Abstract
The identity of the cellular mechanisms through which nitroglycerin (glyceryl trinitrate, GTN) elicits nitric oxide (NO)-based signaling to dilate blood vessels remains one of the longest standing foci of investigation and sources of controversy in cardiovascular biology. Recent evidence suggests an unexpected role for mitochondria. We show here that bioconversion by mitochondria of clinically relevant concentrations of GTN results in activation of guanylate cyclase, production of cGMP, vasodilation in vitro, and lowered blood pressure in vivo, which are eliminated by genetic deletion of the mitochondrial aldehyde dehydrogenase (mtALDH). In contrast, generation of vasoactivity from alternative nitro(so)-vasodilators is unaffected. In mtALDH-/- mice and their isolated vascular tissue, GTN bioactivity can still be generated, but only at substantially higher concentrations of GTN and by a mechanism that does not exhibit tolerance. Thus, mtALDH is necessary and sufficient for vasoactivity derived from therapeutic levels of GTN, and, more generally, mitochondria can serve as a source of NO-based cellular signals that may originate independently of NO synthase activity.
Keywords: nitric oxide, nitrite, S-nitrosothiol, nitrate tolerance
The ability of mammalian cells to convert the manmade organic nitrate, nitroglycerin (glyceryl trinitrate, GTN), to vasoactive nitric oxide (NO) or S-nitrosothiol (SNO) played a significant part in the discovery that NO or its equivalent functions as an endogenous physiological mediator (1, 2), and GTN has long served as a principal therapeutic agent for acute angina and congestive heart disease (3-7). Although multiple cellular activities mediating GTN metabolism have been characterized, the mechanisms that specifically subserve GTN bioactivation have remained elusive. Recent evidence indicates a central role for mitochondria in GTN bioactivation. In particular, it has been proposed that the mitochondrial aldehyde dehydrogenase (mtALDH), aldehyde dehydrogenase (ALDH) 2, may provide a principal enzymatic source of GTN-derived NO vasoactivity (through the intermediacy of mitochondrial nitrite) and that mechanism-based mtALDH inactivation contributes to GTN tolerance (8-10). However, this previously uncharacterized role for mitochondria in the generation of NO bioactivity remains unproven, and the centrality of mtALDH in GTN bioactivation in vivo has been disputed (5-7). The findings described in the present study establish that NO bioactivity originating in mitochondria and generated by mtALDH is necessary and sufficient to account for the vasoactivity of clinically relevant concentrations of GTN. Our results suggest, in addition, that inactivation of mtALDH is a principal component of mechanism-based GTN tolerance.
Materials and Methods
mtALDH-/- Mice. The generation of mtALDH-/- mice has been described in ref. 11. Wild-type (C57BL/6) and mtALDH-/- mice used for comparative measurements were gender- and age-matched (3-4 months). All procedures were approved by the Institutional Animal Care and Use Committee of Duke University Medical Center.
cGMP Assay. Ring segments of thoracic aorta were cleaned and weighed before equilibration for 1 h in Krebs buffer, bubbled continuously with 95% O2/5% CO2. Rings were exposed to GTN for 1 min, snap-frozen in liquid nitrogen, and stored at -80°C until analysis. cGMP was extracted and quantified by an RIA according to the manufacturer's protocol (Amersham Pharmacia RPA 525).
Rat fetal lung fibroblasts (RFL-6 cells) were grown to confluence in F-12 Hamm's nutrient mixture supplemented with 20% FBS/2 mM glutamate. Cells were incubated for 20 min in respiration buffer (0.3 M sucrose/1 mM EGTA/5 mM Mops/5 mM KH2PO4; pH 7.4) containing 3-isobutyl-1-methylxanthine (0.3 mM) before addition of superoxide dismutase (40 activity units), followed immediately by addition of GTN and/or intact mitochondria (≈1 mg mitochondrial protein per 106 cells). Mitochondria were purified from mouse or rat liver disrupted with a teflon-on-glass Potter-Elvejehm homogenizer in 0.22 M mannitol/0.07 M sucrose/0.5 mM EGTA/0.1% BSA/2 mM Hepes, pH 7.4. The homogenate was centrifuged at 600 × g, and the supernatant (S1) was spun at 10,000 × g to generate S2 and P(ellet)2. Mitochondria were purified from P2 by Percoll gradient centrifugation. When used, hemoglobin (10 μM) or chloral hydrate (1 mM) was added 10 min before mitochondria and/or GTN. After incubation for 1 min, the medium was removed, trichloroacetic acid was added (6% wt/vol), and the samples were frozen in liquid nitrogen and stored at -80°C until analysis.
Assay of GTN Metabolism. Mitochondria were purified as above, and S2 was spun at 20,000 × g to generate the postmitochondrial supernatant (S3). Assays were performed in 1 ml of respiration buffer at 37°C. After the addition of glutamate (2 mM), ADP (0.5 mM), and mitochondria or S3 (1 mg of protein), the reaction was initiated by the addition of 1 μM [14C]GTN. After 5 min, reaction mixtures were flash-frozen and stored at -20°C before analysis.
Mouse thoracic aortae were cleaned, weighed, and equilibrated for 1 h in Krebs buffer bubbled continuously with 95% O2/5% CO2. Each assayed sample consisted of aortic segments from two mice (≈5-6 mg of tissue wet weight), which were diced and transferred to 0.2 ml of Krebs buffer (37°C) before the reaction was initiated by the addition of [14C]GTN (0.1-10 μM). After 5 min, reaction mixtures were flash-frozen, and stored at -20°C before analysis. Organic extraction, separation by TLC, and scintillation counting of GTN and its metabolites were carried out as described in ref. 8.
Bioassay of Vasoactivity. Mice were killed by CO2 inhalation, and thoracic aortae were removed, cleaned, and cut into 3-mm rings. Rings were mounted on strain gauge transducers under 1 g of resting tension, and maintained at 37°C in Krebs solution, bubbled continuously with 20% O2/5% CO2/75% N2. Rings were allowed to equilibrate for 1 h before active tension was induced with prostaglandin F2α (2 μM). For induction of GTN tolerance, rings were allowed to equilibrate for 1 h, then exposed to GTN (300 μM) for 0.5 h, followed by five to six changes of Krebs solution over the 1 h before assay.
Systemic Blood Pressure Measurements. Mice were anesthetized by i.p. injection of ketamine/xyalazine, and the carotid artery and jugular vein were cannulated for arterial blood pressure measurement and venous administration of experimental solutions and additional anesthesia. Solutions of GTN or sodium nitroprusside (SNP) were administered as a bolus of 50 μl over ≈20 seconds. After cannulation, the average heart rate and mean blood pressure did not differ significantly between wild-type and mtALDH-/- mice. Body temperature was maintained at 37°C.
Subcellular Localization of mtALDH. Mitochondria and a postmitochondrial supernatant were prepared as described above and analyzed by SDS/PAGE, followed by Western blotting with a rabbit antisera, raised to a synthetic peptide comprising the N-terminal 19 residues of human mtALDH, and affinity-purified on immobilized peptide.
Statistical Analysis. All results were analyzed by Student's t test, and statistical significance was assumed if P < 0.05.
Results
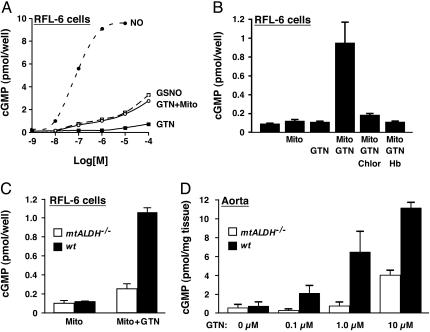
To evaluate directly the ability of mitochondria to generate and export NO-related bioactivity, we used rat fetal lung fibroblast (RFL-6) cells in a reporter assay with mitochondria isolated from rat or mouse liver. RFL-6 cells exhibit only minimal intrinsic GTN metabolism but contain guanylate cyclase that is activated efficiently (to generate cGMP) by exposure to either NO or S-nitrosothiol (12) (Fig. 1A). Coincubation of RFL-6 cells with mitochondria and varying concentrations of GTN resulted in the dose-dependent generation of cGMP, whereas incubation with either GTN or mitochondria had little effect (Fig. 1 A and B). Generation of NO bioactivity by isolated mitochondria (1 μM GTN) was blocked by the ALDH inhibitor chloral hydrate (Fig. 1B). In addition, inclusion of Hb (10 μM) blocked transmission of NO bioactivity from mitochondria to reporter cells (Fig. 1B), consistent with the export of NO or a closely related species. Because (oxy)Hb might scavenge HNO/NO-, S-nitrosothiol, and NO proper, Hb sensitivity cannot serve as definitive evidence for a particular NO-related species. It is nonetheless of interest that the efficiency of guanylate cyclase activation (cGMP production) was similar for GTN and S-nitrosoglutathione (GSNO) (Fig. 1 A). The direct demonstration that intact mitochondria can export NO-related bioactivity adds significantly to the finding of attenuated GTN-induced production of cGMP by ρo cells that are deficient in mitochondrial function (10), and substantiates the report of Kollau et al. (13) describing activation of purified guanylate cyclase by GTN in the presence of mitochondria. Inhibition by chloral hydrate, and by other classes of mtALDH inhibitors (10), indicates further that mtALDH is a mitochondrial generator of NO bioactivity.
Fig. 1.
Generation from GTN of NO bioactivity (guanylate cyclase activation) depends on mtALDH. (A) Mitochondria isolated from mouse or rat liver generate and export GTN-derived NO bioactivity, as revealed by activation of guanylate cyclase (cGMP production) in coincubated RFL-6 cells. The activation of guanylate cyclase by aqueous NO and by S-nitrosoglutathione (GSNO) is shown for comparison. (B) Activation of guanylate cyclase by mitochondria plus GTN is eliminated in the presence of the ALDH inhibitor chloral hydrate (Chlor, 1 mM), and NO bioactivity is scavenged by (oxy)hemoglobin (Hb, 10 μM). (C) Generation of NO bioactivity from GTN (1 μM) is abrogated in mitochondria isolated from mtALDH-/- liver. (D) In segments of intact aorta, deletion of mtALDH eliminates cGMP production from 0.1 and 1.0 μM GTN and substantially inhibits production from 10 μM GTN. In all experiments, GTN was present for 1 min before samples were harvested.
We used mice lacking mtALDH as a result of genetic deletion (mtALDH-/-) (11) to address dispositively the role of mtALDH and additional possible mechanisms in GTN bioactivation. A comparison of mitochondria from wild-type vs. mtALDH-/- mice revealed that the ability to generate cGMP in the RFL-6 reporter assay (1 μM GTN) was almost completely eliminated in mtALDH-/- mitochondria (Fig. 1C). Similarly, in intact segments of thoracic aorta, deletion of mtALDH eliminated cGMP production elicited by exposure to 0.1-1 μM GTN and significantly attenuated production at 10 μM GTN, the highest dose examined. Additional evidence presented below indicates that bioactivation at such high GTN concentrations (which greatly exceed clinically relevant levels in humans; ref. 14) is subserved predominantly by a nonaldehyde dehydrogenase activity.
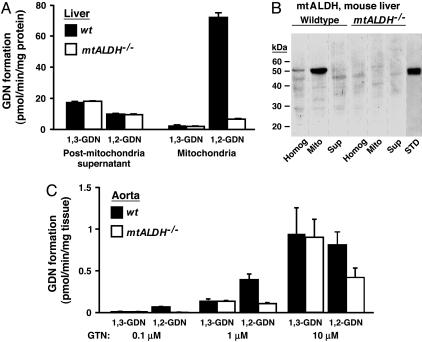
Metabolism of GTN by mammalian cells generates two dinitrates, 1,2-GDN and 1,3-GDN (3). We have shown previously that purified mtALDH catalyzes the stoichiometric formation from GTN of 1,2-GDN and nitrite (8), and production of 1,2-GDN has been associated with GTN bioactivation in vivo and in vitro (15, 16). As shown in Fig. 2A, isolated mitochondria convert GTN (1 μM) to 1,2-GDN in large preference to 1,3-GDN, and production of 1,2-GDN is essentially eliminated in mtALDH-/- mitochondria, whereas production of 1,3-GDN is unaffected. In contrast, metabolism of GTN to 1,2-GDN by a postmitochondrial subcellular fraction is much less efficient, and 1,3-GDN is generated preferentially (Fig. 2 A). The postmitochondrial fraction does not contain mtALDH as evaluated by Western blotting (Fig. 2B). Accordingly, GTN metabolism by nonmitochondrial elements is not affected by deletion of mtALDH (Fig. 2 A). In conjunction with the finding that mtALDH-/- mitochondria lose the ability to stimulate guanylate cyclase by converting GTN, these results indicate that mitochondrial generation of 1,2-GDN is a signature of the bioactivation of GTN by mtALDH. Consistent with this interpretation, generation of 1,2-GDN from 0.1 μM GTN was eliminated in intact vascular tissue (aortic segments) from mtALDH-/- mice and was substantially reduced after exposure to 1-10 μM GTN (Fig. 2C). As for residual activation of guanylate cyclase by GTN in mtALDH-/- aortic segments (Fig. 1D), residual generation of 1,2-GDN (and preferential generation of 1,3-GDN) indicates the operation of an additional mechanism that operates at relatively high GTN concentrations.
Fig. 2.
Metabolism of GTN by mitochondria and intact vascular tissue. (A) Mitochondria isolated from wild-type mouse liver generate 1,2-GDN from GTN (1 μM) in large preference over 1,3-GDN, and generation of 1,2-GDN is virtually eliminated in mitochondria isolated from liver of mtALDH-/- mice. In contrast, a postmitochondrial fraction generates 1,3-GDN in preference to 1,2-GDN, and elimination of mtALDH has no effect. Note that, because the mitochondrial fraction contains only ≈2% of cellular protein, mtALDH will account for only a small proportion of the total GTN-metabolizing activity of liver, consistent with the well established role of hepatocytes in systemic clearance of GTN. (B) mtALDH is absent from the postmitochondrial fraction as assessed by Western blotting (Homog, unfractionated liver homogenate; Mito, isolated mitochondria; Sup, postmitochondrial supernatant; see Materials and Methods). In the example shown (representative of three separate experiments), each lane was loaded with 50 μg of protein with the exception of the lane containing 0.25 μg of purified human mtALDH standard (STD). (C) Generation of 1,2-GDN is selectively reduced vs. 1,3-GDN in aortic segments from mtALDH-/- vs. wild-type mice. Note also that the mtALDH-/- aorta generates 1,2-GDN and 1,3-GDN equally, and that the preference of wild-type aorta for production of 1,2-GDN vs. 1,3-GDN decreases with increasing GTN concentration. In A and C, GTN was present for 5 min before samples were harvested.
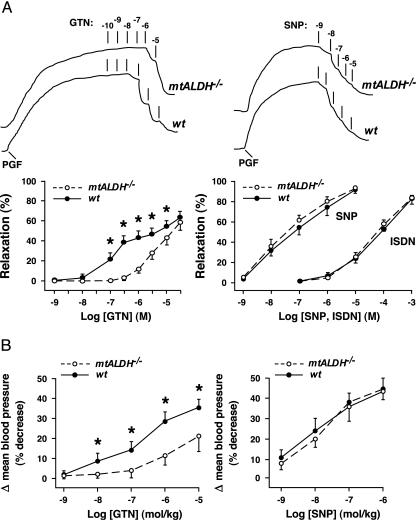
To assess directly the role of mtALDH in GTN-evoked vasodilation, we used a bioassay of aortic ring segments from mtALDH-/- and wild-type mice (Fig. 3A). Consistent with the findings of Malta (17), the dose-response curve for GTN-induced vasorelaxation of wild-type aorta was biphasic, with a distinct region of reduced responsiveness at ≈1 μM GTN. The dose-response curve of mtALDH-/- aorta exhibited a significant right shift with respect to the wild type (indicative of reduced responsiveness) over a large range of GTN concentrations, and, strikingly, vasorelaxation at GTN concentrations less than ≈1 μM was almost completely eliminated. In contrast, vasorelaxation elicited by the NO donor SNP was indistinguishable in wild-type and mtALDH-/- aorta, which indicates that differential responsiveness to GTN results in toto from differences in the generation of, rather than the response to, NO-related vasoactivity. Thus, comparison of the wild-type and mtALDH-/- dose-response curves suggests strongly that two distinct cellular mechanisms generate vasorelaxant activity from GTN and that bioconversion of GTN over a range of concentrations up to ≈1 μM (which extends through and above the clinically relevant range) is subserved predominantly by mtALDH. This interpretation is consistent with an exclusive role for mitochondria as the source and mtALDH as the generator of NO-related bioactivity at relatively low concentrations of GTN, as indicated by guanylate cyclase activation (Fig. 1).
Fig. 3.
mtALDH mediates GTN-induced vasodilation in vitro and in vivo. (A) Aortic relaxation in vitro. (Upper) Polygraph tracings of strain gauge output illustrate typical dose-response relations in vitro for GTN- and SNP-induced vasorelaxation of aortic ring segments from wild-type (wt) and mtALDH-/- mice. Initial tension was induced with prostaglandin F2α (PGF). Dosages of GTN and of SNP are given as log molar concentrations. (Lower) Graphical summaries of dose-response curves illustrate that GTN-induced relaxation (Left) is essentially eliminated in mtALDH-/- aorta at GTN concentrations less than ≈0.5 μM and that the attenuation of responsiveness of mtALDH-/- vs. wild-type aorta decreases with increasing GTN concentration (n = 12; *, P < 0.05). (Note that the dose-response curve for wild-type aorta is biphasic, with a distinct region of reduced slope.) (Right) In contrast, vasorelaxation induced by the NO donor SNP (n = 6) or by the nitrovasodilator ISDN (n = 2-4) is indistinguishable between wild-type and mtALDH-/- aorta. (B) Blood pressure in vivo. The decrease in mean arterial blood pressure induced by i.v. administration of GTN is significantly attenuated in mtALDH-/- vs. wild-type mice, whereas the response to SNP is unaltered (n = 12-13; *, P < 0.05).
Isosorbide dinitrate (ISDN) and isosorbide mononitrate (ISMN) are additional NO-generating nitrovasodilators in widespread clinical use. Aortic bioassays revealed that the deletion of mtALDH had no effect on ISDN-induced vasorelaxation (Fig. 3A), and similar results were obtained for ISMN (data not shown). The threshold concentration for vasorelaxation by ISDN was ≈1 μM, and dose-response curves closely resembled those obtained for GTN-induced relaxation of mtALDH-/- aorta (Fig. 3A). Whether the mtALDH-independent mechanism that generates vasodilatory activity from high doses of GTN is also responsible, at least in part, for bioactivation of isosorbide nitrovasodilators remains to be determined.
We extended our observations of GTN-induced vasodilation in vitro to characterize the effects of mtALDH-mediated GTN bioconversion on systemic blood pressure in vivo (Fig. 3B). Administration of GTN (intrajugular bolus injection) in wild-type mice decreased systemic blood pressure in a dose-dependent fashion with a threshold of ≈1 nmol/kg body weight (representing a theoretical maximum concentration in blood of ≈12.5 nM on the assumption that blood comprises 8% of body weight). In mtALDH-/- mice, GTN elicited substantially smaller decreases in blood pressure at all dosages tested, and similar to the case for vasorelaxation in vitro (Fig. 3A), the effect of GTN was virtually eliminated at dosages less than ≈0.1 μmol/kg (≈1.25 μM theoretical maximum concentration in blood), which decreased mean blood pressure ≈20% in wild-type mice (approximating maximal tolerated effects in normotensive humans). Decreases in blood pressure resulting from systemic administration of SNP were indistinguishable in wild-type and mtALDH-/- mice (Fig. 3B). Thus, the vasodilatory action of GTN administered systemically at clinically relevant doses depends on mtALDH, and bioconversion at higher doses evidently relies on a separate mechanism.
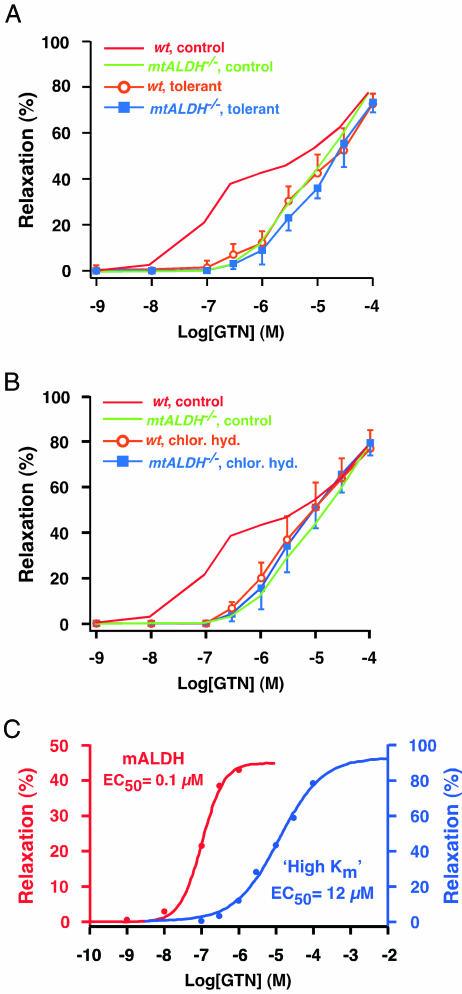
The proposed molecular mechanism of GTN conversion by mtALDH entails a reductase activity that generates 1,2-GDN and nitrite (the intramitochondrial source of NO bioactivity) and results in concomitant oxidation of active site cysteine thiols (8). The endogenous source(s) of the reducing equivalent necessary for redox cycling of those thiols and, thereby, for maintaining GTN metabolism, has not been identified, but it has been suggested that depletion of reducing equivalents (and thus maintained active site thiol oxidation to disulfide or higher oxidation state) represents a principal, mechanism-based component of tolerance induced by sustained exposure to GTN (8) (although additional, mtALDH-independent mechanisms may very well contribute to depressed GTN responsiveness in clinical contexts) (10, 16, 18). If inactivation of mtALDH by GTN is a significant molecular mechanism of tolerance, then responsiveness to GTN should be similar in tolerant wild-type and in mtALDH-/- vasculature, and further, mtALDH-/- vasculature should not exhibit mechanism-based tolerance. These predictions were borne out fully (Fig. 4A): assessment of GTN-induced vasorelaxation in vitro revealed that sustained exposure to GTN (300 μM, 0.5 h) resulted in a right shift in the dose-response curve of wild-type aorta characterized by greater reduction in responsiveness at lower GTN concentrations, such that the resultant curve was indistinguishable from the dose-response curve obtained from mtALDH-/- aorta. In addition, tolerance-inducing exposure to GTN had no effect on the responsiveness to high concentrations of GTN that is retained by mtALDH-/- aorta (Fig. 4A). These results are consistent with our previous demonstration in intact rats that prolonged infusion of relatively low levels of GTN (3 days at 0.48 μmol/hr) resulted in decreased vascular mtALDH activity and GTN biotransformation (10).
Fig. 4.
Inactivation of mtALDH underlies both mechanism-based tolerance to GTN and suppressed responsiveness to GTN induced by chloral hydrate. (A) After induction of tolerance by exposure to GTN before assay (300 μM, 0.5 h), the dose-response curve from wild-type (wt) aorta is superimposable on the curve from mtALDH-/- aorta, reflecting a selective suppression of responsiveness at lower GTN concentrations. The dose-response curve from mtALDH-/- aorta is unaffected by prior exposure to GTN. (B) The effects of the ALDH inhibitor chloral hydrate (chlor. hyd.) on the dose-response curve from wild-type aorta is identical to the effects of tolerance, and chloral hydrate has no effect on the responsiveness of mtALDH-/- aorta. (C) Dose-response curves fitted to the GTN-induced vasodilatory responses of wild-type aorta (1 nM to 1 μM) and of mtALDH-/- aorta (100 nM to 100 μM) indicate a ≈120-fold difference in the functional EC50 of the mtALDH-dependent and mtALDH-independent (high Km) mechanisms of GTN bioactivation.
We also determined that the effects on GTN-induced vasodilation of the ALDH inhibitor chloral hydrate were essentially identical to those of mechanism-based tolerance (Fig. 4B). The dose-response curve for wild-type aorta in the presence of chloral hydrate (1 mM, introduced 20 min before assay) was superimposable upon the dose-response curve for mtALDH-/- aorta, and the response of mtALDH-/- aorta to GTN was unaffected by chloral hydrate. These results support the conclusions that bioactivation of GTN at low (i.e., clinically relevant) concentrations is subserved predominantly by mtALDH and that inactivation of mtALDH is a principal component of classical mechanism-based tolerance (18). In addition, these findings emphasize that the mtALDH-independent mechanism(s) operating at relatively high GTN concentrations does not exhibit tolerance and is not subserved by an alternative form of ALDH (e.g., cytosolic ALDH1).
Discussion
The nature of the cellular processes that generate vasodilatory activity from GTN has remained unresolved and a source of contention for some 150 years, sustained by the difficulty of distinguishing between mechanisms subserving GTN metabolism or bioactivation and of localizing those mechanisms to particular tissues, cell types, or subcellular compartments (3, 4). Demonstration of the necessary and sufficient function of mtALDH in generating vasoactivity from clinically relevant levels of GTN and the enzyme's role in mechanism-based tolerance provides a coherent molecular explanation for the characteristics of GTN bioactivation, including thiol dependence (8), and should resolve these long-standing issues in cardiovascular biology.
Therapeutic administration of GTN in humans sublingually, by patch, as ointment, or by i.v. infusion yields plasma concentrations that rarely exceed ≈90 nM and are in most case substantially lower (except in some hypothermic cardiopulmonary procedures in which concentrations may reach ≈500 nM) (14, 19). Therefore, to the extent that our findings apply to GTN bioconversion in humans, clinically relevant concentrations of GTN are the exclusive purview of mtALDH. In support of this conclusion, purified human mtALDH generates nitrite and 1,2-GDN stoichiometrically from GTN (unpublished observations), 1,2-GTN is the major dinitrate metabolite of GTN in human vascular tissue (14, 16), and 1,2-GDN production is reduced in parallel with vasorelaxation in GTN-tolerant vessels (16). In addition, the GTN-vasorelaxation dose-response curve of human blood vessels in vitro is very similar to that obtained from the mouse (and other mammalian models) (16). It is also clear from our results that, as suggested by the findings of Daiber et al. (20), a mtALDH-independent mechanism(s) operates in mammalian cells to generate vasoactivity from GTN, but only at relatively high GTN concentrations. Evidently, GTN-derived vasoactivity generated independently from mtALDH is also conveyed by NO or S-nitrosothiol, because guanylate cyclase is activated by high concentrations of GTN in mtALDH-/- aorta (Fig. 1D), and the guanylate cyclase inhibitor ODQ blocks GTN-induced vasorelaxation at high concentrations of GTN in wild-type mouse aorta (unpublished observation). Analysis of the in vitro GTN-vasorelaxation dose-response curves from wild-type and mtALDH-/- aortae (Fig. 4C) indicates a functional EC50 for this “high Km” activity of ≈12 μM versus ≈0.1 μM for mtALDH. The molecular mechanism(s) of the high Km activity remain to be determined, and it will be important to ascertain whether it is localized, at least in part, to mitochondria, generates nitrite as a precursor to NO, and plays a role in bioactivation of alternative nitrovasodilators, including isosorbide nitrates. In addition, it will be important to determine whether this activity has a clinically relevant role in GTN-tolerant vasculature, and in particular, whether it is associated with any of the adverse effects of long-term GTN exposure, which include prominently endothelial dysfunction (18) and possibly increased mortality (21).
The identification of mtALDH as the essential source of NO-related vasoactivity that is derived from therapeutic concentrations of GTN indicates the possibility of clinically relevant interactions between GTN and pharmaceutical compounds, including chloral hydrate and acetaminophen, that inhibit mtALDH (22) and, more generally, suggests that both mtALDH polymorphism (e.g., the E487K mutation in Asian populations that results in reduced mtALDH activity; ref. 23), and chronic nitrate therapy (that similarly results in reduced mtALDH activity) may be associated with significant variability in vascular responsiveness to GTN (24). It will be interesting to learn whether patient responsiveness to GTN and comorbidity are related (18, 21) and whether pharmacogenetic profiling can help identify those placed at risk by GTN exposure. In addition, the ability of mitochondria to generate and export NO bioactivity after conversion of GTN to nitrite (8) points to the possibility that mitochondria may serve as a source in many organisms of endogenous NO-based cellular signals that are independent of NO synthase (25), analogous to mitochondrial generation and release of reactive oxygen species that are independent of cellular oxidases.
Author contributions: J.S.S. and Z.C. designed research; Z.C., M.W.F., J.Z., and L.M. performed research; H.A.R., T.K., K.K., and K.I.N. contributed new reagents/analytic tools; Z.C. and D.T.H. analyzed data; and D.T.H. and J.S.S. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ALDH, aldehyde dehydrogenase; GTN, glyceryl trinitrate; mtALDH, mitochondrial ALDH; SNP, sodium nitroprusside.
References
- 1.Arnold, W. P., Mittal, C. K., Katsuki, S. & Murad, F. (1977) Proc. Natl. Acad. Sci. USA 74, 3203-3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gruetter, C. A., Gruetter, D. Y., Lyon, J. E., Kadowitz, P. J. & Ignarro L. J. (1981) J. Pharmacol. Exp. Ther. 219, 181-186. [PubMed] [Google Scholar]
- 3.Bennet, B. M., McDonald, B. J., Nigam, R. & Simon, W. C. (1994) Trends Pharmacol. Sci. 15, 245-249. [DOI] [PubMed] [Google Scholar]
- 4.Ignarro, L. J. (2002) Proc. Natl. Acad. Sci. USA 99, 7816-7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fung, H.-L. (2004) Ann. Rev. Pharmacol. Toxicol. 44, 67-85. [DOI] [PubMed] [Google Scholar]
- 6.Janero, D. R., Bryan, N. S., Saijo, F., Dhawan, V., Schwalb, D. J., Warren, M. C. & Feelisch, M. (2004) Proc. Natl. Acad. Sci. USA 101, 16958-16963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thatcher, G. R., Nicolescu, A. C., Bennett, B. M. & Toader, V. (2004) Free Radic. Biol. Med. 37, 1122-1143. [DOI] [PubMed] [Google Scholar]
- 8.Chen, Z., Zhang, J. & Stamler, J. S. (2002) Proc. Natl. Acad. Sci. USA 99, 8306-8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang, J., Chen, Z., Cobb, F. R. & Stamler, J. S. (2004) Circulation 110, 750-755. [DOI] [PubMed] [Google Scholar]
- 10.Sydow, K., Daiber, A., Oelze, M., Chen, Z., August, M., Wendt, M., Ullrich, V., Mulsch, A., Schulz, E., Keaney, J. F., et al. (2004) J. Clin. Invest. 113, 482-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitagawa, K., Kawamoto, T., Kunugita, N., Tsukiyama, T., Okamoto, K., Yoshida, A, Nakayama, K. & Nakayama, K. (2000) FEBS Lett. 476, 306-311. [DOI] [PubMed] [Google Scholar]
- 12.Forstermann, U. & Ishii, K. (1996) in Methods in Nitric Oxide Research, ed. Stamler, J.S. (Wiley, New York), pp. 555-566.
- 13.Kollau, A., Hofer, A., Russwurm, M., Koesling, D., Keung, W. M., Schmidt, K., Brunner, F. & Mayer, B. (2005) Biochem. J. 385, 769-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto, S. & Kobayashi, A. (2003) Clin. Pharmacokinet. 42, 205-221. [DOI] [PubMed] [Google Scholar]
- 15.Slack, C. J., McLaughlin, B. E., Brien, J. F., Marks, G. S. & Nakatsu, K. (1989) Can. J. Physiol. Pharmacol. 67, 1381-1385. [DOI] [PubMed] [Google Scholar]
- 16.Sage, P. R., de la Lande, I. S., Stafford, I., Bennett, C. L., Phillipov, G., Stubberfield, J. & Horowitz, J.D. (2000) Circulation 102, 2810-2815. [DOI] [PubMed] [Google Scholar]
- 17.Malta, E. (1989) Naunyn Schmiedebergs Arch. Pharmacol. 339, 236-243. [DOI] [PubMed] [Google Scholar]
- 18.Parker, J. D. (2004) J. Clin. Invest. 113, 352-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elkayam, U., Kulick, D., McIntosh, N., Roth, A., Hsueh, W. & Rahimtoola, S. H. (1987) Circulation 76, 577-584. [DOI] [PubMed] [Google Scholar]
- 20.Daiber, A., Oelze, M., Coldewey, M., Bachschmid, M., Wenzel, P., Sydow, K., Wendt, M., Kleschyov, A. L., Stalleicken, D., Ullrich, V. et al. (2004) Mol. Pharmacol. 66, 1372-1382. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura, Y., Moss, A., Brown, M. W., Kinoshita, M. & Kawai, C. (1999) Am. Heart J. 138, 577-585. [DOI] [PubMed] [Google Scholar]
- 22.Landin, J. S., Cohen, S. D. & Khairallah, E. A. (1996) Toxicol. Appl. Pharmacol. 141, 299-307. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida, A., Ikawa, M., Hsu, L.C. & Tani, K. (1985) Alcohol 1, 103-106. [DOI] [PubMed] [Google Scholar]
- 24.Haefeli, W. E., Srivastava, N., Kelsey, K. T., Wiencke, J. K., Hoffman, B. B. & Blaschke, T. F. (1993) Clin. Pharmacol. Ther. 53, 463-468. [DOI] [PubMed] [Google Scholar]
- 25.Modolo, L. V., Augusto, O., Almeida, I. M., Magalhaes, J. R. & Salgado, J. FEBS Lett. (2005) 579, 3814-3820. [DOI] [PubMed] [Google Scholar]