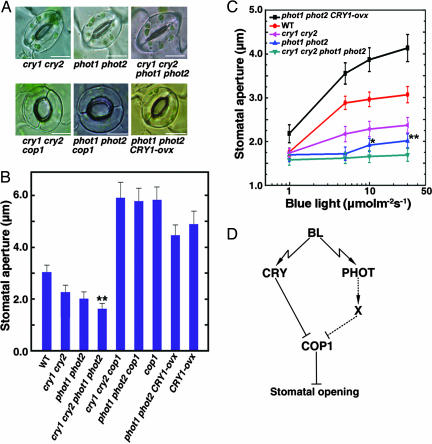
Fig. 5.
Additive roles of cryptochromes and phototropins in the regulation of stomatal opening. (A and B) Confocal images of stomata (A) and stomatal apertures (B) in the cry1 cry2, phot1 phot2, cry1 cry2 phot1 phot2, cry1 cry2 cop1, phot1 phot2 cop1, and phot1 phot2 CRY1-ovx mutant plants under 20 μmol·m-2·s-1 blue light plus 50 μmol·m-2·s-1 red light. Stomata of the cry1 cry2 phot1 phot2 quadruple mutant opened significantly less wide than those of the phot1 phot2 double mutant (**, P ≤ 0.01, Student's t test). (C) Blue light fluence rate response analysis of stomata in the cry1 cry2, phot1 phot2, cry1 cry2 phot1 phot2, and phot1 phot2 CRY1-ovx mutants. Epidermal strips were illuminated with different fluence rates of blue light plus 50 μmol·m-2·s-1 red light. Stomata of the phot1 phot2 mutant open significantly wider than those of the cry1 cry2 phot1 phot2 mutant under fluence rates >10 μmol·m-2·s-1 blue light (*, P ≤ 0.05 at 10 μmol·m-2·s-1; **, P ≤ 0.01 at 30 μmol·m-2·s-1, Student's t test). (D) Signaling pathways illustrating coactions of CRY and PHOT in the regulation of stomatal opening presumably through negative regulation of COP1. Solid line indicates the defined direct CRY-COP1 interaction (28, 29), and the dashed line denotes the presumptive interactions. X, postulated intermediate signaling partner(s) acting between phototropins and COP1.
Light Sources. All experiments involving light illumination of plant materials for stomatal aperture analysis were performed in an E-30 LED growth chamber (Percival, Boone, IA) by using the blue diodes (λmax 469 nm) and/or the red diodes (λmax 680 nm) or the far-red diodes (λmax 730 nm) at 24°C in continuous light. For all blue light illuminations, red light (50 μmol·m-2·s-1) was added. Light spectra and fluence rates were measured by using a HandHeld spectroradiometer (ASD, Boulder, CO) and a Li250 quantum photometer (Li-Cor, Lincoln, NE).
Results
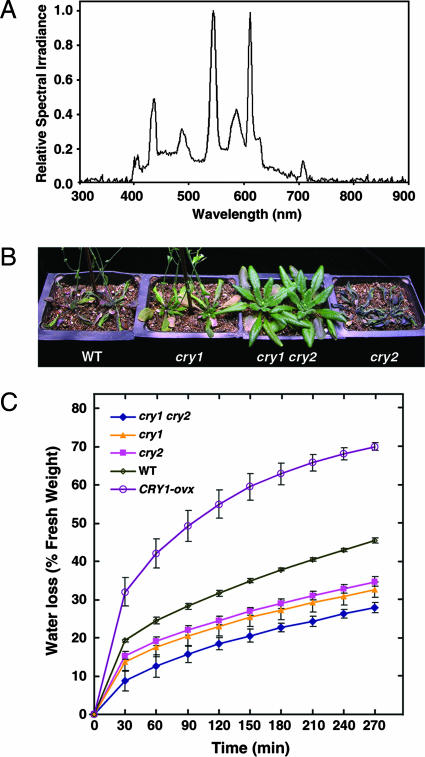
Arabidopsis cry1 cry2 Double Mutant Plants Are Drought-Tolerant. We found in one of our plant growth experiments that, in a tray of the wild-type (WT) and cry1 cry2 double mutant Arabidopsis plants that had not been irrigated for >1 week, the WT plants wilted, whereas the cry1 cry2 double mutant plants thrived. We then initiated drought tolerance studies to confirm this observation by adding the cry1 and cry2 single mutant plants under fluorescent cool white light with the spectrum shown in Fig. 1A. In these studies, the cry1 cry2 double mutant plants were consistently more drought-tolerant than the WT, cry1, and cry2 single mutant plants (Fig. 1B). The cry1 mutant plants were slightly more drought-tolerant than WT, but little difference was observed between the cry2 mutant and WT plants.
Fig. 1.
Reduced wilting of the cry1 cry2 double mutant plants during drought stress. (A) The spectrum of experimental cool white light shown as the relative spectral irradiance in the wavelength range of 300-900 nm. (B) WT, cry1, cry2, and cry1 cry2 mutant plants were grown under normal watering conditions for ≈21 days and then subjected to drought stress by completely terminating irrigation. Photo shows 2 representative plants of 32 after 14 days of drought stress. (C) Water loss is least and greatest from detached leaves of the cry1 cry2 mutant and CRY1-ovx plants, respectively. Water loss is expressed as the percentage of initial fresh weight. Values indicate a mean of three measurements with standard deviations, each with a sample size of five to eight leaves. One of the triplicate trials is shown. Regression analysis confirmed that the WT curve differs significantly from the CRY1-ovx and cry1 cry2 responses (**, P ≤ 0.01, Student's t test).
To further explore the correlation between drought tolerance and CRY1 activity, we made a construct expressing full-length CRY1 (Fig. 2A), and overexpressed it in WT Arabidopsis (Fig. 2B). Expression of the CRY1 protein in CRY1-ovx plants was verified by Western blot using an antibody against CCT1 (Fig. 2C). Then we measured water loss by using detached leaves from different genotypes of plants. The least and greatest water loss was observed in the cry1 cry2 double mutant and CRY1-ovx plants, respectively (Fig. 1C). Water loss in both cry1 and cry2 single mutants was less than that in the WT, and water loss in the cry1 mutant was slightly less than that in the cry2 mutant. These results indicate that both CRY1 and CRY2 are responsible for drought tolerance observed for the cry1 cry2 double mutant plants.
The Drought Tolerance Observed for the cry1 cry2 Mutant Correlates with the Reduced Blue Light-Induced Stomatal Opening. To explore whether drought tolerance observed for the cry1 cry2 double mutant plants correlates with stomata performance, we measured stomatal apertures of the WT, cry1, cry2, cry1 cry2, and CRY1-ovx plants, respectively. Under blue light, stomata of the CRY1-ovx plants opened much wider than those of WT plants (Fig. 2 E and F). Stomata of the WT plants opened wider than those of the cry1 and cry2 single mutants, and stomata of these single mutants opened wider than those of the cry1 cry2 double mutant, indicating an additive role of CRY1 and CRY2 in the regulation of stomatal opening. To further determine CRY2 role in this process, we made a construct expressing Myc-tagged full-length CRY2 (Fig. 2 A) and overexpressed it in the cry1 mutant background (Fig. 2B). Expression of the Myc-CRY2 fusion protein in transgenic CRY2-ovx plants was analyzed by Western blot using an antibody against Myc (Fig. 2D). Although stomata of the CRY2-ovx plants did not open as wide as those of the CRY1-ovx plants, they opened wider than those of the cry1 mutant and WT plants under blue light (Fig. 2 E and F). No difference in stomatal opening was observed among all these genotypes of plants in darkness and under red light (Fig. 2F), indicating that CRY1- and CRY2-mediated stomatal opening is blue light-dependent.
Stomata of the cry1 cry2 Double Mutant Plants Show Reduced Blue Light Response. To further define the function of CRY1 and CRY2 in blue light regulation of stomatal opening, we investigated the dependency of stomatal opening on fluence rate of blue light and obtained clearly different sensitivities for the WT, cry1 cry2 double mutant, and CRY1-ovx plants (Fig. 2G). At the fluence rate 1 μmol·m-2·s-1, stomata of the CRY1-ovx plants showed a very strong response, whereas those of the WT and cry1 cry2 double mutant plants did not respond. When fluence rate was increased to 5 μmol·m-2·s-1, stomata of all of the genotypes responded, with the CRY1-ovx being the most sensitive, the WT less sensitive, and the cry1 cry2 double mutant the least sensitive, respectively. When fluence rate was further increased to >10 μmol·m-2·s-1, stomata of both WT and cry1 cry2 double mutant plants were still responsive, but those of the CRY1-ovx plants were more sensitive. Based on the data shown in Fig. 2G, we estimated that to achieve a stomatal aperture of ≈2.2 μm, the cry1 cry2 double mutant required ≈10-fold more photons per second than WT, and to achieve a stomatal aperture of ≈3.0 μm, the CRY1-ovx plants required ≈6-fold less photons per second than WT under blue light. Therefore, these data suggest that stomata of the cry1 cry2 double mutant show reduced blue light response, whereas those of the CRY1-ovx plants show hypersensitive response to blue light.
Stomata of the cop1 Mutant Are Constitutively Open in Darkness. It is demonstrated that COP1 acts as the downstream signaling partner of CRY in mediating photomorphogenesis (28, 29). To examine whether COP1 might be the downstream partner of CRY in the regulation of stomatal opening, we measured the stomatal apertures of the cop1 mutant and GUS-CCT1 and GUS-CCT2 plants in darkness and blue, red, and far-red lights. Surprisingly, we found that stomata of the cop1 mutant and GUS-CCT1 plants were constitutively wide open in darkness (Fig. 3). Stomata of GUS-CCT2 plants were clearly open in darkness but not as wide as those of the cop1 mutant and GUS-CCT1 plants. It seems likely that the severity of the COP phenotype positively correlates with stomatal opening, because it is shown that the COP phenotype of the GUS-CCT2 lines is less pronounced than that of the GUS-CCT1 lines (11). Little difference in stomatal opening was observed for the cop1 mutant in darkness, blue, red, and far-red lights, and this finding was so for the GUS-CCT1 and GUS-CCT2 plants under these conditions (Fig. 3). These data therefore indicate that COP1 is a repressor of stomatal opening and that the regulation of photomorphogenesis and stomatal opening by CRY is mediated through the same signaling pathway.
Expression of COP1 in the cop1 Mutant Complements the Constitutive Stomatal Opening Phenotype. To confirm that the constitutive stomatal opening phenotype observed for the cop1 mutant indeed resulted from the COP1 mutation, we made a construct expressing full-length COP1 and overexpressed it in the cop1 mutant background. We obtained >30 independent transgenic lines expressing COP1 (COP1-ovx), which were fully etiolated in darkness. We analyzed eight of these lines and found they displayed the morphologies indistinguishable from those of the WT seedlings grown in darkness and blue light, and the WT adult plants grown in the light (Fig. 6 A-C, which is published as supporting information on the PNAS web site). We also overexpressed a construct expressing full-length COP1 fused to the C terminus of GUS in the WT background and obtained >20 independent transgenic GUS-COP1 lines showing the similar reduced blue light response reported in ref. 33 (Fig. 6B). We examined, by Western blot analysis, extracts from the COP1-ovx and GUS-COP1 lines by using α-COP1 antibody and found that COP1 in the COP1-ovx plants was expressed at levels similar to that in the WT, whereas the GUS-COP1 fusion protein in the GUS-COP1 lines was expressed at very high levels (Fig. 6D). Next, we measured the stomatal apertures of the COP1-ovx and GUS-COP1 plants and found that stomatal apertures of the COP1-ovx plants were indistinguishable from those of the WT in both darkness and blue light, whereas stomata of the GUS-COP1 plants were less sensitive to blue light than those of the WT plants (Fig. 4). These data, together with the loss-of-function phenotype of COP1, strongly demonstrate that COP1 performs a role in mediating stomatal closing.
Cryptochromes and Phototropins Act Additively to Regulate Stomatal Opening. With the demonstration that stomata of the cry1 cry2 mutant showed reduced blue light response, whereas those of the CRY1-ovx plants showed hypersensitivity to blue light, and the demonstration that stomata of the cop1 mutant and GUS-CCT plants were constitutively open in darkness, we entertained the possibility that CRY might act in parallel with PHOT to regulate stomatal opening in response to blue light. To test this possibility, we constructed the phot1 phot2 double and cry1 cry2 phot1 phot2 quadruple mutants (Fig. 7, which is published as supporting information on the PNAS web site). As shown in Fig. 5 A and B, stomata of the phot1 phot2 double mutant opened less wide than those of the cry1 cry2 double mutants under 20 μmol·m-2·s-1 blue light. However, stomata of the cry1 cry2 phot1 phot2 quadruple mutant plants opened less wide than those of the phot1 phot2 double mutant.
To further determine the function of CRY in the phot1 phot2 double mutant background in the regulation of stomatal opening, we constructed the phot1 phot2 CRY1-ovx triple mutant and investigated the blue light fluence rate response of the cry1 cry2, phot1 phot2, cry1 cry2 phot1 phot2, and phot1 phot2 CRY1-ovx mutant stomata. As shown in Fig. 5C, stomata of the cry1 cry2 mutant responded to blue light at fluence rates >1 μmol·m-2·s-1, but those of the phot1 phot2 double mutant did not respond to blue light at fluence rates <5 μmol·m-2·s-1. However, when fluence rate was increased to >10 μmol·m-2·s-1, stomata of the phot1 phot2 double mutant clearly responded, but those of the cry1 cry2 phot1 phot2 quadruple mutant hardly responded under all of the fluence rates tested. In contrast, stomata of the phot1 phot2 CRY1-ovx triple mutant were hypersensitive to blue light. Taken together, these data indicate that CRY functions additively with PHOT in the regulation of stomatal opening.
The cop1 Mutation Is Epistatic to the cry1 cry2 and phot1 phot2 Mutations in the Regulation of Stomatal Opening. Previous genetic epistasis analysis has established that COP1 acts downstream of both CRY and phytochrome signaling pathways to regulate photomorphogenesis (34). To determine whether CRY genetically interacts with COP1 in regulating stomatal opening, we constructed the cry1 cry2 cop1 triple mutant. Stomatal aperture measurements showed that stomata of the triple mutant were open as wide as those of the cop1 mutant under blue light (Fig. 5 A Lower and B). This result, together with the constitutive stomatal opening phenotype observed for the cop1 mutant and GUS-CCT plants, and previous CRY-COP1 interaction data (28, 29), demonstrate that the regulation of stomatal opening by CRY is also mediated through negative regulation of COP1.
Next, we constructed the phot1 phot2 cop1 triple mutant (Fig. 7) and determined the interaction of COP1 and PHOT in the regulation of stomatal opening under blue light. As shown in Fig. 5 A and B, although stomata of the phot1 phot2 CRY1-ovx triple mutant were open slightly less wide than those of the CRY1-ovx plants, stomata of the phot1 phot2 cop1 triple mutant were open as wide as those of the cop1 mutant under blue light. These data therefore suggest that the cop1 mutation is epistatic to the phot1 and phot2 mutations in the regulation of stomatal opening.
Discussion
It has been reported that stomata of the phot1 phot2 double mutant showed little blue light response (22), and that CRY1 and CRY2 somehow were not found to be involved in blue light regulation of stomatal opening (24). However, in this study, we have revealed this unrecognized role of Arabidopsis CRY and COP1 through the following demonstrations: (i) Water conservation capacity is enhanced in the cry1 cry2 double mutant plants, whereas it is significantly reduced in the CRY1-ovx plants. (ii) Stomata of the cry1 cry2 double mutant show reduced blue light response, whereas those of the CRY1-ovx plants show hypersensitive response to blue light. (iii) Stomata of the cop1 mutant and GUS-CCT plants are constitutively open in darkness. (iv) Expression of full-length COP1 in the cop1 mutant complements the constitutive stomatal opening phenotype. (v) Stomata of the phot1 phot2 double mutant respond to blue light, which is supported by a recent study (35), where the phot1 phot2 double mutant stomata were also shown to respond to high fluence rate of blue light, but stomata of the cry1 cry2 phot1 phot2 quadruple mutant hardly respond, whereas those of the phot1 phot2 CRY1-ovx triple mutant show hypersensitive response to blue light. (vi) Stomata of the cry1 cry2 cop1 and phot1 phot2 cop1 triple mutants open as wide as those of the cop1 single mutant under blue light. Therefore, these data strongly indicate that Arabidopsis CRY functions additively with PHOT to mediate blue light-induced stomatal opening and that COP1 is a repressor of stomatal opening.
The overlapping functions of CRY and PHOT have been reported in several studies (23-25). The observation that stomata of both cry1 cry2 and phot1 phot2 double mutants showed significantly reduced sensitivity to blue light suggests that both CRY and PHOT might be necessary for blue light regulation of stomatal opening. However, the cry1 cry2 double mutant stomata are able to respond to blue light at fluence rates >1 μmol·m-2·s-1, whereas those of the phot1 phot2 double mutant stomata are not able to respond to blue light at fluence rates <5 μmol·m-2·s-1 (Fig. 5C), indicating that the cry1 cry2 double mutant stomata are more sensitive to blue light than the phot1 phot2 double mutant stomata. These observations might reflect the fact that the native CRY primarily function under relatively high fluence rate of blue light, whereas PHOT functions under both low and high fluence rates of blue light, and that CRY partially depends on PHOT in mediating blue light-induced stomatal opening.
It is interesting to find from our genetic epistasis study that COP1 likely acts downstream of both CRY and PHOT signaling pathways to regulate stomatal opening. Based on the similar constitutive stomatal opening phenotype of the cop1 mutant and GUS-CCT plants (this work) and the earlier CRY-COP1 interaction data (28, 29), we conclude that blue light-induced stomatal opening by CRY is mediated through negative regulation of COP1 (Fig. 5D). It will be of interest to investigate whether PHOT-mediated signals regulate COP1 activity. PHOT1 is localized consistently to the plasma membrane region in etiolated seedlings and, interestingly, a fraction relocates to the cytoplasm in response to blue light (36), and COP1 is predominantly localized to the cytoplasm under light (37). It has been shown that PHOT1 physically interacts with RPT2 to regulate stomatal opening (38). In future studies, it will be worth investigating whether PHOT-mediated signals proceed to COP1 through RPT2 or other intermediate signaling partners (Fig. 5D).
Taken together, this study has defined a previously uncharacterized role of the cryptochromes and COP1 signaling system in the regulation of stomatal opening and its interaction with phototropin signaling pathway in mediating this process. Future studies should identify the components acting downstream of COP1 in the regulation of stomatal opening and work out whether and how phototropin-mediated signals proceed to COP1.
Supplementary Material
Acknowledgments
We are grateful to W. R. Briggs (Carnegie Institution of Washington, Stanford, CA) for critically reading our manuscript, making helpful suggestions, and providing phot1 mutant, and we thank C. Lin (University of California, Los Angeles), X. W. Deng (Yale University, New Haven, CT), and the Arabidopsis Biological Resource Center for providing cry2, cop1, and phot2 mutants; A. R. Cashmore, M. M. Hong, X. Y. Chen, C. K. Wen, and J. R. Huang for giving helpful comments; and H. Q. Zheng and L. J. Wang for microscope imaging. This work was supported by the National Natural Science Foundation of China Grants 90208005 and 30325007 (to H.-Q.Y.) and 30421001, the Chinese Academy of Sciences, and the Shanghai Government.
Author contributions: H.-Q.Y. designed research; J.M., Y.-C.Z., Y.S., and Q.-H.L. performed research; J.M., Y.-C.Z., and H.-Q.Y. analyzed data; and H.-Q.Y. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: COP, constitutive photomorphogenic; CRY, cryptochrome; GUS, β-glucuronidase; PHOT, phototropin.
References
- 1.Blatt, M. R. (2000) Annu. Rev. Cell. Dev. Biol. 16, 221-241. [DOI] [PubMed] [Google Scholar]
- 2.Assmann, S. M. & Wang, X. Q. (2001) Curr. Opin. Plant Biol. 4, 421-428. [DOI] [PubMed] [Google Scholar]
- 3.Dietrich, P., Sanders, D. & Hedrich, R. (2001) J. Exp. Bot. 52, 1959-1967. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder, J. I., Allen, G. J., Hugouvieux, V., Kwak, J. M. & Waner, D. (2001) Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 627-658. [DOI] [PubMed] [Google Scholar]
- 5.Roelfsema, M. R., Hanstein, S., Felle, H. H. & Hedrich, R. (2002) Plant J. 32, 65-75. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad, M. & Cashmore, A. R. (1993) Nature 366, 162-166. [DOI] [PubMed] [Google Scholar]
- 7.Lin, C., Ahmad, M., Gordon, D. & Cashmore, A. R. (1995) Proc. Natl. Acad. Sci. USA 92, 8423-8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin, C., Ahmad, M. & Cashmore, A. R. (1996) Plant J. 10, 893-902. [DOI] [PubMed] [Google Scholar]
- 9.Lin, C., Yang, H., Guo, H., Mockler, T., Chen, J. & Cashmore, A. R. (1998) Proc. Natl. Acad. Sci. USA 95, 2686-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmad, M., Jarillo, J. A. & Cashmore, A. R. (1998) Plant Cell 10, 197-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang, H. Q., Wu, Y. J., Tang, R. H., Liu, D., Liu, Y. & Cashmore, A. R. (2000) Cell 103, 815-827. [DOI] [PubMed] [Google Scholar]
- 12.Bagnall, D. J., King, R. W. & Hangarter, R. P. (1996) Planta 200, 278-280. [DOI] [PubMed] [Google Scholar]
- 13.Guo, H., Yang, H., Mockler, T. C. & Lin, C. (1998) Science 279, 1360-1363. [DOI] [PubMed] [Google Scholar]
- 14.Mockler, T. C., Guo, H., Yang, H., Duong, H. & Lin, C. (1999) Development (Cambridge, U.K.) 126, 2073-2082. [DOI] [PubMed] [Google Scholar]
- 15.Mockler, T., Yang, H., Yu, X., Parikh, D., Cheng, Y.-C., Dolan, S. & Lin, C. (2003) Proc. Natl. Acad. Sci. USA 100, 2140-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Somers, D. E., Devlin, P. F. & Kay, S. A. (1998) Science 282, 1488-1490. [DOI] [PubMed] [Google Scholar]
- 17.Kleine, T., Lockhart, P. & Batschauer, A. (2003) Plant J. 35, 93-103. [DOI] [PubMed] [Google Scholar]
- 18.Kagawa, T., Sakai, T., Suetsugu, N., Oikawa, K., Ishiguro, S., Kato, T., Tabata, S., Okada, K. & Wada, M. (2001) Science 291, 2138-2141. [DOI] [PubMed] [Google Scholar]
- 19.Sakai, T., Kagawa, T., Kasahara, M., Swartz, T. E., Christie, J. M., Briggs, W. R., Wada, M. & Okada, K. (2001) Proc. Natl. Acad. Sci. USA 98, 6969-6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarillo, J. A., Gabrys, H., Capel, J., Alonso, J. M., Ecker, J. R. & Cashmore, A. R. (2001) Nature 410, 952-954. [DOI] [PubMed] [Google Scholar]
- 21.Huala, E., Oeller, P. W., Liscum, E., Han, I. S., Larsen, E. & Briggs, W. R. (1997) Science 278, 2120-2123. [DOI] [PubMed] [Google Scholar]
- 22.Kinoshita, T., Doi, M., Suetsugu, N., Kagawa, T., Wada, M. & Shimazaki, K. (2001) Nature 414, 656-660. [DOI] [PubMed] [Google Scholar]
- 23.Folta, K. M. & Spalding, E. P. (2001) Plant J. 26, 471-478. [DOI] [PubMed] [Google Scholar]
- 24.Ohgishi, M., Saji, K., Okada, K. & Sakai, T. (2004) Proc. Natl. Acad. Sci. USA 101, 2223-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whippo, C. W. & Hangarter, R. P. (2003) Plant Physiol. 132, 1499-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng, X. W., Matsui, M., Wei, N., Wagner, D., Chu, A. M., Feldmann, K. A. & Quail, P. H. (1992) Cell 71, 791-801. [DOI] [PubMed] [Google Scholar]
- 27.Wei, N., Chamovitz, D. A. & Deng, X. W. (1994) Cell 78, 117-124. [DOI] [PubMed] [Google Scholar]
- 28.Wang, H., Ma, L. G., Li, J. M., Zhao, H. Y. & Deng, X. W. (2001) Science 294, 154-158. [DOI] [PubMed] [Google Scholar]
- 29.Yang, H. Q., Tang, R. H. & Cashmore, A. R. (2001) Plant Cell 13, 2573-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sang, Y., Li, Q. H., Rubio, V., Zhang, Y. C., Mao, J., Deng, X. W. & Yang, H. Q. (2005) Plant Cell 17, 1569-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pei, Z. M., Ghassemian, M., Kwak, C. M., McCourt, P. & Schroeder, J. I. (1998) Science 282, 287-290. [DOI] [PubMed] [Google Scholar]
- 32.Leung, J., Merlot, S. & Giraudat, J. (1997) Plant Cell 9, 759-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNellis, T. W., von Arnim, A. G. & Deng, X. W. (1994) Plant Cell 6, 1391-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ang, L. H. & Deng, X. W. (1994) Plant Cell 6, 613-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talbott, L. D., Shmayevich, I. J., Chung, Y., Hammad, J. W. & Zeiger, E. (2003) Plant Physiol. 133, 1522-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakamoto, K. & Briggs, W. R. (2002) Plant Cell 14, 1723-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Arnim, A. G. & Deng, X. W. (1994) Cell 79, 1035-1045. [DOI] [PubMed] [Google Scholar]
- 38.Inada, S., Ohgishi, M., Mayama, T., Okada, K. & Sakai, T. (2004) Plant Cell 16, 887-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.