Abstract
Wnt/β-catenin signaling has been shown to promote self-renewal in a variety of tissue stem cells, including neuronal stem cells and hematopoietic stem cells. However, activation of the Wnt/β-catenin pathway promoted and inhibition of the pathway prevented differentiation of neuronal precursor cells. A clear explanation for the differential effects of Wnt/β-catenin activation on neuronal precursors is not available at present. Presenilin-1 (PS-1) is a polytopic protein comprised of six to eight transmembrane domains. PS-1, as part of the γ-secretase complex, is required for the intramembrane proteolysis of both amyloid precursor protein (APP) and Notch. Additionally, through interactions with β-catenin, PS-1 is associated with modulation of Wnt/β-catenin signaling. A familial Alzheimer's disease-associated PS-1 mutant, PS-1(L286V), causes a dramatic increase in T cell factor (TCF)/β-catenin transcription in PC-12 cells, which prevents normal nerve growth factor (NGF)-induced neuronal differentiation and neurite outgrowth. Selective inhibition of TCF/β-catenin/cAMP-response element-binding protein (CREB)-binding protein (CBP)-mediated transcription, but not TCF/β-catenin/p300, with the recently described small molecule antagonist ICG-001 corrects these defects in neuronal differentiation, highlighting the importance of Wnt/β-catenin signaling in this process. We propose that increased TCF/β-catenin/CBP-mediated transcription, as well as a failure to switch to TCF/β-catenin/p300-mediated transcription, play an important role in decreasing neuronal differentiation.
Keywords: Wnt/β-catenin, Alzheimer's disease
The Wnt/β-catenin pathway initiates a signaling cascade critical in normal development. The pathway plays an important role in both neural proliferation and neuronal differentiation (1-3). It participates in initial formation of the neural plate and neural crest and in later stages of the mature CNS, including regulation of the neuronal cytoskeleton and synaptic differentiation, and also in cell survival and prevention of apoptosis (4).
Canonical Wnt signaling inactivates GSK-3β, preventing β-catenin phosphorylation and leading to accumulation of β-catenin in the cytoplasm and subsequent translocation to the nucleus (5). A key step in the activation of target genes is the formation of a complex between β-catenin and members of the T cell factor (TCF)/lymphoid enhancer factor (LEF) family of transcription factors (6). To generate a transcriptionally active complex, β-catenin recruits the transcriptional coactivator cAMP-response element-binding protein (CREB)-binding protein (CBP) or its closely related homolog, p300 (7, 8), as well as other components of the basal transcription machinery.
Presenilins are transmembrane proteins whose functions are related to trafficking, turnover, and cleavage of the Notch and amyloid precursor protein (APP). Missense mutations in presenilin-1 (PS-1) are associated with early-onset familial Alzheimer's disease (FAD) (9). PS-1 is a critical component of the γ-secretase complex, and mutant PS-1 increases the production of amyloid peptides (10) as well as the proteolysis of Notch intracellular domain (11). PS-1 has also been shown to interact with members of the armadillo family of proteins, including β-catenin (12-14).
PC-12 cells are derived from the neural crest lineage and undergo differentiation to a neuronal phenotype with neurite outgrowth when treated with nerve growth factor (NGF) (15, 16). Expression of AD-linked human PS-1 mutation in cells has been shown to result in significant reduction of neurite outgrowth upon exposure to NGF (17, 18). Here, we report the effects of an FAD-associated PS-1 mutation, Leu-286-Val (L286V), on TCF/β-catenin-mediated transcription. We demonstrate that specifically blocking TCF/β-catenin/CBP transcription using the recently described small molecule inhibitor ICG-001 (19) alleviates the mutant PS-1(L286V)-induced defects in neuronal differentiation.
Materials and Methods
Plasmids. Optimized TOPFLASH luciferase reporter plasmids were provided by Randall T. Moon (University of Washington). Plasmids expressing wild-type human PS-1 and PS-1 containing the human Leu-286-Val mutation were generous gifts from Bryce Sopher (University of Washington).
Cell Culture. PC-12 cells (American Type Culture Collection) were maintained according to recommendations. PC-12 cells stably expressing wild-type PS-1, mutant (L286V) PS-1, and an empty vector control were generated by using a modified protocol described in ref. 20. Briefly, full-length wild-type human PS-1 cDNA and a PS-1 cDNA containing the L286V mutation were cloned into the expression vector pTRE-hyg (Clontech). PC-12 cells were transfected with these constructs as well as an empty vector control by using SuperFect (Qiagen). Expression of the PS-1(L286V) did not affect cell viability during selection. Cells were grown in selection medium containing 0.5 mg/ml hygromycin, and stable clones were isolated after 8 weeks and screened for PS-1 expression. Cell lines that exhibited high levels of expression of PS-1 (data not shown) were used for subsequent experiments.
Experimental Treatments. Cells were differentiated at a density of 15,000 cells per cm2 into a neuronal-like phenotype by incubation in medium with reduced serum (1% FBS) containing 50 ng/ml NGF (Sigma-Aldrich) for 8 days. NGF-containing medium, in the presence or absence of 10 μM ICG-001, was changed every third day. Culture dishes were precoated with 0.25 mg/ml collagen (Cohesion, Palo Alto, CA), 10 μg/ml poly-l-lysine (Sigma-Aldrich), and 12 μg/ml polyethyleneimine (ICN). ICG-001 was dissolved in DMSO at a stock concentration of 100 mM.
Transfection and Luciferase Assays. Cells were transfected by using SuperFect (Qiagen). Transfection efficiencies were normalized with the pRL-null luciferase plasmid. Luciferase assays were performed by using the Dual-Luciferase Reporter Assay System (Promega).
Immunofluorescence. Cells were plated at a density of 10,000 cells per cm2 on sterile, coated, 22 × 22-mm coverslips in a six-well culture plate. Differentiated cells were fixed in methanol for 15 min at -20°C, followed by a 15-min incubation with PBS plus 0.1% Triton X-100. Cells were then incubated with antibodies against Gap-43 (Novus Biologicals, Littleton, CO) for 40 min at 37°C. After a series of washes with PBS-Triton X-100, secondary antibody conjugated to FITC (Jackson ImmunoResearch) was applied. All slide images were acquired by using a Nikon PCM2000 laser scanning confocal microscope mounted on a Nikon Eclipse E600 upright microscope.
RNA Extraction and RT-PCR. Total RNA was extracted (TRIzol, Invitrogen) and reverse-transcribed by using SuperScript II (Invitrogen). RT-PCR was performed with the following primers: 5′-CACAACGCACTTTCTTTCCA-3′ (cyclin D1 forward) and 5′-GACCAGCCTCTTCCTCCA-3′ (cyclin D1 reverse).
Immunoblotting. Lysates from cultured cells were immunoblotted by using monoclonal N-cadherin (BD Bioscience) antibody, which recognizes full-length fragments, and C-terminal fragments 1 and 2 (CTF1 and CTF2), polyclonal CBP A-22, polyclonal p300 N-15 (Santa Cruz Biotechnology), and monoclonal α-tubulin antibodies (Calbiochem). Immune complexes were visualized with enhanced chemiluminescence detection (Amersham Pharmacia).
Results
PC-12/L286V Mutant Cells Do Not Differentiate with NGF. PC-12 cells were exposed to NGF to induce differentiation. Whereas PC-12 cells overexpressing wild-type PS-1 cells (PC-12/WT cells) and vector-control cells (PC-12/Vector cells) had extensive neurite formation (Fig. 1 A and B), similar to PC-12 cell clones from American Type Culture Collection (data not shown), PC-12 cells expressing the mutant PS-1 (PC-12/L286V cells), as has been previously reported (17), exhibited significant reduced neurite formation (Fig. 1C).
Fig. 1.
NGF-treated PC-12 cells. PC-12/Vector control cells (A) and PC-12-overexpressing wild-type PS-1 cells (B) exhibit extensive neurite outgrowth after 8 days of exposure to NGF. (C) PC-12 cells expressing mutant PS-1(L286V) do not display significant neurites under the same culture conditions. Immunofluorescence of GAP-43 (green), a molecular marker of neurite outgrowth, demonstrates intense staining in the neurites of vector-transfected (D) and overexpressing wild-type PS-1 (E) PC-12 cells. (F) Lack of neurite outgrowth corresponds to the weak GAP-43 immunostaining in the PC-12 cells overexpressing mutant PS-1. (G) GAP-43 (green) was significantly elevated in the mutant cells that were treated with NGF and ICG-001. This neuronal protein was also observed in the neurites.
GAP-43 is a neuronal differentiation marker (21, 22) and has recently been shown to be required for appropriate cell fate commitment (23). To validate our morphological observation at the molecular level, we immunostained for GAP-43 expression after NGF-induced differentiation. PC-12/Vector control cells and PC-12/WT cells displayed extensive expression of the neuronal differentiation marker GAP-43 (Fig. 1 D and E), whereas the mutant PC-12/L286V cells were essentially devoid of this neuronal marker (Fig. 1F).
Mutant PS-1 Up-Regulates TCF/β-Catenin-Mediated Signaling. To assess the effects of the L286V PS-1 mutation on TCF/β-catenin-mediated signaling, we transiently transfected NGF-treated PC-12 cells with TOPFLASH, a Wnt-responsive reporter that contains multimerized LEF/TCF binding sites (24). As seen in Fig. 2A, the PC-12/WT cells had similar levels of TCF/β-catenin signaling compared with the PC-12/Vector control cells. However, the PS-1/L286V mutant cells displayed significantly (10-fold) increased TOPFLASH expression.
Fig. 2.
TCF/β-catenin-mediated signaling in PC-12 cells. (A) NGF-treated cells were transfected with TOPFLASH, a TCF/β-catenin reporter construct. PC-12/Vector and PC-12/WT cells display similar levels of TCF/β-catenin-mediated signaling, whereas mutant PC-12/L286V cells have significantly higher levels. Data represent the mean of three independent experiments (±SD). (B) Cyclin D1 promoter activity (Upper) is decreased after NGF treatment in PC-12/Vector control and PC-12/WT cells. PC-12/L286V mutant cells maintain high cyclin D1 activity after NGF exposure, which is significantly reduced upon ICG-001 (10 μM) treatment. Message level for cyclin D1 is down-regulated in NGF-treated PC-12/Vector (Lower, lane 2) and PC-12/WT (lane 4) cells compared with control-treated PC-12/Vector or PC-12/WT cells (lanes 1 and 3, respectively). Cyclin D1 message level in mutant PC-12/L286V cells did not decrease upon NGF exposure (compare control-treated lane 5 to lane 6). However, there is a significant reduction in cells treated with NGF and ICG-001(lane 7). (C) ICG-001 phenotypically corrects deficient neuronal differentiation in mutant PC-1/L286V cells. Mutant cells were exposed to 10 μM ICG-001, in addition to NGF, during the differentiation period. (D) Short interfering RNA (siRNA) to CBP phenotypically corrects deficient neuronal differentiation in mutant PC-1/L286V cells. (E) Quantitation of neurite outgrowth in PC-12 cells. The number of cells with neurite lengths greater than two cell lengths was scored. The number of mutant cells that exhibited the defined neurite length was <25% that of the PC-12/Vector and PC-12/WT cells. After treatment with NGF plus ICG-001, the number of mutant cells of defined neurite lengths was essentially the same as in PC-12/Vector and PC-12/WT cells. The results are the average (±SD) of two independent experiments
Cyclin D1, a critical gene in G1 progression, is a direct target gene of TCF/β-catenin signaling (25). Repression of cyclin D1 transcription and cell cycle arrest are highly coordinated with neurogenesis (26, 27). Exit from the cell cycle is a critical step on the pathway toward neuronal differentiation (26, 28, 29). We investigated whether this increased TCF/β-catenin-mediated signaling (Fig. 2 A) is correlated with an increase in cyclin D1 expression in PC-12/L286V mutant cells. As shown in Fig. 2B, cyclin D1 expression in PC-12/Vector control and PC-12/WT cells was significantly reduced 24 h after NGF treatment, as judged by a cyclin D1 promoter/luciferase construct (Fig. 2B Upper) and by RT-PCR (Fig. 2B Lower, compare lane 1 to lane 2 and compare lane 3 to lane 4). However, treatment of the PC-12/L286V mutant cells with NGF did not significantly decrease cyclin D1 expression (Fig. 2B Lower, lanes 5 and 6).
We hypothesized that dysregulated TCF/β-catenin signaling in the mutant PC-12/L286V cells was responsible for the defective differentiation and neurite outgrowth. To test this hypothesis, we used a recently described specific small-molecule inhibitor of TCF/β-catenin signaling. This small molecule, ICG-001, selectively blocks the β-catenin/CBP interaction but not the β-catenin/p300 interaction, thereby interrupting a subset of TCF/β-catenin-mediated transcription (19). Treatment of the mutant cells with 10 μM ICG-001 in the presence of NGF decreased cyclin D1 reporter gene transcription (Fig. 2B Upper) and message levels (Fig. 2B Lower, compare lanes 5 and 6 to lane 7). Morphologically, treatment of mutant cells with NGF and 10 μM ICG-001 led to essentially normal neurite outgrowth and differentiation (Fig. 2C), similar to that seen in the vector control and overexpressing PC-12/WT cells (Fig. 1 A and B), as compared with the untreated mutant cells (Fig. 1C). Similarly, short interfering RNA (siRNA) to CBP increased neurite outgrowth in PC-12/L286V cells (Fig. 2D) and decreased cyclin D1 message as judged by real-time RT-PCR (data not shown). To confirm that ICG-001-treated mutant cells develop neurites of similar lengths to the vector control or wild-type cells, we scored neurites that were at least twice the length of the cell body. As can be seen in Fig. 2E, treatment with ICG-001 substantially increased the percentage of cells bearing neurites to levels similar to that of the vector-transfected and overexpressing PS-1 wild-type PC-12 cells. Similarly, ICG-001-treated PC-12/L286V mutants showed similar intense GAP-43 staining (Fig. 1G) to the wild-type and vector-transfected cells (Fig. 1 D and E).
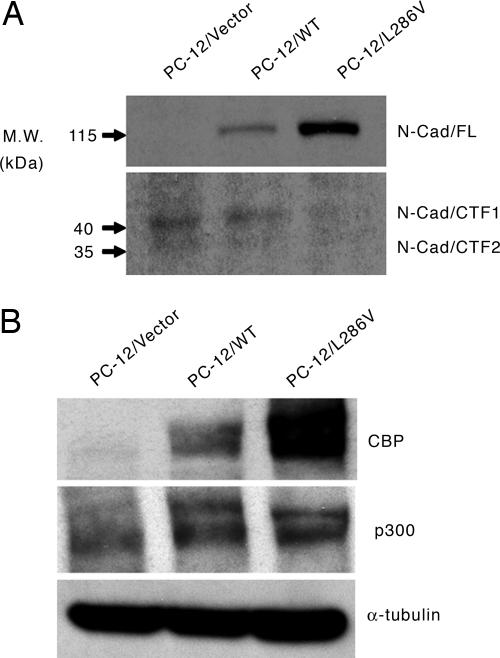
PS-1 Mutations Affect N-Cadherin Processing and CBP Levels and Thereby Dysregulate a Subset of TCF/β-Catenin Transcription. The next question we wished to address was the mechanism by which introduction of the PS-1/L286V mutation affected TCF/β-catenin-mediated transcription. Recently, PS-1, in addition to its well documented role in the γ-secretase-mediated cleavage of both amyloid precursor protein (APP) and Notch, has been reported to promote the ε-cleavage of additional type I membrane proteins including E-cadherin and CD44 (30). In the case of cadherin, the ε-cleavage is significantly stimulated in the absence of calcium homeostasis and by apoptotic induction (31). PS-1-mediated ε-cleavage of N-cadherin affects the intracellular release of an N-cadherin CTF (N-Cad/CTF2). N-Cad/CTF2 subsequently forms a complex with cytoplasmic CBP, thereby promoting CBP's proteasomal degradation (31). Importantly, Marambaud et al. (31) reported that PS-1 FAD mutations inhibit N-Cad/CTF2 processing, thereby increasing nuclear levels of CBP. Based on these observations, we speculated that increased CBP levels in the PS-1/L286V cells may be the cause of the aberrant TCF/β-catenin transcriptional regulation. We found that PC-12/L286V mutant cells, but not those overexpressing wild-type PS-1 or vector, contained almost exclusively full-length N-cadherin and essentially none of the processed N-Cad/CTF1 or N-Cad/CTF2 (Fig. 3A). The decreased ε-cleavage-mediated release of N-Cad/CTF2 additionally correlated with significantly increased levels of CBP, but not p300, in the L286V mutant cells compared with both wild-type overexpressing and vector control cells (Fig. 3B). An intriguing point worth noting is that N-cadherin has recently been implicated as playing an important role in the maintenance of stem cell niches (32). We speculate that this may be because of its role in regulating the CBP/p300 ratio and thereby coactivator usage in stem cells. We conclude that defective N-cadherin processing in the PS-1/L286V mutant cells leads to increased TCF/β-catenin/CBP-dependent transcription at the expense of TCF/β-catenin/p300-mediated transcription, thereby blocking NGF-induced differentiation, and that this transcriptional imbalance is corrected by treatment with ICG-001.
Fig. 3.
Mutant PS-1(L286V) affects N-cadherin processing and CBP levels. (A) Immunoblotting demonstrates the presence of significantly increased full-length N-cadherin in mutant PC-12/L286V cells compared with PC-12/Vector or PC-12/WT cells. (B) Mutant PC-12/L286V cells have significantly increased CBP compared with PC-12/Vector and PC-12/WT cells.
Discussion
Introduction of the FAD mutant PS-1/L286V into PC-12 cells inhibits neurite outgrowth (Fig. 1C) (17). Additionally, introduction of two other PS-1 mutations, P117L and M146L, into N2a neuroblastoma cells has been shown to inhibit neurite outgrowth (18). We now demonstrate that introduction of the FAD mutant PS-1/L286V into PC-12 cells causes dysregulation of TCF/β-catenin signaling, thereby inhibiting neuronal differentiation and neurite outgrowth. Furthermore, the selective small-molecule inhibitor of TCF/β-catenin/CBP transcription, ICG-001, can phenotypically rescue NGF-induced neuronal differentiation and neurite outgrowth in the mutant cells, emphasizing the importance of the TCF/β-catenin signaling pathway on neurite outgrowth and neuronal differentiation.
PS-1 mutations are associated with the majority of FAD cases. Alzheimer's disease (AD) has been suggested to be a manifestation of abnormal neuroplasticity (33, 34). Consistent with this hypothesis, the hippocampus, which is most severely affected in AD, is associated with high synaptic plasticity, axonal and dendritic remodeling (35), and GAP-43 expression (36). A critical role for GAP-43 in neurogenesis has been demonstrated recently. GAP-43 knockout (-/-) P19 embryonic carcinoma cells failed to undergo normal retinoic acid-induced neuronal differentiation (23). Neuronal overexpression of the FAD mutant PS-1(P117L) in transgenic mice affected neurogenesis in the adult hippocampus. Whereas overexpression of the wild-type PS-1 transgene promoted survival and differentiation of progenitor cells, leading to increased granule cell neurons, the mutant transgene did not (37). Furthermore, increased attempts at neurogenic activity may be associated with AD because of attempts to replace hippocampal neurons lost because of toxicity (e.g., Aβ) associated with the disease (38).
Although originally associated with amyloid precursor protein (APP) and Notch processing, it is clear that PS-1 as part of the γ-secretase complex is involved in the proteolytic processing of a variety of additional transmembrane proteins, including both N-cadherin (31) and the protein Deleted in Colon Cancer, both of which are known to modulate various aspects of neuronal development and function (39, 40). We have found, similar to the results of Marambaud et al. (31), that introduction of the PS-1(L286V) mutation into PC-12 cells decreases γ-secretase processing of N-cadherin, thereby increasing nuclear CBP levels. However, it should be noted that conditional double knockout of both PS-1 and PS-2 in mice has been shown to decrease CBP expression (41). Aberrant Wnt signaling has previously been speculated to play a part in AD neuronal degeneration (42-44); however, the complexity of this signaling pathway (45) has complicated the analysis. We propose that the selective increase of a subset of TCF/β-catenin-dependent transcription is associated with defective exit from the cell cycle and NGF-induced neuritogenesis seen in the PC-12/L286V cells. Furthermore, we demonstrate that, phenotypically, this defect can be corrected by selectively antagonizing TCF/β-catenin/CBP-dependent transcription using ICG-001. Additionally, the expression of the important marker of neuronal development GAP-43 is dramatically increased in the mutant cells treated with ICG-001 during NGF-induced differentiation compared with untreated cells.
Within the broader context of AD, our results prompt us to speculate that increased TCF/β-catenin/CBP-mediated transcription may decrease the rate at which neuronal precursor populations differentiate to neurons in AD brains. This finding may be applicable not only to individuals with PS-1 FAD mutations but also to general AD patients (46). This decline in neuronal differentiation, together with enhanced apoptotic susceptibility (20, 47), may exacerbate the decline in neuronal plasticity seen in normal aging.
Intriguingly, Goodman and Pardee (48) recently proposed that decreased retinoid activity in the CNS is a contributing factor to late-onset AD. Retinoic acid potentiates early events in neuronal differentiation and enhances the response to neurotrophic factors (49). Although retinoids are pleiotrophic factors, one of the known effects of retinoids is to antagonize TCF/β-catenin transcription (50). This activity may be associated with the beneficial effects of retinoids on memory and neuronal plasticity (51, 52). We have mapped the binding of ICG-001 to the N-terminal 110 aa of CBP (19). Interestingly, the consensus (LXXLL) retinoic acid receptor/retinoid X receptor binding domain also lies within this region of CBP (residues 70-74), to which ICG-001 binds. These results lay the groundwork for further investigations concerning TCF/β-catenin/CBP signaling in AD.
It is becoming apparent that, despite their high degree of homology and similar patterns of expression, that CBP and p300 play unique and distinct roles in gene regulation. We recently demonstrated that CBP and p300 have distinct functions in the regulation of TCF/β-catenin-mediated survivin transcription (53). The results are consistent with recent publications suggesting nonredundant roles for CBP and p300 in cell growth, differentiation, and development, despite their high degree of homology (54-58). Rebel et al. (59), using a hematopoietic stem cell (HSC) model, concluded that CBP (and not p300) is essential for HSC self-renewal, whereas p300 is critical for proper hematopoietic differentiation. Kawasaki et al. (54) found that p300, but not CBP, is absolutely required for retinoic acid-induced F9 differentiation. Based on our results in the PC-12 model of neuronal differentiation, as well as differentiation studies in other model systems (M. Kahn, unpublished results), we propose a model (Fig. 4) whereby TCF/β-catenin/CBP-mediated transcription is critical for stem cell/progenitor cell proliferation, whereas a switch to TCF/β-catenin/p300-mediated transcription, whether induced chemogenomically with ICG-001 or naturally, is critical to initiate a differentiative program with a more limited proliferative capacity. Aberrant regulation of the balance between these two related transcriptional programs may be associated with a wide array of diseases, including cancer and AD (19, 53).
Fig. 4.
Model for differential coactivator usage in TCF/β-catenin transcription and its role in initiating differentiation.
Acknowledgments
This work was partially supported by National Institutes of Health Grant R01 HL073722.
Author contributions: J.-L.T., H.M., C.N., and C.L. performed research; J.-L.T., H.M., and M.K. analyzed data; J.-L.T. and M.K. wrote the paper; and M.K. designed research.
Abbreviations: CBP, cAMP-response element-binding protein (CREB)-binding protein; TCF, T cell factor; PS-1, presenilin-1; NGF, nerve growth factor; AD, Alzheimer's disease; FAD, familial AD; CTF, C-terminal fragment.
References
- 1.Zechner, D., Fujita, Y., Hulsken, J., Muller, T., Walther, I., Taketo, M. M., Crenshaw, E. B., III, Birchmeier, W. & Birchmeier, C. (2003) Dev. Biol. 258, 406-418. [DOI] [PubMed] [Google Scholar]
- 2.Otero, J. J., Fu, W., Kan, L., Cuadra, A. E. & Kessler, J. A. (2004) Development 131, 3545-3557. [DOI] [PubMed] [Google Scholar]
- 3.Hirabayashi, Y., Itoh, Y., Tabata, H., Nakajima, K., Akiyama, T., Masuyama, N. & Gotoh, Y. (2004) Development 131, 2791-2801. [DOI] [PubMed] [Google Scholar]
- 4.Patapoutian, A. & Reichardt, L. F. (2000) Curr. Opin. Neurobiol. 10, 392-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behrens, J. (2000) Ann. N.Y. Acad. Sci. 910, 21-33. [DOI] [PubMed] [Google Scholar]
- 6.Brantjes, H., Barker, N., van Es, J. & Clevers, H. (2002) Biol. Chem. 383, 255-261. [DOI] [PubMed] [Google Scholar]
- 7.Hecht, A., Vleminckx, K., Stemmler, M. P., van Roy, F. & Kemler, R. (2000) EMBO J. 19, 1839-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takemaru, K. I. & Moon, R. T. (2000) J. Cell Biol. 149, 249-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraser, P. E., Yu, G., Levesque, L., Nishimura, M., Yang, D.S., Mount, H. T., Westaway, D. & St George-Hyslop, P. H. (2001) Biochem. Soc. Symp. 67, 89-100. [DOI] [PubMed] [Google Scholar]
- 10.De Strooper, B., Saftig, P., Craessaerts, K., Vanderstichele, H., Guhde, G., Annaert, W., Von Figura, K. & Van Leuven, F. (1998) Nature 391, 387-390. [DOI] [PubMed] [Google Scholar]
- 11.Berezovska, O., Xia, M. Q. & Hyman, B. T. (1998) J. Neuropathol. Exp. Neurol. 57, 738-745. [DOI] [PubMed] [Google Scholar]
- 12.Levesque, G., Yu, G., Nishimura, M., Zhang, D. M., Levesque, L., Yu, H., Xu, D., Liang, Y., Rogaeva, E., Ikeda, M., et al. (1999) J. Neurochem. 72, 999-1008. [DOI] [PubMed] [Google Scholar]
- 13.Kang, D. E., Soriano, S., Frosch, M. P., Collins, T., Naruse, S., Sisodia, S. S., Leibowitz, G., Levine, F. & Koo, E. H. (1999) J. Neurosci. 19, 4229-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Killick, R., Pollard, C. C., Asuni, A. A., Mudher, A. K., Richardson, J. C., Rupniak, H. T., Sheppard, P. W., Varndell, I. M., Brion, J. P., Levey, A. I. et al. (2001) J. Biol. Chem. 276, 48554-48561. [DOI] [PubMed] [Google Scholar]
- 15.Tischler, A. S. & Greene, L. A. (1975) Nature 258, 341-342. [DOI] [PubMed] [Google Scholar]
- 16.Greene, L. A. & Tischler, A. S. (1976) Proc. Natl. Acad. Sci. USA 73, 2424-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furukawa, K., Guo, Q., Schellenberg, G. D. &Mattson, M. P. (1998) J. Neurosci. Res. 52, 618-624. [DOI] [PubMed] [Google Scholar]
- 18.Dowjat, W. K., Wisniewski, T., Efthimiopoulos, S. & Wisniewski, H. M. (1999) Neurosci. Lett. 267, 141-144. [DOI] [PubMed] [Google Scholar]
- 19.Emami, K. H., Nguyen, C., Ma, H., Kim, D. H., Jeong, K. W., Eguchi, M., Moon, R. T., Teo, J. L., Oh, S. W., Kim, H. Y. et al. (2004) Proc. Natl. Acad. Sci. USA 101, 12682-12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo, Q., Sopher, B. L., Furukawa, K., Pham, D. G., Robinson, N., Martin, G. M. & Mattson, M. P. (1997) J. Neurosci. 17, 4212-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorgels, T. G., Oestreicher, A. B., de Kort, E. J. & Gispen, W. H. (1987) Neurosci. Lett. 83, 59-64. [DOI] [PubMed] [Google Scholar]
- 22.Shen, Y., Mani, S., Donovan, S. L., Schwob, J. E. & Meiri, K. F. (2002) J. Neurosci. 22, 239-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen, Y., Mani, S. & Meiri, K. F. (2004) Mol. Cell Neurosci. 26, 390-405. [DOI] [PubMed] [Google Scholar]
- 24.Korinek, V., Barker, N., Morin, P. J., van Wichen, D., de Weger, R., Kinzler, K. W., Vogelstein, B. & Clevers, H. (1997) Science 275, 1784-1787. [DOI] [PubMed] [Google Scholar]
- 25.Tetsu, O. & McCormick, F. (1999) Nature 398, 422-426. [DOI] [PubMed] [Google Scholar]
- 26.Canzoniere, D., Farioli-Vecchioli, S., Conti, F., Ciotti, M. T., Tata, A. M., Augusti-Tocco, G., Mattei, E., Lakshmana, M. K., Krizhanovsky, V., Reeves, S. A., et al. (2004) J. Neurosci. 24, 3355-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kowalczyk, A., Filipkowski, R. K., Rylski, M., Wilczynski, G. M., Konopacki, F. A., Jaworski, J., Ciemerych, M. A., Sicinski, P. & Kaczmarek, L. (2004) J. Cell Biol. 167, 209-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farah, M. H., Olson, J. M., Sucic, H. B., Hume, R. I., Tapscott, S. J. & Turner, D. L. (2000) Development 127, 693-702. [DOI] [PubMed] [Google Scholar]
- 29.Hippenmeyer, S., Kramer, I. & Arber, S. (2004) Trends Neurosci. 27, 482-488. [DOI] [PubMed] [Google Scholar]
- 30.Weidemann, A., Eggert, S., Reinhard, F. B., Vogel, M., Paliga, K., Baier, G., Masters, C. L., Beyreuther, K. & Evin, G. (2002) Biochemistry 41, 2825-2835. [DOI] [PubMed] [Google Scholar]
- 31.Marambaud, P., Wen, P. H., Dutt, A., Shioi, J., Takashima, A., Siman, R. & Robakis, N. K. (2003) Cell 114, 635-645. [DOI] [PubMed] [Google Scholar]
- 32.Zhang, J., Niu, C., Ye, L., Huang, H., He, X., Tong, W.G., Ross, J., Haug, J., Johnson, T., Feng, F. Q., et al. (2003) Nature 425, 836-841. [DOI] [PubMed] [Google Scholar]
- 33.Mesulam, M. M. (1999) Neuron 24, 521-529. [DOI] [PubMed] [Google Scholar]
- 34.Selkoe, D. J. (2002) Science 298, 789-791. [DOI] [PubMed] [Google Scholar]
- 35.Arendt, T. (2001) Int. J. Dev. Neurosci. 19, 231-245. [DOI] [PubMed] [Google Scholar]
- 36.Lin, L. H., Bock, S., Carpenter, K., Rose, M. & Norden, J. J. (1992) Brain Res. Mol. Brain Res. 14, 147-153. [DOI] [PubMed] [Google Scholar]
- 37.Wen, P. H., Shao, X., Shao, Z., Hof, P. R., Wisniewski, T., Kelley, K., Friedrich, V. L., Jr., Ho, L., Pasinetti, G. M., Shioi, J., et al. (2002) Neurobiol. Dis. 10, 8-19. [DOI] [PubMed] [Google Scholar]
- 38.Jin, K., Peel, A. L., Mao, X. O., Xie, L., Cottrell, B. A., Henshall, D. C. & Greenberg, D. A. (2004) Proc. Natl. Acad. Sci. USA 101, 343-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hjorth, J. T., Gad, J., Cooper, H. & Key, B. (2001) Mech. Dev. 109, 105-109. [DOI] [PubMed] [Google Scholar]
- 40.Kiryushko, D., Berezin, V. & Bock, E. (2004) Ann. N.Y. Acad. Sci. 1014, 140-154. [DOI] [PubMed] [Google Scholar]
- 41.Saura, C. A., Choi, S. Y., Beglopoulos, V., Malkani, S., Zhang, D., Shankaranarayana, B. S., Chattarji, S., Kelleher, R. J., III, Kandel, E. R., Duff, K., et al. (2004) Neuron 42, 23-36. [DOI] [PubMed] [Google Scholar]
- 42.Inestrosa, N., De Ferrari, G. V., Garrido, J. L., Alvarez, A., Olivares, G. H., Barria, M. I., Bronfman, M. & Chacon, M. A. (2002) Neurochem. Int. 41, 341-344. [DOI] [PubMed] [Google Scholar]
- 43.Caricasole, A., Copani, A., Caruso, A., Caraci, F., Iacovelli, L., Sortino, M. A., Terstappen, G. C. & Nicoletti, F. (2003) Trends Pharmacol. Sci. 24, 233-238. [DOI] [PubMed] [Google Scholar]
- 44.Uemura, K., Kitagawa, N., Kohno, R., Kuzuya, A., Kageyama, T., Shibasaki, H., Shimohama, S. (2003) J. Neurosci. Res. 73, 166-175. [DOI] [PubMed] [Google Scholar]
- 45.Nelson, W. J. & Nusse, R. (2004) Science 303, 1483-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caille, I., Allinquant, B., Dupont, E., Bouillot, C., Langer, A., Muller, U. & Prochiantz, A. (2004) Development 131, 2173-2178. [DOI] [PubMed] [Google Scholar]
- 47.Keller, J. N., Guo, Q., Holtsberg, F. W., Bruce-Keller, A. J. & Mattson, M. P. (1998) J. Neurosci. 18, 4439-4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodman, A. B. & Pardee, A. B. (2003) Proc. Natl. Acad. Sci. USA 100, 2901-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahashi, J., Palmer, T. D. & Gage, F. H. (1999) J. Neurobiol. 38, 65-81. [PubMed] [Google Scholar]
- 50.Easwaran, V., Pishvaian, M. & Byers, S. (1999) Curr. Biol. 9, 1415-1418. [DOI] [PubMed] [Google Scholar]
- 51.Chiang, M. Y., Misner, D., Kempermann, G., Schikorski, T., Giguere, V., Sucov, H. M., Gage, F. H., Stevens, C. F. & Evans, R. M. (1998) Neuron 21, 1353-1361. [DOI] [PubMed] [Google Scholar]
- 52.Misner, D. L., Jacobs, S., Shimizu, Y., de Urquiza, A. M., Solomin, L., Perlmann, T., De Luca, L. M., Stevens, C. F. & Evans, R. M. (2001) Proc. Natl. Acad. Sci. USA 98, 11714-11719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma, H., Nguyen, C., Lee K. S. & Kahn, M. (2005) Oncogene 24, 3619-3631. [DOI] [PubMed] [Google Scholar]
- 54.Kawasaki, H., Altieri, D. C., Lu, C. D., Toyoda, M., Tenjo, T. & Tanigawa, N. (1998) Cancer Res. 58, 5071-5074. [PubMed] [Google Scholar]
- 55.Yao, T. P., Oh, S. P., Fuchs, M., Zhou, N. D., Ch'ng, L. E., Newsome, D., Bronson, R. T., Li, E., Livingston, D. M. & Eckner, R. (1998) Cell 93, 361-372. [DOI] [PubMed] [Google Scholar]
- 56.Kung, A. L., Rebel, V. I., Bronson, R. T., Ch'ng, L. E., Sieff, C. A., Livingston, D. M. & Yao, T. P. (2000) Genes Dev. 14, 272-277. [PMC free article] [PubMed] [Google Scholar]
- 57.Yamauchi, T., Oike, Y., Kamon, J., Waki, H., Komeda, K., Tsuchida, A., Date, Y., Li, M. X., Miki, H., Akanuma, Y., et al. (2002) Nat. Genet. 31, 221-226. [DOI] [PubMed] [Google Scholar]
- 58.Roth, J. F., Shikama, N., Henzen, C., Desbaillets, I., Lutz, W., Marino, S., Wittwer, J., Schorle, H., Gassmann, M. & Eckner, R. (2003) EMBO J. 19, 5186-5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rebel, V. I., Kung, A. L., Tanner, E. A., Yang, H., Bronson, R. T. & Livingston, D. M. (2002) Proc. Natl. Acad. Sci. USA 99, 14879-14894. [DOI] [PMC free article] [PubMed] [Google Scholar]