Abstract
Background
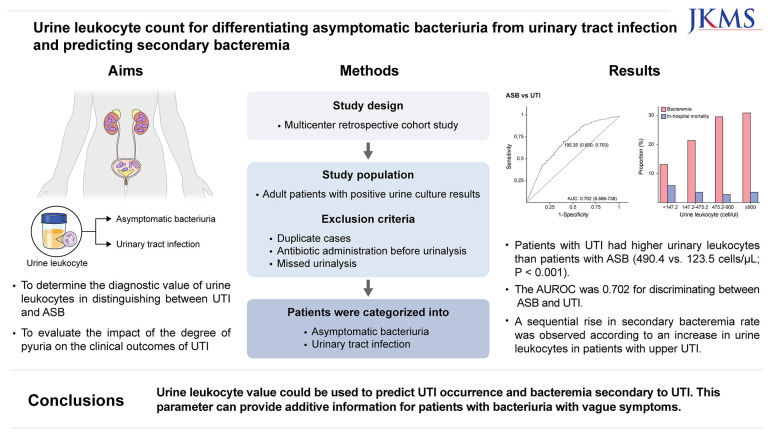
Differentiating between asymptomatic bacteriuria (ASB) and urinary tract infection (UTI) is difficult in patients who have difficulty communicating their symptoms. This study aimed to evaluate the diagnostic accuracy of urine leukocytes in distinguishing between UTI and ASB, and the clinical outcomes of patients with UTI according to the degree of pyuria.
Methods
This retrospective cohort study included patients with positive urine cultures between July 2022 and June 2023 at two hospitals. UTI and ASB were diagnosed through a comprehensive review of medical records. We evaluated the differences in urine leukocyte counts between patients with UTI and ASB. The diagnostic performance of urine leukocytes to differentiate between UTI and ASB was evaluated. To investigate the clinical outcomes based on the degree of pyuria, we classified patients with upper UTI according to their urine leukocyte counts.
Results
Of the 1,793 eligible patients with bacteriuria included, 1,464 had UTI and 329 had ASB. Patients with UTI had higher urinary leukocytes than patients with ASB did (490.4 vs. 123.5 cells/µL; P < 0.001). The area under the receiver operating characteristic curve was 0.702 for discriminating between ASB and UTI. The optimal urine leukocyte cutoff was 195.35 cells/µL, with a sensitivity and specificity of 0.70 and 0.60, respectively. A sequential rise in secondary bacteremia rate was observed according to an increase in urine leukocytes in patients with upper UTI, whereas in-hospital mortality showed no corresponding trend.
Conclusion
Urine leukocyte counts could be used to predict UTI occurrence and bacteremia secondary to UTI. Higher degrees of pyuria were associated with bacteremia but not with mortality. Urine leukocyte counts can provide additive information for patients with bacteriuria with vague symptoms.
Keywords: Asymptomatic Bacteriuria, Urinary Tract Infection, Leukocyte, Urine, Pyuria, Bacteremia
Graphical Abstract
INTRODUCTION
Urinary tract infection (UTI) is a common medical condition that affects 60% of women at least once in their lifetime.1 UTI can be diagnosed based on the presence of pyuria along with clinical symptoms.2 The positive predictive value of pyuria for lower UTI, such as acute cystitis, is 90% when new-onset frequency, dysuria, and urgency are present3 Unlike UTI, asymptomatic bacteriuria (ASB) is a benign condition that usually does not require antibiotic treatment. ASB is diagnosed when the urine has a marked amount of bacteria without UTI symptoms or signs.4
However, in practice, differentiating between ASB and UTI is difficult. First, diagnosis is challenging in patients with cognitive impairment who may have difficulty communicating their symptoms. Second, the symptoms of lower UTI are sometimes vague and can be confused with those caused by functional urinary tract disorders or superficial genital infections.5 Finally, the high prevalence of ASB complicates UTI diagnosis. The prevalence of ASB in women aged ≥ 80 years is 20% and approaches 100% in those using long-term indwelling catheters.6,7 Therefore, these conditions can lead to UTI misdiagnosis and inappropriate antibiotic use in ASB, resulting in adverse drug effects, Clostridioides difficile infection, and infection from antibiotic-resistant pathogens.8,9,10
Pyuria has been used for UTI diagnosis because of having high sensitivity for diagnosing UTIs.11 The current cutoff value for pyuria is 10 leukocytes/µL, based on a study in which the leukocyte excretion rate was measured in women with bacteriuria.12 Using current cutoffs, 90% of older women with ASB are found to have pyuria, which lowers the positive predictive value of pyuria in diagnosing UTIs in patients who are unable to articulate symptoms.13,14 Bilsen et al.15 suggested that a cutoff value of 264 leukocytes/µL is optimal for differentiating between UTI and ASB. However, the study included only women aged ≥ 65 years and excluded patients with indwelling urinary catheters and those receiving immunosuppressants. These conditions are frequently encountered when differentiating UTIs, thus validation using real-world data from these populations is needed to determine the diagnostic accuracy of pyuria for the diagnosis of UTIs.
Therefore, this study aimed to determine the diagnostic value of urine leukocytes in distinguishing between UTI and ASB using a real-world cohort that included patients who underwent urinary catheterization and to evaluate the impact of the degree of pyuria on the clinical outcomes of UTI, offering useful information to clinicians.
METHODS
Study design and study population
This retrospective study was performed between July 2022 and June 2023 at two hospitals in Korea. The study hospitals are both university-affiliated hospitals, with 708 and 638 beds, respectively. We identified patients with positive urine culture results, and their medical records were retrospectively reviewed by data reviewers at each hospital. The study included only patients aged ≥ 16 years. The exclusion criteria were duplicate cases, concurrent other infections, antibiotic administration before the urine test, incomplete information, and missed urinalysis. Patients with ≥ 3 organisms identified from a urine culture, bacterial counts < 105/mL for ASB (< 102/mL with a urinary catheter), or candiduria were also excluded.16,17
Variables and definition
The collected data included age, sex, body mass index, date of death, comorbidities, use of a urinary catheter, microbiological results, urinalysis results, and antibiotic use. Urine leukocytes were quantified using automated microscopy of mid-stream urine, and the upper limit of detection was set at 900 cells/µL. Automated microscopy was performed using the Atellica® 1500 Automated Urinalysis System (Siemens Healthineers, Forchheim, Germany) and the UC-3500 and UF-5000 (Sysmex, Kobe, Japan).18,19 For patients with urinary catheters, sampling port was used for sampling following manufacturer’s instructions.20,21 The use of a urinary catheter was defined as the retention of any urological device for at least 2 days before culture. The types of urinary catheter included Foley, suprapubic indwelling, percutaneous nephrostomy, double-J catheters, and clean intermittent catheterization.
ASB was defined as a bacterial count ≥ 105/mL (≥ 102/mL for patients with urinary catheters) from urine culture without symptoms or signs of UTI. Patients with bacteriuria are classified as having lower UTI (cystitis) when they presented with one or more of the following clinical features: urinary frequency, urgency, dysuria, suprapubic pain, or gross hematuria. Patients with bacteriuria were diagnosed with upper UTI if the image results were consistent with upper UTI or when they presented with one or more of the following symptoms or signs: unexplained fever, shock, quick Sequential Organ Failure Assessment (qSOFA) ≥ 2, flank pain, or costovertebral angle tenderness.22 At each center, investigators (HWP, IJY, JHH, and JES) initially classified the cases. For cases with discrepancies between the primary investigator’s judgment and the attending physician’s diagnosis, secondary investigators (YCK and YKK) made the final evaluation and decision.
Outcome variables for the upper UTI were secondary bacteremia and in-hospital mortality.23,24 Secondary bacteremia was defined as the identification of the same organism in both the urine and blood cultures within three days.23
Microbiological tests
Microbiological tests were conducted on urine samples collected mid-stream in sterile containers. For patients with urinary catheters, sampling port was used for sampling following manufacturer’s instructions. Non-centrifuged urine (1 µL) was inoculated onto blood agar and MacConkey agar plates and incubated at 35°C for 1 day. A culture result was deemed positive in cases of growth ≥ 104 CFU/mL, and an identification test was performed. A Bruker Biotyper matrix-assisted laser desorption ionization time-of-flight mass spectrometry system was used for the identification of microbes (Bruker Daltonics, Bremen, Germany).
Statistical analysis
Continuous variables were presented as mean ± standard deviation for normally distributed data or as median (interquartile range [IQR]) for non-normally distributed data. Categorical variables were presented as count (percentage). Two-tailed independent t-tests or Mann–Whitney U tests were used to compare continuous variables, whereas Pearson’s χ2 or Fisher’s exact were used to compare categorical variables. The Shapiro–Wilk test was performed to determine normality, and non-parametric tests were used to analyze non-normally distributed data.
Subgroups were divided according to sex, age, use of urinary catheters, and microorganisms. Differences in urine leukocyte counts and diagnostic performance were evaluated for each subgroup. The optimal cutoff value was determined using the Youden index in all patients. Sensitivity and specificity with 95% confidence intervals (CIs) were assessed using the calculated cutoff value and the conventional cutoff value of 10 cells/µL. The area under the receiver operating characteristic (ROC) curve (AUC) was calculated for each subgroup. DeLong’s test was used to compare AUCs between the two ROC curves.
Patients with upper UTI were grouped according to urine leukocyte counts to evaluate the clinical outcomes based on the degree of pyuria. Quartiles of urine leukocyte count were used to divide the groups. In-hospital mortality and secondary bacteremia, were used to assess the clinical outcomes. Multivariate logistic regression was performed to determine the association between the degree of pyuria and clinical outcomes. Variables with P values < 0.05 in univariable analysis were included in the multivariable logistic regression model. The variables of sex and urinary catheter were not significant in the univariable analysis, however, they were included in the multivariate analysis at the discretion of the investigators given the clinical importance of the variables for UTI. Differences were considered statistically significant at P < 0.05. No adjustments were made for multiple comparisons. All statistical analyses were performed using R, V.4.2.2 (The R Foundation for Statistical Computing, Vienna, Austria).
Ethics statement
This study was approved by the Institutional Review Board of Yonsei University Health System Clinical Trial Center (9-2023-0127) and Inje University Ilsan Paik Hospital (2024-09-001). The need for patient consent was waived owing to the retrospective nature of the study.
RESULTS
Clinical and microbiological characteristics of patients
A total of 4,418 positive urine culture cases were identified (Supplementary Fig. 1). Of these, 1,793 eligible patients were included in the analysis. Among this cohort, 1,464 patients (81.7%) had UTI, whereas 329 (18.3%) had ASB. Among the UTI cases, lower and upper UTIs accounted for 26.1% and 73.9%, respectively.
The median age of patients with UTI was 79 years (IQR, 68.0–85.0 years), whereas the median age of patients with ASB was 68 years (IQR, 55.0–82.6 years; P < 0.001) (Table 1). Distribution with respect to sex did not differ significantly. The use of urinary catheters was more prevalent in patients with ASB than in patients with UTI (34.0% vs. 20.8%; P < 0.001). Most urinary catheters were Foley catheters, and clean intermittent catheterization was performed in six patients in the UTI group and in one patient in the ASB group. Patients with ASB had a higher proportion of comorbidities, including diabetes, cerebrovascular disease, hemiplegia, and congestive heart failure, compared with patients with UTI.
Table 1. Comparison of clinical characteristics between patients with ASB and UTI.
| Characteristics | Total (N = 1,793) | ASB (n = 329) | UTI (n = 1,464) | P value | |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age, yr | 74.9 (56.7–83.0) | 79.0 (68.0–85.0) | 73.0 (55.0–82.6) | < 0.001 | |
| < 60 | 516 (28.8) | 56 (17.0) | 460 (31.4) | < 0.001 | |
| 60–70 | 246 (13.7) | 39 (11.9) | 207 (14.1) | 0.276 | |
| 70–80 | 374 (20.9) | 87 (26.4) | 287 (19.6) | 0.006 | |
| ≥ 80 | 657 (36.6) | 147 (44.7) | 510 (34.8) | 0.001 | |
| Female sex | 1,363 (76.1) | 248 (75.4) | 1,115 (76.2) | 0.749 | |
| BMI | 22.8 (20.1–25.7) | 23.5 (20.5–26.1) | 22.6 (20.1–25.6) | 0.106 | |
| Lower UTI | 382 (21.3) | - | 382 (26.1) | - | |
| Upper UTI | 1,082 (60.3) | - | 1,082 (73.9) | - | |
| Use of urinary tract catheter | 417 (23.3) | 112 (34.0) | 305 (20.8) | < 0.001 | |
| Receipt of antibiotics | 1,519 (84.7) | 76 (23.1) | 1,443 (98.6) | < 0.001 | |
| Urine sample collection location | |||||
| General ward | 1,346 (75.1) | 289 (87.8) | 1,057 (72.2) | < 0.001 | |
| Emergency department | 418 (23.3) | 34 (10.3) | 384 (26.2) | < 0.001 | |
| Outpatient clinic | 29 (1.6) | 6 (1.8) | 23 (1.6) | 0.743 | |
| Comorbidities | |||||
| Diabetes mellitus | 568 (31.7) | 122 (37.1) | 446 (30.5) | 0.020 | |
| Cerebrovascular disease | 355 (19.8) | 98 (29.8) | 257 (17.6) | < 0.001 | |
| Hemiplegia | 102 (5.7) | 30 (9.1) | 72 (4.9) | 0.003 | |
| Congestive heart failure | 182 (10.2) | 46 (14.0) | 136 (9.3) | 0.011 | |
| Renal disease | 82 (4.6) | 19 (5.8) | 63 (4.3) | 0.250 | |
| Chronic liver disease | 53 (3.0) | 10 (3.0) | 43 (2.9) | 0.921 | |
| Cancer | 306 (17.1) | 67 (20.4) | 239 (16.3) | 0.078 | |
| Charlson comorbidity index | 1.0 (0.0–3.0) | 2.0 (1.0–3.0) | 1.0 (0.0–3.0) | < 0.001 | |
| Microorganisms isolated in urine culture | |||||
| Escherichia coli | 1,215 (67.8) | 161 (48.9) | 1,054 (72.0) | < 0.001 | |
| Klebsiella pneumoniae | 174 (9.7) | 36 (10.9) | 138 (9.4) | 0.401 | |
| Pseudomonas aeruginosa | 44 (2.5) | 12 (3.6) | 32 (2.2) | 0.122 | |
| Enterococcus spp. | 153 (8.5) | 71 (21.6) | 82 (5.6) | < 0.001 | |
| Othersa | 262 (14.6) | 61 (18.5) | 201 (13.7) | 0.026 | |
| Secondary bacteremia | 238 (13.3) | - | 238 (22.7) | - | |
| In-hospital mortality | 57 (3.2) | 9 (2.7) | 48 (3.3) | 0.612 | |
Data are expressed as median (IQR) or number (%).
ASB = asymptomatic bacteriuria, UTI = urinary tract infection, BMI = body mass index, IQR = interquartile range.
aOthers include Staphylococcus aureus, coagulase-negative Staphylococci, Citrobacter spp., Proteus spp., Serratia spp., and Acinetobacter baumannii.
Regarding the microbiological characteristics, Escherichia coli was the most common microorganism in both groups; however, the rate of E. coli infection was significantly higher in the UTI group than in the ASB group (72.0% vs. 48.9%; P < 0.001). Patients with ASB showed higher rates of Enterococcus species infections, compared with patients with UTI (21.6% vs. 5.6%; P < 0.001). The incidences of Klebsiella pneumoniae and Pseudomonas aeruginosa infections were not significantly different between the two groups.
Urine leukocyte counts
Urine leukocyte counts were compared between the ASB and UTI groups (Supplementary Table 1). Overall, urine leukocyte counts were significantly higher in the UTI group than in the ASB group. The median count of urine leukocytes was 490.4 cells/µL (IQR, 147.2–900.0 cells/µL) in the UTI group, whereas the value was 123.5 cells/µL (IQR, 18.1–480.4 cells/µL) in the ASB group.
In all subgroups, urine leukocyte counts were significantly higher in the UTI group than in the ASB group; however, differences were observed in the degree depending on the causative organism. Patients with P. aeruginosa infections had higher urine leukocyte counts (median, 900.0 cells/µL; IQR, 441.3–900.0 cells/µL) and those with enterococcal infections had lower urine leukocyte counts (median, 142.5 cells/µL; IQR, 34.5–787.7 cells/µL) than those in the remaining groups.
Diagnostic accuracy
Fig. 1 shows the ROC curves for urine leukocytes. The AUC for discriminating ASB and UTI was 0.702 (95% CI, 0.666–0.738). The AUCs for discriminating upper and lower UTI from ASB were 0.701 (95% CI, 0.664–0.738) and 0.705 (95% CI, 0.662–0.748), respectively. No significant difference was observed between the two values based on the DeLong’s test (P = 0.882).
Fig. 1. Receiver operating characteristic curves of urine leukocytes for the diagnosis of UTI. The optimal cutoff points were marked with their corresponding values (cells/µL), specificity, and sensitivity.
ASB = asymptomatic bacteriuria, UTI = urinary tract infection, AUC = area under the receiver operating characteristic curve.
Diagnostic performance was evaluated using 195.35 cells/µL as a cutoff value (Table 2). In all patients, the sensitivity and specificity were 0.70 (95% CI, 0.68–0.73) and 0.60 (95% CI, 0.54–0.66), respectively. Patients with a urinary catheter showed a sensitivity of 0.66 (95% CI, 0.59–0.71) and specificity of 0.53 (95% CI, 0.43–0.64), with an AUC of 0.645 (95% CI, 0.578–0.711).
Table 2. Diagnostic statistics of urine leukocytes for the diagnosis of UTI with cutoff 195.35 cells/µL.
| Variables | Cutoff = 195.35 cells/µL | AUC (95% CI) | |
|---|---|---|---|
| Sensitivity (95% CI) | Specificity (95% CI) | ||
| Overall | 0.70 (0.68–0.73) | 0.60 (0.54–0.66) | 0.702 (0.666–0.738) |
| Age ≥ 65 yr | 0.68 (0.64–0.71) | 0.58 (0.51–0.65) | 0.676 (0.634–0.719) |
| Age < 65 yr | 0.75 (0.70–0.79) | 0.68 (0.54–0.80) | 0.773 (0.706–0.841) |
| Male | 0.76 (0.70–0.80) | 0.67 (0.54–0.79) | 0.766 (0.697–0.835) |
| Female | 0.69 (0.66–0.72) | 0.58 (0.51–0.65) | 0.682 (0.640–0.724) |
| Upper UTI | 0.70 (0.67–0.73) | 0.60 (0.54–0.66) | 0.701 (0.664–0.738) |
| Lower UTI | 0.72 (0.66–0.77) | 0.60 (0.54–0.66) | 0.705 (0.662–0.748) |
| With urinary catheter | 0.66 (0.59–0.71) | 0.53 (0.43–0.64) | 0.645 (0.578–0.711) |
| Without urinary catheter | 0.72 (0.69–0.75) | 0.64 (0.56–0.71) | 0.725 (0.681–0.769) |
| Escherichia coli | 0.73 (0.69–0.76) | 0.56 (0.47–0.65) | 0.703 (0.654–0.751) |
| Klebsiella pneumoniae | 0.70 (0.60–0.79) | 0.68 (0.49–0.83) | 0.691 (0.586–0.796) |
| Pseudomonas aeruginosa | 0.87 (0.66–0.97) | 0.27 (0.06–0.61) | 0.723 (0.543–0.903) |
| Enterococcus spp. | 0.45 (0.33–0.58) | 0.71 (0.57–0.83) | 0.675 (0.575–0.775) |
AUC = area under receiver operating characteristic curve, CI = confidence interval, UTI = urinary tract infection.
Patients without urinary catheters showed a significantly higher AUC (0.725; 95% CI, 0.681–0.769) than did those with urinary catheters (DeLong’s test: P = 0.050). In the subgroup analysis for P. aeruginosa infections, the sensitivity was 0.87 (95% CI, 0.66–0.97); however, the specificity was low, at 0.27 (95% CI, 0.06–0.61). For patients with Enterococcus species infections, the sensitivity was low, at 0.45 (95% CI, 0.33–0.58), whereas the specificity was 0.71 (95% CI, 0.57–0.83). Using a cutoff value of 10.00 cells/µL, the sensitivity and specificity were 0.97 (95% CI, 0.95–0.98) and 0.15 (95% CI, 0.11–0.20), respectively (Supplementary Table 2).
Clinical outcomes based on the degree of pyuria
The clinical outcomes of upper UTI were evaluated with respect to the degree of pyuria. A sequential increase in the incidence of secondary bacteremia was observed across the quartiles of urine leukocytes, ranging from 13.2% in the lowest quartile to 30.9% in the highest quartile (Fig. 2). In contrast, no discernible trend was observed in in-hospital mortality across the urine leukocyte quartiles.
Fig. 2. Clinical outcomes of patients with upper urinary tract infection according to the degree of pyuria.
Furthermore, multivariate logistic regression analysis revealed a sequential increase in the adjusted odds ratio (aOR) according to the degree of pyuria (Table 3). The aOR for bacteremia was 1.87 (P = 0.024), 2.81 (P = 0.001), and 3.01 (P < 0.001) for the second, third, and the highest quartiles, respectively, with the lowest quartile as the reference. The results of the univariable analysis are included in Supplementary Table 3.
Table 3. Multivariate analysis of risk factors for bacteremia in patients with upper UTI.
| Variables | Multivariate analysis | ||
|---|---|---|---|
| aOR (95% CI) | P value | ||
| Age | 1.02 (1.01–1.03) | 0.002 | |
| Female sex | 0.88 (0.60–1.29) | 0.508 | |
| Urinary catheter | 0.75 (0.48–1.15) | 0.190 | |
| Diabetes mellitus | 1.47 (1.03–2.09) | 0.033 | |
| Microorganisms | |||
| Escherichia coli | 1.37 (0.86–2.23) | 0.195 | |
| Othersa | 0.41 (0.19–0.83) | 0.018 | |
| Urine leukocytes, cells/µL | |||
| < 147.2 | 1.00 (Reference) | ||
| 147.2–475.2 | 1.87 (1.09–3.26) | 0.024 | |
| 475.2–900.0 | 2.81 (1.58–5.07) | 0.001 | |
| ≥ 900 | 3.01 (1.85–5.05) | < 0.001 | |
Variables with a P value of < 0.05 in univariable analysis were included in multivariable analysis. The variables of sex and urinary catheter were not significant in the univariable analysis, however, they were included in the multivariate analysis at the discretion of the investigators given the clinical importance of the variables for UTI.
aOR = adjusted odds ratio, CI = confidence interval, UTI = urinary tract infection.
aOthers include Staphylococcus aureus, coagulase-negative Staphylococci, Citrobacter spp., Proteus spp., Serratia spp., and Acinetobacter baumannii.
DISCUSSION
This study was conducted to evaluate the diagnostic accuracy of urine leukocytes for differentiating between UTI and ASB. The AUC for discriminating ASB and UTI was 0.702; however, it was significantly lower (0.645) in those with a urinary catheter compared with those without. Differences were observed in sensitivity and specificity depending on the causative organism, with low specificity for P. aeruginosa infections and low sensitivity for enterococcal infections. Higher degrees of pyuria were associated with bacteremia but not with mortality.
This study is significant because it validates real-world data, showing that the quantitative value of urine leukocytes can help differentiate between UTI and ASB. The AUC reported in the present study is lower than that reported in a previous study (0.93)15; two reasons are likely responsible for this low AUC. First, the previous study included only older women, and immunocompromised patients and patients with urinary catheters were excluded from the analysis; these aspects limit the generalizability of the study. Additionally, the control group included patients with culture-negative and mixed flora results, and only 18% population of the control group had ASB. The low inclusion rate of patients with ASB contributed to the high AUC value, making it difficult to determine the diagnostic accuracy of urine leukocytes in differentiating ASB from UTI. Our study demonstrates that urine leukocytes can be used to distinguish between UTI and ASB in different patient populations. This parameter is likely to be useful as an additional marker to existing diagnostic criteria when bacteriuria is difficult to classify.
Patients with P. aeruginosa infections had higher urine leukocyte counts, whereas those with enterococcal infections had lower values. Monsen and Rydén25 and Kim et al.26 quantified urine leukocytes according to the microorganisms present in UTI. Monsen and Rydén25 reported that infections caused by enterococci had the lowest value (120 cells/µL), whereas those caused by P. aeruginosa had the highest value (1,175 cells/µL). Kim et al.26 also reported that enterococcal infections resulted in the lowest urine leukocyte count (33.9 cells/µL), compared with that by other strains. These results are consistent with our findings. Virulence factors, such as exotoxins, and at least four different proteases, have been reported in Pseudomonas spp., which can cause bleeding and tissue necrosis.27,28,29 On the contrary, enterococci are generally considered to be less virulent, suggesting that the virulence of the strain in UTI influences the degree of inflammation of the urinary tract.30,31 These findings suggest that urine leukocyte counts should be interpreted according to the causative organism.
To the best of our knowledge, no studies have been conducted using urine leukocyte counts to differentiate ASB from UTI in patients with urinary catheters. Tambyah and Maki32 reported a lower sensitivity of pyuria in catheter-associated UTI, with less urine leukocyte counts observed for gram-positive cocci and yeast infections. However, the definition of catheter-associated UTI in that study was a positive urine culture result regardless of symptoms, and most patients were asymptomatic. Therefore, distinguishing between ASB and UTI using pyuria based on the results of this study may be challenging. In our study, a low AUC (0.645) was observed for the use of urinary catheters. The catheters may have caused mucosal inflammation, resulting in poor diagnostic performance for pyuria; however, to validate this finding, further studies are required.
Inappropriate use of antibiotics among patients with UTI is a common issue. Gupta et al.33 reported that antibiotics were used in 25% of asymptomatic patients with pyuria who underwent routine preoperative urinalysis. In our study, antibiotics were used in 23.1% of patients with ASB, mostly without a clear indication. Patient’s symptoms play a crucial role in diagnosing UTI; however, many inpatients are often unable to effectively communicate their symptoms owing to various systemic conditions such as delirium and sedation. Lower UTIs typically lack systemic manifestations. No standardized method is currently available to diagnose this condition in patients who are unable to report symptoms accurately. This study revealed comparable diagnostic accuracy in differentiating ASB from lower UTI, addressing a current unmet need that has significant value in clinical and antibiotic stewardship.
This study showed an increased risk of secondary bacteremia with increasing urine leukocyte counts but no effect on mortality. Armbruster et al.34 found that high urinary cytokine and chemokine levels are predictors of UTI severity in mouse models. In UTI, toll-like receptor activation induces a potent proinflammatory cytokine response that attracts neutrophils to the site of infection.35 Consistent with previous findings, the results of our study could be attributed to increased urinary cytokines by disease severity, resulting in increased neutrophil attraction. Our results may provide additional diagnostic value of urinalysis, which is inexpensive and universally performed in clinical practice. Therefore, the additional value of urine leukocyte tests as predictors of disease severity in UTI is significant.
The strengths of our study include the use of a UTI definition with symptoms and signs of UTI instead of a urine culture. Second, we evaluated the diagnostic accuracy using real-world data, including most patients, regardless of age, sex, and urinary catheterization status. Finally, we used ASB as a control group, which addressed a significant unmet clinical need.
However, this study has limitations. First, it included only patients hospitalized in university-affiliated hospitals, which may be prone to selection bias. Second, the retrospective design of the study may have introduced an information bias, particularly for urinary symptoms, which could have been missed in the medical records and led to misclassification of UTI and ASB. In addition, diagnosing upper UTIs based on unexplained fever, shock, or elevated qSOFA in patients with bacteriuria may lead to potential overdiagnosis. However, the study protocol was substantiated by experienced researchers and the possibility of concurrent infections was thoroughly investigated, which reduced the likelihood of misclassification. Third, residual confounding factors may have been present. Other important confounders, including urolithiasis, underlying urinary disorders (e.g., urinary incontinence and benign prostatic hyperplasia), immunocompromised status, treatment factors such as antibiotics, and progression to sepsis, were not considered in the analysis. Finally, most urinary catheters were Foley catheters, which may limit the generalizability of the findings to other types of devices. To address these limitations, future research that includes prospective data on urinary symptoms and encompasses possible confounders, such as urolithiasis and the use of immunosuppressive agents, is needed.
In conclusion, urine leukocyte counts can serve as predictors of the occurrence of UTI and secondary bacteremia resulting from UTI. This finding may be beneficial in providing additional information to conventional diagnostic criteria for patients with bacteriuria who present with unclear signs or vague UTI symptoms. Further research focusing on the impact of using a higher pyuria cutoff value on changes in antibiotic use or clinical outcomes is necessary.
ACKNOWLEDGMENTS
We appreciate the Medical Illustration & Design (MID) team, a member of Medical Research Support Services of Yonsei University College of Medicine, for their excellent support with medical illustration.
Footnotes
Funding: This work was supported by a new faculty research seed money grant of Yonsei University College of Medicine for 2024 (2024-32-0049).
Disclosure: The authors have no potential conflicts of interest to disclose.
Data Availability Statement: The datasets used for the current study are available from the corresponding author upon reasonable request.
- Conceptualization: Lee Y.
- Data curation: Park HW, Yun IJ, Hyun J, Song JE.
- Formal analysis: Lee Y.
- Funding acquisition: Kim YC.
- Investigation: Lee Y.
- Supervision: Kwak YG, Kim YC.
- Writing - original draft: Lee Y.
- Writing - review & editing: Lee Y, Hyun J, Song JE, Kwak YG, Kim YC.
SUPPLEMENTARY MATERIALS
Comparison of urine leukocyte (cells/µL) between patients with UTI and ASB
Diagnostic statistics of urine leukocytes for the diagnosis of UTI with cutoff 10.00 cells/µL
Univariable analysis of risk factors for bacteremia in patients with upper urinary tract infection
Flowchart of the study.
References
- 1.Nicolle LE. Uncomplicated urinary tract infection in adults including uncomplicated pyelonephritis. Urol Clin North Am. 2008;35(1):1–12. doi: 10.1016/j.ucl.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Stamm WE. Measurement of pyuria and its relation to bacteriuria. Am J Med. 1983;75(1):53–58. doi: 10.1016/0002-9343(83)90073-6. [DOI] [PubMed] [Google Scholar]
- 3.Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S. Does this woman have an acute uncomplicated urinary tract infection? JAMA. 2002;287(20):2701–2710. doi: 10.1001/jama.287.20.2701. [DOI] [PubMed] [Google Scholar]
- 4.Nicolle LE. Asymptomatic bacteriuria. Curr Opin Infect Dis. 2014;27(1):90–96. doi: 10.1097/QCO.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 5.Foxman B. Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am. 2014;28(1):1–13. doi: 10.1016/j.idc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Nicolle LE. Urinary tract infections in the elderly. Clin Geriatr Med. 2009;25(3):423–436. doi: 10.1016/j.cger.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Nicolle LE. Asymptomatic bacteriuria and bacterial interference. Microbiol Spectr. 2015;3(5) doi: 10.1128/microbiolspec.UTI-0001-2012. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell SL, Shaffer ML, Loeb MB, Givens JL, Habtemariam D, Kiely DK, et al. Infection management and multidrug-resistant organisms in nursing home residents with advanced dementia. JAMA Intern Med. 2014;174(10):1660–1667. doi: 10.1001/jamainternmed.2014.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rotjanapan P, Dosa D, Thomas KS. Potentially inappropriate treatment of urinary tract infections in two Rhode Island nursing homes. Arch Intern Med. 2011;171(5):438–443. doi: 10.1001/archinternmed.2011.13. [DOI] [PubMed] [Google Scholar]
- 10.Lin E, Bhusal Y, Horwitz D, Shelburne SA, 3rd, Trautner BW. Overtreatment of enterococcal bacteriuria. Arch Intern Med. 2012;172(1):33–38. doi: 10.1001/archinternmed.2011.565. [DOI] [PubMed] [Google Scholar]
- 11.Lachs MS, Nachamkin I, Edelstein PH, Goldman J, Feinstein AR, Schwartz JS. Spectrum bias in the evaluation of diagnostic tests: lessons from the rapid dipstick test for urinary tract infection. Ann Intern Med. 1992;117(2):135–140. doi: 10.7326/0003-4819-117-2-135. [DOI] [PubMed] [Google Scholar]
- 12.Mabeck CE. Studies in urinary tract infections. IV. Urinary leucocyte excretion in bacteriuria. Acta Med Scand. 1969;186(1-6):193–198. doi: 10.1111/j.0954-6820.1969.tb01463.x. [DOI] [PubMed] [Google Scholar]
- 13.Boscia JA, Abrutyn E, Levison ME, Pitsakis PG, Kaye D. Pyuria and asymptomatic bacteriuria in elderly ambulatory women. Ann Intern Med. 1989;110(5):404–405. doi: 10.7326/0003-4819-110-5-404. [DOI] [PubMed] [Google Scholar]
- 14.Hooton TM, Roberts PL, Cox ME, Stapleton AE. Voided midstream urine culture and acute cystitis in premenopausal women. N Engl J Med. 2013;369(20):1883–1891. doi: 10.1056/NEJMoa1302186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bilsen MP, Aantjes MJ, van Andel E, Stalenhoef JE, van Nieuwkoop C, Leyten EM, et al. Current pyuria cutoffs promote inappropriate urinary tract infection diagnosis in older women. Clin Infect Dis. 2023;76(12):2070–2076. doi: 10.1093/cid/ciad099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40(5):643–654. doi: 10.1086/427507. [DOI] [PubMed] [Google Scholar]
- 17.Whelan P, Nelson A, Kim CJ, Tabib C, Preminger G, Turner NA, et al. Investigating risk factors for urine culture contamination in outpatient clinics: a new avenue for diagnostic stewardship. Antimicrob Steward Healthc Epidemiol. 2022;2(1):e29. doi: 10.1017/ash.2021.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iezzi P, Cappellini F, Intra J, Carnicelli S, Fossati L, Basta F, et al. The diagnostic performances of the Atellica® 1500 automated urinalysis system for ruling out bacterial urinary tract infection. Clin Chim Acta. 2023;548:117494. doi: 10.1016/j.cca.2023.117494. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Zhang Z, Diao Y, Wang W, Zhu Y, Li J, et al. Combination of UC-3500 and UF-5000 as a quick and effective method to exclude bacterial urinary tract infection. J Infect Chemother. 2023;29(7):667–672. doi: 10.1016/j.jiac.2023.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Shah PS, Cannon JP, Sullivan CL, Nemchausky B, Pachucki CT. Controlling antimicrobial use and decreasing microbiological laboratory tests for urinary tract infections in spinal-cord-injury patients with chronic indwelling catheters. Am J Health Syst Pharm. 2005;62(1):74–77. doi: 10.1093/ajhp/62.1.74. [DOI] [PubMed] [Google Scholar]
- 21.Roodhouse A, Wellsted A. Safety in urine sampling: maintaining an infection-free environment. Br J Nurs. 2006;15(16):870–872. doi: 10.12968/bjon.2006.15.16.21851. [DOI] [PubMed] [Google Scholar]
- 22.Madrazo M, López-Cruz I, Zaragoza R, Piles L, Eiros JM, Alberola J, et al. Prognostic accuracy of Quick SOFA in older adults hospitalised with community acquired urinary tract infection. Int J Clin Pract. 2021;75(10):e14620. doi: 10.1111/ijcp.14620. [DOI] [PubMed] [Google Scholar]
- 23.Choi MH, Kim D, Park Y, Jeong SH. Impact of urinary tract infection-causative microorganisms on the progression to bloodstream infection: a propensity score-matched analysis. J Infect. 2022;85(5):513–518. doi: 10.1016/j.jinf.2022.08.039. [DOI] [PubMed] [Google Scholar]
- 24.Foxman B, Klemstine KL, Brown PD. Acute pyelonephritis in US hospitals in 1997: hospitalization and in-hospital mortality. Ann Epidemiol. 2003;13(2):144–150. doi: 10.1016/s1047-2797(02)00272-7. [DOI] [PubMed] [Google Scholar]
- 25.Monsen T, Rydén P. Flow cytometry analysis using Sysmex UF-1000i classifies uropathogens based on bacterial, leukocyte, and erythrocyte counts in urine specimens among patients with urinary tract infections. J Clin Microbiol. 2015;53(2):539–545. doi: 10.1128/JCM.01974-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H, Kim HR, Kim TH, Lee MK. Age-specific cutoffs of the Sysmex UF-1000i automated urine analyzer for rapid screening of urinary tract infections in outpatients. Ann Lab Med. 2019;39(3):322–326. doi: 10.3343/alm.2019.39.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ben Haj Khalifa A, Moissenet D, Vu Thien H, Khedher M. [Virulence factors in Pseudomonas aeruginosa: mechanisms and modes of regulation] Ann Biol Clin (Paris) 2011;69(4):393–403. doi: 10.1684/abc.2011.0589. [DOI] [PubMed] [Google Scholar]
- 28.Mittal R, Aggarwal S, Sharma S, Chhibber S, Harjai K. Urinary tract infections caused by Pseudomonas aeruginosa: a minireview. J Infect Public Health. 2009;2(3):101–111. doi: 10.1016/j.jiph.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Park SR, Lee KD, Kim UK, Gil YG, Oh KS, Park BS, et al. Pseudomonas aeruginosa exotoxin A reduces chemoresistance of oral squamous carcinoma cell via inhibition of heat shock proteins 70 (HSP70) Yonsei Med J. 2010;51(5):708–716. doi: 10.3349/ymj.2010.51.5.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Billström H, Lund B, Sullivan A, Nord CE. Virulence and antimicrobial resistance in clinical Enterococcus faecium. Int J Antimicrob Agents. 2008;32(5):374–377. doi: 10.1016/j.ijantimicag.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 31.Pai CH, Kim MN. Antimicrobial resistance in enterococci. Yonsei Med J. 1998;39(6):554–561. doi: 10.3349/ymj.1998.39.6.554. [DOI] [PubMed] [Google Scholar]
- 32.Tambyah PA, Maki DG. The relationship between pyuria and infection in patients with indwelling urinary catheters: a prospective study of 761 patients. Arch Intern Med. 2000;160(5):673–677. doi: 10.1001/archinte.160.5.673. [DOI] [PubMed] [Google Scholar]
- 33.Gupta K, O’Brien W, Gallegos-Salazar J, Strymish J, Branch-Elliman W. How testing drives treatment in asymptomatic patients: level of pyuria directly predicts probability of antimicrobial prescribing. Clin Infect Dis. 2020;71(3):614–621. doi: 10.1093/cid/ciz861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armbruster CE, Smith SN, Mody L, Mobley HL. Urine cytokine and chemokine levels predict urinary tract infection severity independent of uropathogen, urine bacterial burden, host genetics, and host age. Infect Immun. 2018;86(9):e00327-18. doi: 10.1128/IAI.00327-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spencer JD, Schwaderer AL, Becknell B, Watson J, Hains DS. The innate immune response during urinary tract infection and pyelonephritis. Pediatr Nephrol. 2014;29(7):1139–1149. doi: 10.1007/s00467-013-2513-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of urine leukocyte (cells/µL) between patients with UTI and ASB
Diagnostic statistics of urine leukocytes for the diagnosis of UTI with cutoff 10.00 cells/µL
Univariable analysis of risk factors for bacteremia in patients with upper urinary tract infection
Flowchart of the study.