Abstract
Objective
Recurrence continues to be a pivotal challenge among hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) breast cancers. In the international consensus guidelines, HR+/HER2− breast cancer relapse patterns are divided into three distinct types: primary resistant, secondary resistant, and endocrine sensitive. However, owing to the lack of cohorts with treatment and follow-up data, the heterogeneity among different recurrence patterns remains uncharted. Current treatments still lack precision.
Methods
This analysis included data from a large-scale multiomics study of a HR+/HER2− breast cancer cohort (n=314). Through the analysis of transcriptomics (n=312), proteomics (n=124), whole-exome sequencing (n=290), metabolomics (n=217), and digital pathology (n=228) data, we explored distinctive molecular features and identified putative therapeutic targets for patients experiencing recurrence.
Results
We explored distinct clinicopathological characteristics, biological heterogeneity, and potential therapeutic strategies for recurrence. Based on a shared relapse signature, we stratified patients into high- and low-recurrence-risk groups. Patients with different relapse patterns presented unique molecular features in primary tumors. Specifically, receptor tyrosine kinase (RTK) pathway activation in the primary resistant group suggested the utility of RTK inhibitors, whereas mammalian target of rapamycin (mTOR) and cell cycle pathway activation in the secondary resistant group highlighted the potential of mTOR and CDK4/6 inhibitors. Interestingly, the endocrine-sensitive group displayed a quiescent state and high genomic instability, suggesting that targeting quiescent cells and using poly-ADP-ribose polymerase (PARP) inhibitors could be effective strategies.
Conclusions
These findings illuminate the clinicopathological and molecular landscape of HR+/HER2− breast cancer patients with distinct recurrence patterns, highlighting potential targeted therapies.
Keywords: HR+/HER2– breast cancer, endocrine resistance, cancer recurrence, multiomics analysis, precise treatment
Introduction
As the most prevalent cancer affecting women, breast cancer not only impacts physical well-being but also imposes substantial psychological stress and burden (1,2). Hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) breast cancers constitute two-thirds of diagnosed cases and present a stable risk of recurrence lasting up to 20 years, contributing significantly to patient mortality (3,4). At present, the dominant treatment for HR+/HER2− breast cancer is adjuvant chemoendocrine therapy, which aims to mitigate the risk of recurrence. However, endocrine therapy is not foolproof: approximately 25% of patients with either primary or metastatic breast cancer develop resistance to endocrine therapy, posing a great clinical challenge (5). This underscores the urgency of understanding the clinical and molecular diversity of resistance and developing more effective therapeutic options for HR+/HER2− breast cancers. In the context of neoadjuvant therapy, the relationship between the clinical and molecular diversity of HR+/HER2− breast cancer and patient prognosis has been elucidated (6). However, a knowledge gap remains in understanding this heterogeneity during the adjuvant therapy phase.
Recurrent HR+/HER2− breast cancers are classified as primary resistant, secondary resistant or endocrine sensitive on the basis of their prior exposure to adjuvant endocrine therapy and relapse timing according to international consensus guidelines (7). This classification influences first-line treatment decisions and patient eligibility for clinical trials. However, this approach largely stems from expert consensus rather than substantial evidence due to the scarcity of patient cohorts with comprehensive clinical information and prolonged follow-up periods. As a result, although several studies have outlined the clinicopathologic differences in recurrence patterns classified by relapse timing (8-11), only one study has validated the current clinical classification scheme (12). Additionally, for the analysis of the intrinsic features of different recurrence patterns, most studies of estrogen receptor-positive (ER+) breast cancer recurrence have utilized cohorts with short-term antiestrogen therapy or short follow-up durations (approximately 50 months) (13,14). As more than 50% of all recurrences occur after five or more years of adjuvant endocrine therapy (3,15), the reliability of these omics analyses remains to be verified. To date, significant gaps remain in our understanding of the endocrine resistance patterns of HR+/HER2− breast cancer.
Here, to provide broader knowledge about the mechanisms underlying the recurrence pattern classification scheme given in the international consensus guidelines, we established a comprehensive multiomics cohort of Chinese patients afflicted with HR+/HER2− breast cancer. By integrating detailed therapy data and relapse timing, we divided patients into no relapse, primary resistant, secondary resistant, and endocrine-sensitive groups. Through analysis of the clinicopathological and endocrine therapy resistance-related pathways, this study validated the disparate heterogeneity of the different endocrine resistance/sensitivity classifications, especially in previously neglected endocrine-sensitive groups, and offers a theoretical basis for tailored treatment strategies.
Materials and methods
Patients and clinical data collection
We generated a multiomics cohort based on our previously developed HR+/HER2− multiomics dataset without intentional selection. The cohort comprised 351 HR+/HER2− patients treated at the Fudan University Shanghai Cancer Center (FUSCC) between January 2009 and December 2016 (The National Omics Data Encyclopedia: OEP003358) (16). The hematoxylin-eosin (H&E) staining stained tumor tissues of all patients were evaluated by experienced pathologists. Moreover, after applying the exclusion criteria of missing follow-up data and incomplete treatment information, a total of 314 samples from 314 patients were enrolled in the present study. RNA-seq, proteome, whole-exome sequencing (WES), metabolome, and digital pathology data were available for 312, 124, 290, 217, and 228 samples, respectively. All samples were from previously untreated primary breast cancer patients. All patients included in the cohort received standard adjuvant endocrine therapy. Overall survival (OS) was defined as the time from the date of surgery to the date of death from any cause. Patients without events were censored at the time of the last follow-up.
The NCT02712723 cohort included 20 patients administered femara (letrozole) plus ribociclib (LEE011) or placebo as neoadjuvant endocrine therapy for women with ER+, HER2− early breast cancer (FELINE trial) (17). Patients exhibiting either a sustained shrinkage in tumor size during treatment or an initial shrinkage followed by a plateau during treatment were classified as sensitive to therapy. Alternatively, tumors classified as resistant showed either 1) no change in size during treatment, 2) a rebound indicated by initial tumor shrinkage followed by growth during treatment or 3) continually increased growth.
Omics data generation and analysis
The generation of omics data, including RNA and DNA sequencing data, copy number alterations (CNAs), and metabolomics and lipidomics data, has been described in details in our previous study (16). Detailed information about the analysis of immune cell abundance, mutational signatures, homologous recombination deficiency (HRD) scores, gene set enrichment analysis (GSEA) and single-sample GSEA (ssGSEA) has also been described in our previous study (16,18).
Significance of drug sensitivity
To evaluate the drug sensitivity of different relapse groups, we predicted the clinical therapeutic response using baseline tumor gene expression data, which were obtained by developing statistical models based on the gene expression and drug sensitivity data from cell lines in the Cancer Genome Project with the R package “pRRophetic” (19). The differences in the IC50s of common anti-tumor drugs in different relapse groups were compared using the Wilcoxon signed-rank test, and the results are shown as box plots.
Evaluation of CDK4/6 inhibitor sensitivity
To further evaluate the CDK4/6 inhibitor sensitivity of the different relapse groups, RNA-seq data were used to assess the expression of candidate predictive biomarkers of the response to CDK4/6 inhibition, including XCL1, KRT6A, CNTFR, SIRT3, IL24, SP1, IL19, IL17A, and ATF6 (20).
Differential abundance (DA) score
The DA score captures the tendency for a pathway to have increased levels of metabolites relative to a control group (21). The score is calculated by first applying a non-parametric DA test (in this study, Benjamini-Hochberg corrected Mann-Whitney U tests) to all metabolites in a pathway. After determining which metabolites are significantly increased/decreased in abundance, the DA score is defined as follows: DA = (number of metabolites increased − number of metabolites decreased)/number of measured metabolites in the pathway. Thus, the DA score varies from −1 to 1. A score of −1 indicates that all metabolites in a pathway decreased in abundance, whereas a score of 1 indicates that all metabolites increased.
Single-cell RNA sequencing (scRNA-seq) analysis
We used the R package Seurat (Version 4.3.0; https://cran.r-project.org/web/packages/Seurat/index.html) to perform downstream analysis of the scRNA-seq data from the NCT02712723 cohort. All cells expressing <200 or >7,500 genes were removed, as were those containing >20% mitochondrial counts. The anchor-based canonical correlation analysis (CCA) method in the Seurat package was performed for dataset integration and batch effect correction after normalization. The integrated data were subsequently adopted for dimensional reduction, clustering, and data visualization using default parameters. The R package GSVA (Version 1.52.3; https://bioconductor.org/packages/release/bioc/html/GSVA.html) was used to perform ssGSEA on the hallmark, Reactome and Gene Ontology (GO) gene sets from the molecular signatures database (MSigDB).
Immunohistochemistry (IHC)
We performed IHC staining on formalin-fixed, paraffin-embedded tumor specimens from the FUSCC HR+/HER2− breast cancer cohort (n=76) to evaluate the expression of p-ERK1/2. The sections were deparaffinized and rehydrated through a graded series of xylene-ethanol baths. Antigen retrieval was performed using citrate buffer (pH=6.0) for 20 min at 95 °C. The tissue sections were incubated with primary antibody for 1 h at room temperature. The anti-phospho-p44/42 MAPK (Erk1/2) (Cell Signaling Technology, Boston, USA) antibodies were used at a 1:200 dilution. Visualization was performed using the Novolink Polymer Detection System (Leica Biosystems, Nussloch, Germany). The tissue sections were counterstained with hematoxylin, dehydrated, and mounted. The analysis was performed in a blinded fashion. Images were organized in folders identified by patient ID by one person and quantified by another. The results were recorded as the percentage of IHC-stained cells and were assessed by two pathologists.
Statistical analysis
Differences between continuous variables were assessed by Student’s t test, the Wilcoxon rank-sum test, and the Kruskal-Wallis test. The Shapiro-Wilk test was first used to check whether the data followed a normal distribution. Categorical variables were compared using the Chi-square test or Fisher’s exact test when needed. A permutation test was conducted when comparing gene mutation frequencies among different groups. Pearson or Spearman correlation was used for correlation analysis. All of the tests were two-sided, and a P<0.05 indicated significance unless otherwise stated. False discovery rate correction was used for multiple tests to decrease the false-positive rate. Statistical analysis was performed using R software (Version 4.2.1; http://www.R-project.org/).
Study approval
All procedures were conducted in accordance with the Helsinki Agreement and with the approval of the independent Ethics Committee/Institutional Review Board of the Fudan University Shanghai Cancer Center Ethical Committee. Ethical approval and informed consent were obtained [No. 2011226-17 (2010-ZZK-31)].
Results
Overview of multiomics profiling data from FUSCC HR+/HER2− breast cancer cohort
In our natural Chinese Breast Cancer Genome Atlas (CBCGA) cohort (22), we observed a general decline in the likelihood of breast cancer recurrence over time. However, patients with HR+/HER2− breast cancer demonstrated a conspicuous “bimodal” pattern of recurrence, characterized by a persistent risk of recurrence beyond the initial five years post-surgery (Supplementary Figure S1A). This distinctive pattern of delayed recurrence in HR+/HER2− breast cancer patients was further verified by data from a public cohort (TCGA cohort) (Supplementary Figure S1B). This special recurrence phenomenon drew our attention to HR+/HER2− breast cancer, with the aim of identifying its underlying causes and formulating potential therapeutic strategies for different patterns of recurrence (Figure 1A).
Figure 1.
Overview of multiomics profiling data from FUSCC HR+/HER2− breast cancer cohort. (A) Workflow of the analytical process performed in this study used to investigate the characteristics and druggable targets in different relapse groups; (B) Overview of multiomics information acquired for FUSCC HR+/HER2− cohort; (C) Distribution of relapse groups among FUSCC HR+/HER2− cohort; (D) Relapse rates of each SNF subtype of HR+/HER2− breast cancer in FUSCC HR+/HER2− cohort; (E) Relationships between proportion of SNF subtypes and relapse groups in FUSCC HR+/HER2− cohort (P=0.016), determined by Fisher’s exact test. FUSCC, Fudan University Shanghai Cancer Center; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; RTK, receptor tyrosine kinase; PARP, poly-ADP-ribose polymerase; WES, whole-exome sequencing; SNF, similarity network fusion.
For the abovementioned purposes, we utilized data on HR+/HER2− breast cancer patients with comprehensive follow-up records and detailed medication histories from the FUSCC (16). A total of 314 patients were included. All patients underwent surgical intervention and received standard endocrine therapy for more than five years between January 2009 and December 2016. Furthermore, our study benefited from the availability of multiomics data, including transcriptomics (n=312), proteomics (n=124), WES (n=290), metabolomics (n=217), and digital pathology (n=228) data (Figure 1B).
Following the international consensus guidelines (7), we divided the patients into four distinct groups: the no relapse group (64.0%, n=201), primary resistant group (15.6%, n=49), secondary resistant group (13.1%, n=41), and endocrine-sensitive group (7.3%, n=23) (Figure 1B,C, Supplementary Table S1). In brief, primary resistance is defined as a relapse within the first two years of adjuvant endocrine therapy. Secondary resistance is characterized by relapse after the initial two years of therapy or within 12 months following the completion of adjuvant endocrine therapy, whereas endocrine sensitivity is characterized by relapse subsequent to 12 months of therapy. In addition, we utilized transcriptomic data to evaluate the relevance of the recurrence patterns among the HR+/HER2− breast cancer similarity network fusion (SNF) subtypes (i.e., SNF1, SNF2, SNF3 and SNF4) (16). Notably, the SNF1 subtype was prominently distributed in the no relapse group. For SNF2 patients, the onset of relapse was relatively early, and patients who experienced relapse were mainly in the primary resistant group, whereas SNF3 patients were mainly classified into either the secondary resistant group or the endocrine-sensitive group. SNF4 patients presented the most adverse prognosis, exhibiting a bimodal distribution in the timing of relapse. Correspondingly, recurrent SNF4 patients were allocated to either the primary resistant group or the secondary resistant group (Figure 1D,E). Building upon this multiomics cohort, we subsequently analyzed clinical and molecular characteristics of their relapse patterns.
Clinicopathological features of HR+/HER2− breast cancer patients with different relapse patterns
We further explored the clinicopathological features of patients with different relapse patterns. Overall, the key indicators of relapse were ER status, progesterone receptor (PR) status, age, pT, pN and lymphovascular invasion (LVI) (Figure 2A, Supplementary Figure S2A−G), in agree with previous published data (23,24). A more comprehensive overview of the clinical parameters is provided in Table 1. Notably, each group displayed specific clinical features. The primary resistant group generally consisted of younger patients than did the no relapse group, with a median age at diagnosis of 47 years as opposed to 54 years (P=0.030). Moreover, this group had the most advanced lymph node stage and the highest occurrence of LVI (Figure 2A, Supplementary Figure S2E). Interestingly, the endocrine-sensitive group tended to have a relatively higher tumor stage (P<0.001) but a lower Ki-67 index (P=0.723), hinting an inert signature (Figure 2A). Furthermore, breast tumors with different relapse patterns corresponded with distinct PAM50 subtypes (Supplementary Figure S2H). As reported, the PAM50 subtype is heterogeneous with respect to recurrence (25). Specifically, the no relapse group presented a notably greater percentage of patients with the LumA subtype than the other groups did. The primary resistant group appeared to have a higher proportion of patients with the HER2 subtype, whereas the secondary resistant group tended to have a greater proportion of patients with the Basal subtype. Within the endocrine-sensitive group, the LumB subtype was the most prevalent. Correspondingly, clinical data from the TCGA cohort (ER status, PR status, pT stage, and pN stage) also supported our findings (Supplementary Figure S2I−L).
Figure 2.
Heterogeneity of clinical characteristics in four relapse patterns. (A) Clinicopathological features of four relapse patterns. HER2-0 was defined as a HER2 IHC score of 0; HER2-low was defined as a HER2 IHC score of 1+ or 2+ with in situ hybridization (–); (B) Kaplan-Meier curves of OS among the relapse subgroups in FUSCC HR+/HER2− cohort. IHC, immunohistochemistry; HR, hormone receptor; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; LVI, lymphovascular invasion; mOS, overall survival computed from the date of distant relapse to the date of death from any cause. P values were determined by Fisher’s exact test (A) or log-rank test (B). *, P<0.05; **, P<0.01; ***, P<0.001.
Table 1. Patient characteristics of FUSCC HR+HER2− breast cancer cohort among recurrence group.
| Variables | n (%) | P | ||||
| Overall (N=314) |
No relapse (N=201) |
Primary resistant (N=49) |
Secondary resistant (N=41) |
Endocrine sensitive (N=23) |
||
| FUSCC, Fudan University Shanghai Cancer Center; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; ER, estrogen receptor; IDC, infiltrating ductal carcinoma; HER2-low, HER2 immunohistochemistry 1+ or HER2 IHC 2+ with in situ hybridization (−); LVI, lymphovascular invasion; SNF, similarity network fusion. | ||||||
| Age (year) | 0.006 | |||||
| ≤50 | 142 (45.2) | 87 (43.3) | 29 (59.2) | 22 (53.7) | 4 (17.4) | |
| >50 | 172 (54.8) | 114 (56.7) | 20 (40.8) | 19 (46.3) | 19 (82.6) | |
| Menopause | 0.121 | |||||
| Premenopause | 137 (43.6) | 84 (41.8) | 25 (51.0) | 22 (53.7) | 6 (26.1) | |
| Postmenopausal | 176 (56.1) | 117 (58.2) | 24 (49.0) | 18 (43.9) | 17 (73.9) | |
| Unknown | 1 (0.3) | 0 (0) | 0 (0) | 1 (2.4) | 0 (0) | |
| Laterality | 0.625 | |||||
| Left | 166 (52.9) | 104 (51.7) | 30 (61.2) | 21 (51.2) | 11 (47.8) | |
| Right | 148 (47.1) | 97 (48.3) | 19 (38.8) | 20 (48.8) | 12 (52.2) | |
| Histology | 0.201 | |||||
| IDC | 293 (93.3) | 184 (91.5) | 47 (95.9) | 41 (100) | 21 (91.3) | |
| Others | 21 (6.7) | 17 (8.5) | 2 (4.1) | 0 (0) | 2 (8.7) | |
| pT | <0.001 | |||||
| pT1 | 132 (42.0) | 106 (52.7) | 14 (28.6) | 10 (24.4) | 2 (8.7) | |
| pT2 | 175 (55.7) | 93 (46.3) | 32 (65.3) | 31 (75.6) | 19 (82.6) | |
| pT3 | 6 (1.9) | 2 (1.0) | 2 (4.1) | 0 (0) | 2 (8.7) | |
| Unknown | 1 (0.3) | 0 (0) | 1 (2.0) | 0 (0) | 0 (0) | |
| pN | <0.001 | |||||
| pN0 | 125 (39.8) | 103 (51.2) | 8 (16.3) | 11 (26.8) | 3 (13.0) | |
| pN1 | 94 (29.9) | 59 (29.4) | 10 (20.4) | 16 (39.0) | 9 (39.1) | |
| pN2 | 52 (16.6) | 29 (14.4) | 14 (28.6) | 5 (12.2) | 4 (17.4) | |
| pN3 | 43 (13.7) | 10 (5.0) | 17 (34.7) | 9 (22.0) | 7 (30.4) | |
| Grade | 0.216 | |||||
| Grade I | 3 (1.0) | 3 (1.5) | 0 (0) | 0 (0) | 0 (0) | |
| Grade II | 195 (62.1) | 135 (67.2) | 26 (53.1) | 23 (56.1) | 11 (47.8) | |
| Grade III | 88 (28.0) | 49 (24.4) | 17 (34.7) | 12 (29.3) | 10 (43.5) | |
| Unknown | 28 (8.9) | 14 (7.0) | 6 (12.2) | 6 (14.6) | 2 (8.7) | |
| HER2 status | 0.060 | |||||
| HER2-0 | 59 (18.8) | 31 (15.4) | 15 (30.6) | 9 (22.0) | 4 (17.4) | |
| HER2-low | 237 (75.5) | 163 (81.1) | 34 (69.4) | 32 (78.0) | 8 (34.8) | |
| Unknown | 18 (5.7) | 7 (3.5) | 0 (0) | 0 (0) | 11 (47.8) | |
| ER status | 0.029 | |||||
| ER>10% | 266 (84.7) | 201 (100) | 24 (49.0) | 22 (53.7) | 19 (82.6) | |
| ER 1%−10% | 3 (1.0) | 0 (0) | 2 (4.1) | 0 (0) | 1 (4.3) | |
| Unknown | 45 (14.3) | 0 (0) | 23 (46.9) | 19 (46.3) | 3 (13.0) | |
| PR status | 0.192 | |||||
| PR>10% | 216 (68.8) | 164 (81.6) | 20 (40.8) | 17 (41.5) | 15 (65.2) | |
| PR 1%−10% | 36 (11.5) | 21 (10.4) | 6 (12.2) | 4 (9.8) | 5 (21.7) | |
| Unknown | 62 (19.7) | 16 (8.0) | 23 (46.9) | 20 (48.8) | 3 (13.0) | |
| Ki-67 status | 0.528 | |||||
| Ki-67>25% | 152 (48.4) | 96 (47.8) | 24 (49) | 19 (46.3) | 13 (56.5) | |
| Ki-67≤25% | 155 (49.4) | 105 (52.2) | 21 (42.9) | 20 (48.8) | 9 (39.1) | |
| Unknown | 7 (2.2) | 0 (0) | 4 (8.2) | 2 (4.9) | 1 (4.3) | |
| LVI | <0.001 | |||||
| Yes | 166 (52.9) | 84 (41.8) | 39 (79.6) | 27 (65.9) | 16 (69.6) | |
| No | 146 (46.5) | 116 (57.7) | 10 (20.4) | 13 (31.7) | 7 (30.4) | |
| Unknown | 2 (0.6) | 1 (0.5) | 0 (0) | 1 (2.4) | 0 (0) | |
| SNF subtype | <0.001 | |||||
| SNF1 | 50 (15.9) | 36 (17.9) | 5 (10.2) | 7 (17.1) | 2 (8.7) | |
| SNF2 | 51 (16.2) | 29 (14.4) | 14 (28.6) | 3 (7.3) | 5 (21.7) | |
| SNF3 | 71 (22.6) | 40 (19.9) | 10 (20.4) | 13 (31.7) | 8 (34.8) | |
| SNF4 | 35 (11.1) | 13 (6.5) | 12 (24.5) | 8 (19.5) | 2 (8.7) | |
| Unknown | 107 (34.1) | 83 (41.3) | 8 (16.3) | 10 (24.4) | 6 (26.1) | |
| PAM50 subtype | <0.001 | |||||
| Basal | 12 (3.8) | 8 (4.0) | 1 (2.0) | 3 (7.3) | 0 (0) | |
| HER2 | 13 (4.1) | 8 (4.0) | 2 (4.1) | 1 (2.4) | 2 (8.7) | |
| LumA | 98 (31.2) | 86 (42.8) | 4 (8.2) | 4 (9.8) | 4 (17.4) | |
| LumB | 99 (31.5) | 81 (40.3) | 3 (6.1) | 6 (14.6) | 9 (39.1) | |
| Normal | 17 (5.4) | 12 (6.0) | 2 (4.1) | 3 (7.3) | 0 (0) | |
| Unknown | 75 (23.9) | 6 (3.0) | 37 (75.5) | 24 (58.5) | 8 (34.8) | |
We subsequently identified a correlation between recurrence status and patient outcomes. Among 314 patients with a median follow-up interval of 86 (range: 22−139) months, 32% experienced recurrence, with a median time to recurrence of 32 (range: 4−81) months. Strikingly, patients with different recurrence types demonstrated differences in prognostic features. The primary resistant group had a significantly shorter OS than the secondary resistant group (82.5 vs. 107.0 months, P=0.024; Figure 2B). However, despite the responsiveness to endocrine therapy, the prognosis of the endocrine-sensitive group essentially mirrored that of the primary resistant group and they showed the shortest OS in the CBCGA cohort (76.3 vs. 82.5 months, P=0.645). These findings suggest a malignant nature of endocrine-sensitive tumors and the urgent need for more precise treatment. Next, we tried to verify the prognostic value of the relapse groups in the TCGA cohort. The primary resistant group had a poorer prognosis than the secondary resistant group (28.3 vs. 107.2 months, P<0.001; Supplementary Figure S2M). Owing to the limited amount of follow-up data, we cannot determine the prognostic value of endocrine-sensitive patients in the TCGA cohort. Overall, these findings suggest an association of the prognostic and clinical features of HR+/HER2− breast cancer patients with their relapse patterns.
Molecular features of HR+/HER2− breast cancer patients with different relapse patterns
As mentioned previously, HR+/HER2− breast cancer patients with different relapse patterns presented distinct PAM50 subtypes. This implies that each relapse group may have specific molecular characteristics. Therefore, we further resolved the molecular features of the four relapse patterns through multiomics analysis.
First, as patients with germline pathogenic variants, such as BRCA1/2, have a particular predisposition to develop breast cancer (26), we focused on genomic alterations across the four relapse patterns. The top-ranked mutated genes are illustrated in Figure 3A. Interestingly, we observed a prominent occurrence of MUC5B [odds ratio (OR)=84; 95% confidence interval (95% CI), 2.56−191.26] in the primary resistant group. In the secondary resistant group, the frequency of USH2A (OR=21.31; 95% CI, 1.15−93.45) mutations was significantly greater than that in the other groups, whereas FUT3 (OR=60.75; 95% CI, 2.11−91.91) and IGFN1 (OR=3.71; 95% CI, 1.83−7.85) mutations were more prevalent in the endocrine-sensitive group (Figure 3B). Furthermore, certain CNAs were identified across different relapse groups (Supplementary Table S2, Supplementary Figure S3A). In the primary resistant subgroup, amplification of 3p22.2−26.2 was more frequent than that in the no relapse subgroup. This chromosomal fragment contains several classical oncogenes implicated in key biological pathways: FBLN2, which is involved in NOTCH signaling; WNT7A, which plays a role in WNT/β-catenin signaling; RAF1, a crucial component of the RAF/MEK/ERK pathway; and ATG7, which is associated with autophagy signaling (27-29). In the endocrine-sensitive group, amplification of 7q11.21−11.23 and 7q21.11−21.3 proved to be the most prevalent CNAs. These amplified regions contain a series of neuropeptide receptors (CRCP and CALCR) and neural signal transmitters (KCTD7, HGF, PCLO, SEMA3A, SEMA3D, and SEMA3E), suggesting the potential influence of the neural microenvironment on endocrine-sensitive relapses. Conversely, a region-specific deletion on 8q21.13−22.1 (comprising CA1, CA2, CA3, and CA13) was observed in the secondary resistant group. Carbonic anhydrases (CAs) are enzymes that catalyze the hydration of CO2 and play key roles in ion exchange and pH homeostasis (30). Thus, the loss of CAs may contribute to ion and pH homeostasis disorders in the secondary resistant group. Additionally, endocrine-sensitive-specific deletions were noted in the regions of 9q21.11−21.33 and 9q22.31−22.33 (encompassing FBP1, FBP2, ANXA1, and FXN), suggesting that DNA repair deficiency may partially lead to endocrine-sensitive relapses.
Figure 3.
Comparison of multiomics features among four relapse patterns. (A) Mutation profile classified by the relapse subgroups and annotated with the variation type and mutation frequency; (B) Significant enrichment of mutations in different relapse groups. The size of the dot represents the odds ratio; (C) Endocrine sensitivity in relapse subgroups (primary resistant, secondary resistant, endocrine sensitive) vs. no relapse group, determined by two-sided unpaired Student’s t-test; (D) Heatmap of the normalized single-sample GSEA enrichment score of top 10 upregulated pathways among the four relapse patterns; (E) Dot plot showing comparison of the relapse-associated signatures between each group and no relapse group; (F) A pathway-based analysis of metabolomic alterations between the primary resistant, secondary resistant, and endocrine-sensitive groups and the no relapse group. The DA score captured the overall change in a metabolic pathway. A score of 1 indicated that all metabolites in this pathway increased in the relapse group compared with no relapse group, and a score of −1 indicated that all metabolites in this pathway decreased. TMB, tumor mutation burden; GSEA, gene set enrichment analysis; RTK, receptor tyrosine kinase; DA, differential abundance. ns, no significant; *, P<0.05; **, P<0.01.
Second, we delved into transcriptomic alterations. As expected, tumors in the primary resistant cohort presented the lowest endocrine sensitivity score, which is consistent with their resistance to endocrine therapy. Interestingly, tumors in the secondary resistant cohort also presented significant downregulation of endocrine sensitivity compared with those in the no relapse cohort, suggesting an intrinsic feature (Figure 3C).
Moreover, single-sample gene set enrichment analysis (ssGSEA) revealed that multiple metabolic changes, such as changes in tyrosine metabolism and metal ion response pathways, were upregulated specifically in relapsed patients (Figure 3D). More precisely, the primary resistant group presented distinct activation of copper ion detoxification and tyrosine metabolism processes. The secondary resistant group appeared to have an elevated level of RNA splicing and degradation. Additionally, the endocrine-sensitive group presented increased magnesium ion transport activity. These modifications in signal transduction pathways might be associated with the different recurrence patterns observed after endocrine therapy.
We also explored the predictive value of the recurrence-related signatures between each relapse group and the no relapse group using logistic regression (Figure 3E). In all relapse patients, the 21-gene signature (8) and sensitivity to endocrine therapy (SET) score (31) were significantly positively associated with recurrence, whereas increased CDK sensitivity usually indicated a better prognosis. With respect to the primary relapse group, we observed that endocrine resistance and the receptor tyrosine kinase (RTK) pathway score suggested a high risk of relapse, suggesting that primary relapse patients might benefit from RTK inhibitors. In the secondary relapse group, patients with activation of CDK resistance, the cell cycle, the RTK pathway, and 70-gene signature (32) tended to have a better treatment response. Additionally, we also found that the score of the cell cycle, the SET score, and cisplatin resistance were able to predict the recurrence of sensitive relapse patients. In general, the predictive value of these molecular biomarkers varied across different subtypes, highlighting the complexity of the drug sensitivity and resistance mechanisms.
Finally, significant metabolic and ion transport alterations at the transcriptomic level prompted us to conduct DA score analysis (Figure 3F). These analyses investigated differences in metabolite and lipid profiles among samples derived from the primary resistant cohort, secondary resistant cohort, and endocrine-sensitive cohort compared with those of the no relapse cohort. Intriguingly, despite varying types, similar trends were observed among the three relapse groups in terms of changes in some metabolic pathways. We detected an increased abundance of glycine and histamine and a decrease in other amino acids across all three relapse groups. Moreover, the pathways involved in carbohydrate metabolism consistently increased across the three relapse groups (Supplementary Table S3). To emphasize this finding, we outlined the mRNA expression of central metabolic enzymes and corresponding metabolite abundances involved in the tricarboxylic acid cycle and glycolysis. As depicted in Supplementary Figure S3B,C, all three relapse groups exhibited heightened glucose transport and metabolism, whereas lactate transport was diminished. This imbalance led to an accumulation of carbohydrate metabolic intermediates within cells, such as glucose, pyruvate, isocitrate, and succinate, which eventually contributed to the relapse of HR+/HER2− breast cancer. Conversely, the profile of lipid metabolism disorders varied across the relapse groups, indicating a particular dependency on specific lipids. The primary resistant group consistently demonstrated downregulated lipid metabolism. However, in the secondary resistant group, fatty acid biosynthesis was activated. In tumors in the endocrine-sensitive group, glycerophospholipid metabolism and fatty acid degradation tended to increase overall.
The above findings revealed both shared and unique alterations across the three relapse groups. However, their potential as therapeutic targets remain questionable at present. To pinpoint clinically validated biomarkers and formulate effective treatments for assorted relapse groups, we subsequently conducted a series of comprehensive omics analyses, which were specifically designed to address potential targetable pathways.
Potential application of RTK inhibitors in primary resistant group
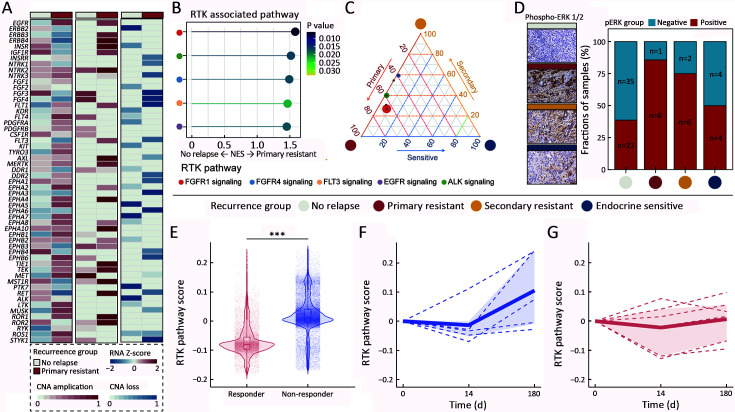
Initially, we analyzed the putatively druggable pathways that exhibited noticeable dysregulation in the primary resistant group. We observed copy number amplification and increased mRNA levels of RTK-related genes within the primary resistant group, indicating their specific role in primary endocrine resistance (Figure 4A). In addition, GSEA and ternary analyses revealed overactivation of RTK signaling in this group (Figure 4B,C). To bolster this finding, we performed immunohistochemical staining for ERK1/2 and detected peak expression levels of ERK1/2 in the primary resistant group (Figure 4D, Supplementary Table S4). Using scRNA-seq data from HR+/HER2− patients receiving endocrine treatment with letrozole (17), we observed activation of the RTK pathway in non-responders at baseline (Figure 4E). Intriguingly, letrozole treatment further upregulated RTK pathway activity in non-responders by d 180, a trend not observed among responders (t=12.9, P<0.001) (Figure 4F,G). Consequently, RTK inhibitors could be potential therapeutic options for patients exhibiting primary resistance. In summary, we elucidated a connection between RTK signaling overactivation and primary resistance and proposed plausible therapeutic targets for patients suffering from primary resistance.
Figure 4.
RTK pathway activation and potential application of RTK inhibitors in primary resistant group. (A) Heatmap of RTK-relative genes expression or CNA between no relapse group and primary resistant group; (B) ssGSEA analysis of RTK pathways between no relapse group and primary resistant group; (C) Ternary plot of RTK pathways among primary resistant, secondary resistant, and endocrine-sensitive groups; (D) IHC detection of phospho-ERK1/2 and IHC staining score quantification among four relapse patterns. Scale bar: 50 μm; (E) Relative RTK pathway scores between responders and non-responders who received endocrine therapy (GSE158724); (F,G) Relative RTK pathway score in responder (F) and non-responder (G) in endocrine therapy on d 0, 14 and 180 in GSE158724. P values were determined by Fisher’s exact test (D) or two-sided unpaired Student’s t-test (E). CNA, copy number alteration; RTK, receptor tyrosine kinase; ssGSEA, single sample gene set enrichment analysis; IHC, immunohistochemical. ***, P<0.001.
Promising application of mammalian target of rapamycin (mTOR) inhibitors and CDK4/6 inhibitors in secondary resistant group
To investigate promising treatment strategies for patients experiencing secondary resistance, we analyzed ten common targetable pathways (33). Through the prediction of drug sensitivity, we discovered that both the mTOR and cell cycle pathways hold potential as targets for patients suffering from secondary resistance (Figure 5A). We subsequently performed a comprehensive analysis of these two pathways separately.
Figure 5.
Enhanced mTOR inhibitors and CDK4/6 inhibitors sensitivity in secondary resistant group. (A) Estimated sensitivity of 10 drugs targeting 10 oncogene pathways in no relapse group and secondary resistant group; (B) Somatic mutation landscape of AKT pathway-related genes. Genes marked with * represent genes differentially mutated between the no relapse group and the secondary resistant group; (C) Pie plots of the three gene mutations (PIK3CA, AKT1, PTEN) related with AKT pathway in four relapse patterns; (D) Hallmark analysis upregulated in secondary resistant group; (E) Heatmap of cell cycle-related genes in no relapse group and secondary resistant group; (F) Comparation of CDK inhibitors response score between three relapse groups (primary resistant, secondary resistant, and endocrine-sensitive group) and no relapse group, respectively. P values were determined by two-sided unpaired Student’s t-test (A,F) or Fisher’s exact test (B). mTOR, mammalian target of rapamycin; TMB, tumor mutation burden. ns, no significant; *, P<0.05.
Initially, we explored the mutation landscape of the mTOR-related genes in our cohort, which might have contributed to the upregulation of mTOR signaling (34). Interestingly, we recorded the highest rates of PIK3CA mutation in patients with secondary resistance (Figure 5B). When the overall mutation status of three main genes (PIK3CA, AKT1, and PTEN) was examined, we found that, compared with the other three groups, the secondary resistant group presented the highest mutation frequency (Figure 5C). This trend was corroborated in the TCGA cohort (Supplementary Figure S4A). At the mRNA level, the expression of most mTOR-related genes was correspondingly elevated in the secondary resistant group (Supplementary Figure S4B), indicating the involvement of cis effects in the regulation of the mTOR pathway.
We subsequently noted enrichment of the cell cycle pathway and upregulation of cell cycle-related genes at the transcriptomic level in the secondary resistant group (Figure 5D,E, Supplementary Figure S4C,D). Excessive cell cycle stimulation is a key cancer development factor, especially in HR+/HER2− breast cancer (35). Concomitantly, CDK4/6 inhibitors, such as albociclib and abemaciclib, have been utilized as first-line therapies in advanced HR+/HER2− breast cancer to arrest cell cycle progression. Our examination of previously reported CDK4/6 inhibitor response markers revealed that patients with secondary resistance exhibited greater sensitivity to CDK4/6 inhibitors than no relapse patients did (Figure 5F). These observations indicate a potential response to mTOR inhibitors and CDK4/6 inhibitors in patients experiencing secondary resistance.
Unique genomic instability and quiescent-cell signature of endocrine-sensitive group
As expected, the results from our drug sensitivity analysis revealed that endocrine-sensitive patients were sensitive to tamoxifen (P<0.05) (Figure 6A). However, these patients still experience relapses after adjuvant therapy. Intriguingly, we observed specific sensitivity to olaparib, a poly-ADP-ribose polymerase (PARP) inhibitor, among endocrine-sensitive patients (P<0.05) (Figure 6B), indicating a deficiency in DNA damage repair. Since impaired DNA damage repair can induce high genomic instability, we further investigated the mutation load among the relapse groups. Unsurprisingly, the endocrine-sensitive group presented the highest tumor mutation burden (TMB) and the highest HRD level (P<0.05) (Figure 6C,D).
Figure 6.
Endocrine-sensitive group showed unique genomic instability and quiescent state. (A) Estimated sensitivity of tamoxifen among four relapse patterns; (B) Estimated sensitivity of PARP inhibitor, olaparib, among four relapse patterns; (C) Distribution of the median TMB in four relapse patterns; (D) Distribution of allele-specific copy-number profile-based HRD scores in four relapse patterns; (E) Distribution of COSMIC ID8 mutational signature in four relapse patterns; (F) Distribution of COSMIC SBS mutational signature in four relapse patterns; (G) Estimated sensitivity of AZD7762 targeting quiescent cells among four relapse patterns; (H) ssGSEA analysis of cell proliferation related pathways between no relapse group and endocrine-sensitive group; (I,J) Kaplan-Meier curves showing RFS of patients with non-standard or standard adjuvant endocrine therapy in high quiescent cell signature subgroup (P=0.768) (I) and low quiescent cell signature subgroup (P=0.034) (J). P values were determined by two-sided unpaired Student’s t-test (A−G) or log-rank test (I,J). TMB, tumor mutation burden; HRD, homologous recombination deficiency; COSMIC, the catalogue of somatic mutations in cancer; ID, insertions and deletion; SBS, single-base substitution; ssGSEA, single sample gene set enrichment analysis; RFS, relapse-free survival. ns, no significant; *, P<0.05; **, P<0.01.
We further analyzed the mutational signatures among the four relapse patterns. For small insertions and deletions (IDs), the ID8 signature, which is associated with the repair of DNA double-strand breaks, was significantly mutated in the endocrine-sensitive group (P<0.05) (Figure 6E). Similarly, mutation analysis of single-base substitutions (SBSs) revealed an enrichment of age-related (SBS1) and APOBEC-related (SBS2/13) signatures (Figure 6F). Collectively, these findings illustrate the prominent genomic instability characteristic of the endocrine-sensitive group, thereby suggesting that PARP inhibitors are plausible therapeutic interventions.
Drug sensitivity analysis further revealed high sensitivity to AZD7762 in the endocrine-sensitive group (P<0.05) (Figure 6G). AZD7762 is a CHK1 inhibitor that eradicates quiescent cancer cells (QCCs). QCCs are cancer cells that temporarily suspend cell cycle progression (G0 phase) but have the ability to reinitiate cell division and thereby spur tumor growth, recurrence and metastasis (36). The reactivation of quiescent cancer cells has been recognized as a cause of cancer recurrence. The sensitivity to AZD7762 implies that the endocrine-sensitive group may harbor a substantial pool of quiescent cells, which might contribute to long-term recurrence and resistance to endocrine therapy.
To verify this hypothesis, we investigated the molecular divergence among these four groups. With GO analysis for biological process (BP), we found that the endocrine-sensitive group presented common features of proliferation arrest, with downregulation of cell proliferation in almost all cell types (Figure 6H). Moreover, we performed ssGSEA with a quiescent cell gene set and divided our cohort into high- and low-quiescent-cell signature subsets based on the median quiescent-cell signature. Interestingly, no difference in recurrence-free survival was observed between patients receiving standard therapy and those receiving non-standard therapy in the high-quiescent-cell signature subset (Figure 6I). However, in the low-quiescent-cell signature subset, standard therapy significantly improved patient outcomes compared with non-standard therapy (P=0.034) (Figure 6J), suggesting that quiescent cells play a role in treatment resistance. Thus, while endocrine therapies can effectively target actively proliferating hormone-dependent tumor cells, dormant cells might evade this therapeutic pressure. After therapy ends, quiescent breast cancer cells may reactivate, and patients with high levels of quiescent cells ultimately relapse.
Overall, endocrine therapy was effective for the primary lesions of endocrine-sensitive patients. However, these patients displayed both elevated genomic instability and a dominant quiescent-cell signature. Consequently, quiescent cells escape adjuvant endocrine therapy. Under stress, these dormant cells acquire proliferative capabilities because of their high genomic instability. Our study supports the potential of PARP inhibitors and AZD7762 to reduce the recurrence rate in endocrine-sensitive patients (Supplementary Figure S5).
Discussion
The recurrence time of HR+/HER2− breast cancer varies greatly, often demonstrating a prolonged recurrence timeline compared with that of other subtypes. However, this understanding has been historically limited by gaps in therapeutic follow-up data. In our study, employing the FUSCC HR+/HER2− cohort with well-documented medication information, we categorized HR+/HER2− breast cancer patients (n=314) into distinct groups: no relapse, primary resistant, secondary resistant, and endocrine sensitive. We conducted a comprehensive analysis of clinicopathological features, multiomics profiles, and pathways associated with drug resistance. Our findings revealed that the primary tumors of relapsed patients exhibited profound biological differences from those of no relapsed patients, with markedly divergent mutational and copy number features. Furthermore, we clarified the heterogeneity within the relapse groups and proposed treatment strategies for each group (Supplementary Figure S6).
Our study has noteworthy potential for clinical translation. Specifically, within the primary resistant group, we observed activation of the RTK pathway. Elevated RTK levels in breast cancer are associated with a more aggressive and malignant phenotype, as well as decreased disease-free survival (37). In line with this observation, we detected a significant enrichment of primary resistant patients in our SNF4 subtype, an RTK-driven subtype. According to the SNF classification system, this RTK-driven subtype has a poor prognosis (16). Thus, administering RTK inhibitors to patients who experience primary resistance may improve clinical outcomes.
In the secondary resistant group, we detected activation of the PI3K/AKT/mTOR and cell cycle signaling pathways. This finding is consistent with accumulating evidence for extensive crosstalk between PI3K/AKT/mTOR signaling and cell cycle regulators (38). This dysregulation of the cell cycle pathway is a fundamental biological trait of breast cancer. The role of estrogen in enhancing cyclin D1 expression, which in turn activates CDK4/6 and thereby facilitates cell cycle progression in HR+/HER2− breast cancer, highlights this point (39). Currently, the adoption of CDK4/6 inhibitors has transformed the treatment landscape for patients with advanced-stage HR+/HER2− breast cancer, but the optimal treatment strategy is still under investigation. The PI3K/AKT/mTOR pathway plays an important role in the dysregulation of cell proliferation, migration and apoptosis, and its activation leads to endocrine resistance and poor outcomes of patients with breast cancer (40). With full verification from phase III trials, the integration of the mTOR inhibitor everolimus and the PIK3CA inhibitor alpelisib has been substantiated as standard second-line treatments following the inefficacy of CDK4/6 inhibitors (41,42). Furthermore, preclinical studies endorsed the concurrent use of CDK4/6 and PI3K inhibitors (35). Owing to the observed activation of the CDK4/6 and PI3K/AKT/mTOR pathways in the secondary resistant group, we highlighted a more precise application of CDK4/6 and PI3K inhibitors in treating patients with secondary resistance.
In the endocrine-sensitive group, our findings indicated that these patients had relatively poorer prognoses, characterized by the presence of quiescent cells and genomic instability. The development of therapeutic approaches that target these two features may improve clinical outcomes in this population. Indeed, previous studies have revealed the important role of quiescent cells in tumor recurrence (36,43-45). Quiescent breast cancer cells are resistant to T-cell killing (46). A critical, yet unresolved, aspect involves the mechanisms behind the reactivation of QCCs. Our data suggest that the QCCs in the endocrine-sensitive group displayed high genomic instability, which might have contributed to the reactivation of the QCCs. The combination of targeted QCCs and genomic instability may expand our molecular armamentarium against relapse.
Our study addresses and mitigates some of the shortcomings encountered in previous research on the recurrence of HR+/HER2− breast cancer. Several recent reports have enriched our understanding of the recurrence landscape in HR+/HER2− breast cancer through traditional single-omic analyses. For example, investigations into the genomic landscape of endocrine-resistant advanced breast cancers revealed the key role of the MAPK pathway and identified prevalent gene mutations in ERBB2 and NF1 in endocrine-resistant tumors (47). WES of HR+/HER2− breast cancer patients receiving short-term preoperative estrogen deprivation revealed a correlation between high Ki-67 expression and gene amplification of FGFR1 and CCND1 (13). However, these studies have often been limited in scope, focusing primarily on genomic or transcriptomic analyses, utilizing small sample sizes, and relying on datasets consisting of samples from patients after short-term treatments only. These constraints have resulted in a loss of valuable information and impeded a deeper understanding, reinforcing the need for a thorough characterization of HR+/HER2− breast cancer recurrence. Our study overcomes these limitations by comprehensively evaluating the multiomics data from our large dataset of breast cancer samples with complete drug treatment records and follow-up information. Our detailed analysis sheds light on genes that undergo preferential mutation and CNAs across different relapse scenarios. Additionally, our study reveals preferentially activated oncogenic pathways, elucidating the key driving events behind breast cancer recurrence and proposing corresponding therapeutic avenues. An important contribution of our work is the discovery of altered pathways in the endocrine-sensitive group, corroborating the potential effectiveness of targeting quiescent cells with specific drugs. Overall, by addressing specific vulnerabilities in each relapse group, our findings emphasize the importance of personalized medicine in the management of HR+/HER2− breast cancers.
Despite the above strengths, there are limitations to this study. The retrospective nature and the limited sample size of patients and events within each subgroup highlight the need for validation in larger and more diverse datasets. Moreover, although this investigation boasts a substantial median follow-up period of seven years, it is important to note that recurrences can still manifest beyond this interval. This fact underscores the continued need for prolonged follow-up periods in patient registries to capture late recurrence events comprehensively. While we performed multiomics analysis on recurrent breast cancer tumors and verified RTK pathway activity in the primary resistant group by IHC, further experimental verification is necessary.
Conclusions
We discovered that the characteristics of primary tumors in HR+/HER2− breast cancer significantly influence the type of recurrence. Using multiomics profiling, we identified the heterogeneity among the various relapse groups and revealed unique therapeutic targets within each group. Targeting these features in different relapse groups could revolutionize the treatment landscape for HR+/HER2− breast cancer and reinforce the potential of precision medicine.
Figure S1.
Public cohort information used in our study, related to Figure 1. Relapse rates according to clinical subtype in breast cancer patients in CBCGA cohort (A) and TCGA cohort (B). CBCGA, Chinese Breast Cancer Genome Atlas; TCGA, The Cancer Genome Atlas; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer.
Figure S2.
Heterogeneity of clinical characteristics in relapse groups, related to Figure 2. (A−E) Clinical features of four relapse patterns in FUSCC HR+/HER2− breast cancer cohort, including ER status (P<0.001) (A), PR status (P<0.001) (B), T stage (P<0.001) (C), N stage (P<0.001) (D) and LVI (P<0.001) (E); (F,G) ER index (F) and PR index (G) in relapse subgroups (primary resistant, secondary resistant, endocrine sensitive) vs. no relapse group; (H) Relationships between proportion of PAM50 subtypes and the relapse groups in TCGA cohort; (I-L) Clinical features of the four relapse patterns in the TCGA cohort, including ER status (P=0.090) (I), PR status (P=0.258) (J), T stage (P=0.034) (K), N stage (P=0.003) (L); (M) Kaplan-Meier curves of overall survival among the relapse groups in TCGA cohort. P values were determined by Fisher’s exact test (A−E, H−L), two-sided unpaired Student’s t-test (F,G) or log-rank test (M). FUSCC, Fudan University Shanghai Cancer Center; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; ER, estrogen receptor; PR, progesterone receptor; LVI, lymphovascular invasion; TCGA, The Cancer Genome Atlas; mOS, overall survival computed from the date of distant relapse to the date of death from any cause; NA, not available. ns, no significant; *, P<0.05; **, P<0.01.
Figure S3.
Multiomics characteristics between non-relapse patients and relapsed patients, related to Figure 3. (A) Comparison of somatic CNAs between the three relapse groups and the no relapse group. The upper plot illustrates the frequency of the amplification (dark red), gain (light red), loss (light blue), and deletion (dark blue) of each gene in each subgroup, and the lower plot illustrates the log10 P value of each gene when compared between the three relapse subgroups and the no relapse group in the amplification/gain-centric (yellow) or deletion/loss-centric (green) calculations; (B) Diagram summarizing metabolic genes and metabolites involved in the TCA cycle and glycolysis. Alteration scores of each gene or metabolite are depicted as log ratios [fold-change, expressed as log2(ratio of average mRNA expression or metabolite abundance in relapse group vs. the paired normal samples)]. Red, upregulated genes or metabolites; blue, downregulated genes or metabolites; (C) Metabolite abundance differences involved in TCA cycle among the four relapse patterns, P values were determined by two-sided unpaired Student’s t-test. CNA, copy number alteration; TCA, tricarboxylic acid. ns, no significant; *, P<0.05; **, P<0.01; ***, P<0.001.
Figure S4.
Metabolic disorder and AKT pathway activation in secondary resistant group, related to Figure 5. (A) Genomic alteration on AKT-related genes in TCGA cohort; (B) Transcriptomic alteration on AKT-related genes in TCGA cohort, P values were determined by Fisher’s exact test; (C,D) Hallmark_E2F_Targets (C) and Hallmark_G2M_Checkpoint (D), the two upregulated pathways about cell cycle in FUSCC HR+/HER2− breast cancer cohort. TMB, tumor mutation burden; TCGA, The Cancer Genome Atlas; FUSCC, Fudan University Shanghai Cancer Center; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; CDKi, cyclin-dependent kinase inhibitor; NES, normalized enrichment score. *, P<0.05; **, P<0.01.
Figure S5.
Schematic diagram showing how quiescent cell and genome instability involved in endocrine sensitivity of luminal breast cancer, related to Figure 6. PARP, poly-ADP-ribose polymerase.
Figure S6.
Summary of characteristics across different recurrence groups in the HR+/HER2− breast cancer patients. Red up arrows denote increased levels or potentially better response, while Blue down arrows represent the opposite. HR, hormone receptor; HER2, human epidermal growth factor receptor 2; LVI, lymphovascular invasion; mTOR, mammalian target of rapamycin; HRD, homologous recombination deficiency; SNF, similarity network fusion.
Table S4. IHC score of ERK1/2.
| Patient code | Recurrence group | ERK1/2 | SNF subtype |
| IHC, immunohistochemical; SNF, similarity network fusion. | |||
| PEYS | No relapse | 0 | SNF2 |
| ZLPB | No relapse | 0 | SNF2 |
| KELR | Primary resistant | 10 | SNF3 |
| UKMR | Primary resistant | 0 | SNF2 |
| UBSN | No relapse | 0 | SNF3 |
| NUVQ | No relapse | 0 | SNF4 |
| NVIY | No relapse | 0 | SNF4 |
| AYKR | No relapse | 0 | SNF2 |
| SAEI | No relapse | 0 | SNF1 |
| PBLW | No relapse | 0 | SNF2 |
| GTNE | No relapse | 0 | SNF1 |
| COUE | No relapse | 0 | SNF3 |
| XPVH | No relapse | 0 | SNF4 |
| TOCM | No relapse | 0 | SNF3 |
| NIDW | No relapse | 0 | SNF3 |
| IEQH | No relapse | 0 | SNF2 |
| XKIR | No relapse | 0 | SNF3 |
| XSJM | No relapse | 0 | SNF3 |
| ZPWX | Secondary resistant | 5 | SNF1 |
| JLWB | No relapse | 0 | SNF3 |
| LWBT | No relapse | 0 | SNF1 |
| BTJD | No relapse | 0 | SNF1 |
| TLKM | No relapse | 0 | SNF1 |
| WVAK | No relapse | 0 | SNF3 |
| LRZM | No relapse | 0 | SNF2 |
| QWJP | No relapse | 0 | SNF3 |
| FWMQ | No relapse | 0 | SNF2 |
| COUB | Secondary resistant | 30 | SNF2 |
| QAGJ | No relapse | 0 | SNF1 |
| PHRN | No relapse | 0 | SNF3 |
| MNXT | No relapse | 0 | SNF2 |
| ZRXI | No relapse | 0 | SNF3 |
| WRNK | No relapse | 0 | SNF1 |
| QLHW | No relapse | 0 | SNF4 |
| FENY | No relapse | 0 | SNF1 |
| YIGX | No relapse | 0 | SNF2 |
| HAIV | No relapse | 0 | SNF3 |
| QGCM | Primary resistant | 5 | SNF2 |
| QLJI | No relapse | 0 | SNF3 |
| JTUY | Endocrine sensitive | 0 | SNF2 |
| JLDQ | No relapse | 0 | SNF3 |
| ICKO | Endocrine sensitive | 1 | SNF3 |
| NBPH | No relapse | 1 | SNF1 |
| YJDI | Primary resistant | 8 | SNF1 |
| FQCZ | Secondary resistant | 5 | SNF4 |
| VPHD | Secondary resistant | 0 | SNF3 |
| GQCV | Primary resistant | 4 | SNF3 |
| NXYB | Endocrine sensitive | 0 | SNF1 |
| TXRL | No relapse | 4 | SNF1 |
| WYJE | Primary resistant | 5 | SNF2 |
| VEBY | No relapse | 4 | SNF1 |
| OPBQ | No relapse | 4.5 | SNF1 |
| EVNI | Endocrine sensitive | 15 | SNF4 |
| NVIW | No relapse | 5 | SNF2 |
| EFWC | No relapse | 5 | SNF2 |
| XMIT | No relapse | 5 | SNF2 |
| VBIT | No relapse | 5 | SNF1 |
| CLAV | No relapse | 5 | SNF2 |
| UWIR | Secondary resistant | 4 | SNF3 |
| INMG | No relapse | 5 | SNF2 |
| MBAU | No relapse | 5 | SNF2 |
| ORNF | Primary resistant | 4 | SNF3 |
| UPLH | No relapse | 7 | SNF1 |
| VQKJ | Endocrine sensitive | 15 | SNF3 |
| TBSH | No relapse | 8 | SNF1 |
| ZIAO | No relapse | 8 | SNF1 |
| SPTV | No relapse | 10 | SNF1 |
| YENU | No relapse | 10 | SNF2 |
| IZVH | No relapse | 10 | SNF1 |
| KBPY | Secondary resistant | 20 | SNF1 |
| RNCI | No relapse | 10 | SNF3 |
| MNDC | No relapse | 10 | SNF2 |
| KCIF | No relapse | 10 | SNF1 |
| LASO | No relapse | 15 | SNF1 |
| WEPS | Secondary resistant | 15 | SNF3 |
| WDRH | No relapse | 25 | SNF2 |
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
Acknowledgements
This work is supported by the National Key Research and Development Program of China (No. 2020YFA0112304), the National Natural Science Foundation of China (No. 82373167, 82341003 and 92159301), the Natural Science Foundation of Shanghai (No. 22ZR1479200), the Shanghai Key Laboratory of Breast Cancer (No. 12DZ2260100), the SHDC Municipal Project for Developing Emerging and Frontier Technology in Shanghai Hospitals (No. SHDC12021103), Shanghai Medical Innovation Research Project (No. 22Y11912700), and Shanghai Anticancer Association EYAS PROJECT (No. SACA-CY22A05). This work was supported by the Medical Science Data Center of Fudan university. Illustrations were created with BioRender.com.
Acknowledgments
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Contributor Information
Yi Xiao, Email: yixiao11@fudan.edu.cn.
Yizhou Jiang, Email: yizhoujiang@fudan.edu.cn.
References
- 1.Siegel RL, Miller KD, Fuchs HE, et al Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Xu H, Xu B Breast cancer: Epidemiology, risk factors and screening. Chin J Cancer Res. 2023;35:565–83. doi: 10.21147/j.issn.1000-9604.2023.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan H, Gray R, Braybrooke J, et al 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377:1836–46. doi: 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tevaarwerk AJ, Gray RJ, Schneider BP, et al Survival in patients with metastatic recurrent breast cancer after adjuvant chemotherapy: little evidence of improvement over the past 30 years. Cancer. 2013;119:1140–8. doi: 10.1002/cncr.27819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeselsohn R, Buchwalter G, De Angelis C, et al ESR1 mutations — a mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol. 2015;12:573–83. doi: 10.1038/nrclinonc.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huppert LA, Wolf D, Yau C, et al Pathologic complete response (pCR) rates for patients with HR+/HER2- high-risk, early-stage breast cancer (EBC) by clinical and molecular features in the phase II I-SPY2 clinical trial. Ann Oncol. 2025;36:172–84. doi: 10.1016/j.annonc.2024.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardoso F, Paluch-Shimon S, Senkus E, et al 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31:1623–49. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowsett M, Sestak I, Buus R, et al Estrogen receptor expression in 21-gene recurrence score predicts increased late recurrence for estrogen-positive/HER2-negative breast cancer. Clin Cancer Res. 2015;21:2763–70. doi: 10.1158/1078-0432.Ccr-14-2842. [DOI] [PubMed] [Google Scholar]
- 9.Luen SJ, Asher R, Lee CK, et al Identifying oncogenic drivers associated with increased risk of late distant recurrence in postmenopausal, estrogen receptor-positive, HER2-negative early breast cancer: results from the BIG 1-98 study. Ann Oncol. 2020;31:1359–65. doi: 10.1016/j.annonc.2020.06.024. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Schroeder BE, Jerevall PL, et al A novel breast cancer index for prediction of distant recurrence in HR+ early-stage breast cancer with one to three positive nodes. Clin Cancer Res. 2017;23:7217–24. doi: 10.1158/1078-0432.Ccr-17-1688. [DOI] [PubMed] [Google Scholar]
- 11.Early Breast Cancer Trialists’ Collaborative Group Reductions in recurrence in women with early breast cancer entering clinical trials between 1990 and 2009: a pooled analysis of 155 746 women in 151 trials. Lancet. 2024;404:1407–18. doi: 10.1016/s0140-6736(24)01745-8. [DOI] [PubMed] [Google Scholar]
- 12.Lambertini M, Blondeaux E, Bisagni G, et al Prognostic and clinical impact of the endocrine resistance/sensitivity classification according to international consensus guidelines for advanced breast cancer: an individual patient-level analysis from the Mammella InterGruppo (MIG) and Gruppo Italiano Mammella (GIM) studies. EClinicalMedicine. 2023;59:101931. doi: 10.1016/j.eclinm.2023.101931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giltnane JM, Hutchinson KE, Stricker TP, et al Genomic profiling of ER+ breast cancers after short-term estrogen suppression reveals alterations associated with endocrine resistance. Sci Transl Med. 2017;9:eaai7993. doi: 10.1126/scitranslmed.aai7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeselsohn R, Yelensky R, Buchwalter G, et al Emergence of constitutively active estrogen receptor-α mutations in pretreated advanced estrogen receptor-positive breast cancer. Clin Cancer Res. 2014;20:1757–67. doi: 10.1158/1078-0432.Ccr-13-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sgroi DC, Sestak I, Cuzick J, et al Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol. 2013;14:1067–76. doi: 10.1016/s1470-2045(13)70387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin X, Zhou YF, Ma D, et al Molecular classification of hormone receptor-positive HER2-negative breast cancer. Nat Genet. 2023;55:1696–708. doi: 10.1038/s41588-023-01507-7. [DOI] [PubMed] [Google Scholar]
- 17.Griffiths JI, Chen J, Cosgrove PA, et al Serial single-cell genomics reveals convergent subclonal evolution of resistance as early-stage breast cancer patients progress on endocrine plus CDK4/6 therapy. Nat Cancer. 2021;2:658–71. doi: 10.1038/s43018-021-00215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Ding XH, Liu CL, et al Genomic alterations affecting tumor-infiltrating lymphocytes and PD-L1 expression patterns in triple-negative breast cancer. J Natl Cancer Inst. 2023;115:1586–96. doi: 10.1093/jnci/djad154. [DOI] [PubMed] [Google Scholar]
- 19.Geeleher P, Cox N, Huang RS pRRophetic: an R package for prediction of clinical chemotherapeutic response from tumor gene expression levels. PLoS One. 2014;9:e107468. doi: 10.1371/journal.pone.0107468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner NC, Liu Y, Zhu Z, et al Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2019;37:1169–78. doi: 10.1200/jco.18.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao Y, Ma D, Yang YS, et al Comprehensive metabolomics expands precision medicine for triple-negative breast cancer. Cell Res. 2022;32:477–90. doi: 10.1038/s41422-022-00614-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang YZ, Ma D, Jin X, et al Integrated multiomic profiling of breast cancer in the Chinese population reveals patient stratification and therapeutic vulnerabilities. Nat Cancer. 2024;5:673–90. doi: 10.1038/s43018-024-00725-0. [DOI] [PubMed] [Google Scholar]
- 23.Bi Z, Wang Y Advances in regional nodal management of early-stage breast cancer. Chin J Cancer Res. 2024;36:215–25. doi: 10.21147/j.issn.1000-9604.2024.02.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin DS, Lee J, Kang E, et al Age and late recurrence in young patients with ER-positive, ERBB2-negative breast cancer. JAMA Netw Open. 2024;7:e2442663. doi: 10.1001/jamanetworkopen.2024.42663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lænkholm AV, Jensen MB, Eriksen JO, et al PAM50 risk of recurrence score predicts 10-year distant recurrence in a comprehensive danish cohort of postmenopausal women allocated to 5 years of endocrine therapy for hormone receptor-positive early breast cancer. J Clin Oncol. 2018;36:735–40. doi: 10.1200/jco.2017.74.6586. [DOI] [PubMed] [Google Scholar]
- 26.Hirose T, Ikegami M, Kida K, et al Cancer risk assessment of premalignant breast tissues from patients with BRCA mutations by genome profiling. NPJ Breast Cancer. 2024;10:87. doi: 10.1038/s41523-024-00693-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai J, Li R, Xu X, et al CK1α suppresses lung tumour growth by stabilizing PTEN and inducing autophagy. Nat Cell Biol. 2018;20:465–78. doi: 10.1038/s41556-018-0065-8. [DOI] [PubMed] [Google Scholar]
- 28.Griveau A, Seano G, Shelton SJ, et al A glial signature and Wnt7 signaling regulate glioma-vascular interactions and tumor microenvironment. Cancer Cell. 2018;33:874–89.e7. doi: 10.1016/j.ccell.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simanshu DK, Nissley DV, McCormick F RAS proteins and their regulators in human disease. Cell. 2017;170:17–33. doi: 10.1016/j.cell.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Samir S, Papadopoulos S, Scheibe RJ, et al Activity and distribution of intracellular carbonic anhydrase II and their effects on the transport activity of anion exchanger AE1/SLC4A1. J Physiol. 2013;591:4963–82. doi: 10.1113/jphysiol.2013.251181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Symmans WF, Hatzis C, Sotiriou C, et al Genomic index of sensitivity to endocrine therapy for breast cancer. J Clin Oncol. 2010;28:4111–9. doi: 10.1200/jco.2010.28.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piccart M, van’t Veer LJ, Poncet C, et al 70-gene signature as an aid for treatment decisions in early breast cancer: updated results of the phase 3 randomised MINDACT trial with an exploratory analysis by age. Lancet Oncol. 2021;22:476–88. doi: 10.1016/s1470-2045(21)00007-3. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez-Vega F, Mina M, Armenia J, et al Oncogenic signaling pathways in The Cancer Genome Atlas. Cell. 2018;173:321–37.e10. doi: 10.1016/j.cell.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hua H, Kong Q, Zhang H, et al Targeting mTOR for cancer therapy. J Hematol Oncol. 2019;12:71. doi: 10.1186/s13045-019-0754-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Leary B, Finn RS, Turner NC Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13:417–30. doi: 10.1038/nrclinonc.2016.26. [DOI] [PubMed] [Google Scholar]
- 36.Xie XP, Laks DR, Sun D, et al Quiescent human glioblastoma cancer stem cells drive tumor initiation, expansion, and recurrence following chemotherapy. Dev Cell. 2022;57:32–46.e8. doi: 10.1016/j.devcel.2021.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butti R, Das S, Gunasekaran VP, et al Receptor tyrosine kinases (RTKs) in breast cancer: signaling, therapeutic implications and challenges. Mol Cancer. 2018;17:34. doi: 10.1186/s12943-018-0797-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glaviano A, Foo ASC, Lam HY, et al PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol Cancer. 2023;22:138. doi: 10.1186/s12943-023-01827-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spring LM, Wander SA, Andre F, et al Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: past, present, and future. Lancet. 2020;395:817–27. doi: 10.1016/s0140-6736(20)30165-3. [DOI] [PubMed] [Google Scholar]
- 40.Verret B, Cortes J, Bachelot T, et al. Efficacy of PI3K inhibitors in advanced breast cancer. Ann Oncol 2019;30(Suppl_10):x12-20.
- 41.André F, Ciruelos E, Rubovszky G, et al Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380:1929–40. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 42.Baselga J, Campone M, Piccart M, et al Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Albrengues J, Shields MA, Ng D, et al Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361:eaao4227. doi: 10.1126/science.aao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Emens LA, Cimino-Mathews A Quiescent cancer cells: Therapeutic targets to overcome immunotherapy resistance. Med. 2022;3:358–60. doi: 10.1016/j.medj.2022.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Hu J, Sánchez-Rivera FJ, Wang Z, et al STING inhibits the reactivation of dormant metastasis in lung adenocarcinoma. Nature. 2023;616:806–13. doi: 10.1038/s41586-023-05880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quiescent breast cancer cells are resistant to T-cell killing. Cancer Discov 2022;12:OF5.
- 47.Razavi P, Chang MT, Xu G, et al The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell. 2018;34:427–38.e6. doi: 10.1016/j.ccell.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.