Abstract
Garlic belongs to the Allium family of plants that produce organosulfur compounds, such as allicin and diallyl disulfide (DADS), which account for their pungency and spicy aroma. Many health benefits have been ascribed to Allium extracts, including hypotensive and vasorelaxant activities. However, the molecular mechanisms underlying these effects remain unknown. Intriguingly, allicin and DADS share structural similarities with allyl isothiocyanate, the pungent ingredient in wasabi and other mustard plants that induces pain and inflammation by activating TRPA1, an excitatory ion channel on primary sensory neurons of the pain pathway. Here we show that allicin and DADS excite an allyl isothiocyanate-sensitive subpopulation of sensory neurons and induce vasodilation by activating capsaicin-sensitive perivascular sensory nerve endings. Moreover, allicin and DADS activate the cloned TRPA1 channel when expressed in heterologous systems. These and other results suggest that garlic excites sensory neurons primarily through activation of TRPA1. Thus different plant genera, including Allium and Brassica, have developed evolutionary convergent strategies that target TRPA1 channels on sensory nerve endings to achieve chemical deterrence.
Keywords: inflammation, pain, TRP channel, vasodilation, natural products
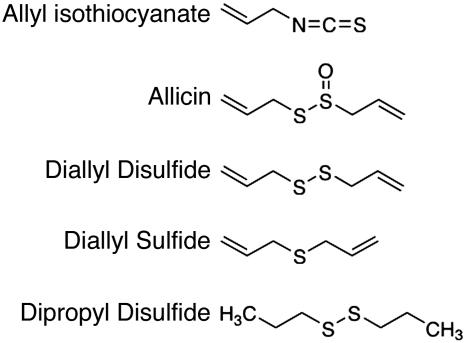
Garlic belongs to the plant genus Allium that also includes onion, leek, chives, and shallots. Allium plants contain a variety of sulfur-based natural products that are responsible for their pungency, lachrymatory effects, and spicy aroma (1). One such compound is the thiosulfinate allicin (2-propenyl 2-propene thiosulfinate), which is especially prevalent in garlic. When the bulb is crushed, allicin is generated by a chemical reaction catalyzed by the vacuolar enzyme, alliinase (2, 3). Allicin and other thiosulfinates are short-lived in aqueous solution, yielding organosulfur biproducts, such as diallyl sulfides (DAS), ajoene, and dithiines (4). Thus, the characteristic pungencies of different Allium bulbs depend on distinctive mixtures of organosulfur compounds that they produce (3, 4). Interestingly, these compounds bear structural similarity to isothiocyanates, the pungent ingredients of wasabi, yellow mustard, and other Brassica plants (Fig. 1) (5).
Fig. 1.
Chemical structures of allyl isothiocyanate (mustard oil), and the garlic derivatives, allicin, DADS, DAS, and dipropyl disulfide.
For many centuries Allium plants, and especially garlic, have been used as herbal medicines for treating a wide range of ailments, including hypertension, high blood cholesterol, and thrombosis (6). However, garlic can also produce adverse effects, such as cutaneous irritation, edema, and allergic contact dermatitis (7-9). In aqueous medium, allicin and other garlic derivatives are highly reactive compounds that can inhibit enzymes, modify nucleic acids, and alter membrane fluidity. Despite their widespread culinary and medicinal use, relatively little is known about the cellular and molecular mechanisms through which garlic extracts produce their physiological effects. Sensory nerve endings that innervate skin, mucous membranes, and vascular smooth muscle are likely targets given the irritant and vasodilatory actions of these pungent extracts. Indeed, sensory nerve endings are targeted by a variety of other plant-derived irritants, such as capsaicin, the pungent ingredient in chili peppers, or allyl isothiocyanate (AITC), the pungent principle in wasabi and other mustard oils (10). Both capsaicin and AITC excite primary sensory neurons by activating members of the TRP channel family (TRPV1 and TRPA1, respectively) (11-13). These nonselective cation channels are expressed by a subset of unmyelinated C-fiber nociceptors (12, 14-16), whose activation elicits acute pain accompanied by vasodilation, vascular leakage, and inflammation due to peripheral release of peptides [substance P and calcitonin gene-related peptide (CGRP)] from the activated nerve terminals (17-20).
Here we show that garlic extracts, as well as purified allicin and diallyl disulfide (DADS), excite a subset of cultured sensory neurons that also respond to capsaicin and AITC. Moreover, like AITC, these compounds depolarize sensory neurons by serving as agonists for TRPA1 channels. We also show that allicin and DADS induce vasorelaxation through a mechanism involving release of CGRP from capsaicin-sensitive nerve terminals. These data provide a mechanistic explanation for the pungency of Allium-derived natural products and suggest that activation of vascular sensory neurons contributes to their cardiovascular effects.
Methods
Neuronal Cell Culture and Calcium Imaging. Trigeminal ganglia were dissected as described (12). Calcium imaging by using fura-2/AM (Molecular Probes) was performed as described (12), and analysis was conducted with automated routines written in Igor Pro (Wavemetrics, Lake Oswego, OR). Extracellular Ringer's solution contained: 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 10 mM d[scap][r]-glucose, and 10 mM Na-Hepes (pH 7.4).
Expression in HEK293t Cells and Oocytes. HEK293t cells were plated on poly-(d)-lysine-coated chamberslides (Nalge-Nunc). Cells were transfected with Lipofectamine 2000 (Invitrogen) by using 5-25 ng of human TRPA1 plasmid per cm2; pcDNA3 vector was added to bring the total amount of plasmid DNA to 100 ng/cm2. After transfection (16 h), cells were loaded with fura-2/AM (10 μM) for 60 min and imaged in Ringer's solution. For oocyte expression, constructs were linearized with MluI and transcribed with T7 polymerase (Ambion, Austin, TX). Currents were recorded in ND96 (96 mM NaCl/2 mM KCl/1.8 mM CaCl2/1 mM MgCl2/5 mM Hepes, pH 7.6).
Recording of Tension. Experiments were performed on mesenteric arteries (200-μm outer diameter) from female Wistar-Hannover rats (200-250 g) as described (20). In brief, arterial ring segments were mounted in tissue bath containing aerated (5% CO2 and 95% O2) physiological salt solution (119 mM NaCl/4.6 mM KCl/1.5 mM CaCl2/1.2 mM MgCl2/15 mM NaHCO3/1.2 mM NaH2PO4/6 mM d-glucose) at 37°C (pH 7.4) in the presence of NG-nitro-l-arginine (0.3 mM) and indomethacin (10 μM) to eliminate contributions from nitric oxide and cyclooxygenase products, respectively. Preparations were contracted with phenylephrine, and agonists were added cumulatively to determine concentration-response relationships. Relaxant responses are expressed as percentage reversal of the phenylephrine-induced contraction. pEC50 and area under the curve were calculated (prism 3.0, GraphPad, San Diego) and used for comparison of drug treatments. Two tailed, paired Student's t test or ANOVA followed by Dunnett's post test (prism 3.0) was used for statistical comparison. Statistical significance, P < 0.05.
Chemicals and Garlic Extracts. Allicin was synthesized or purchased from LKT Laboratories (St. Louis). Capsaicin and capsazepine were purchased from Tocris (Bristol, U.K.). Indomethacin (Confortide) was obtained from Dumex (Copenhagen). All other chemicals were purchased from Sigma. Capsaicin, capsazepine, and the sulfur compounds were dissolved in ethanol and added cumulatively to the organ baths in volumes of 2.5 μl. Final ethanol concentrations were <1%. Garlic extract was prepared by squeezing 4 g of fresh garlic into 10 ml of saline through a kitchen garlic press. The mixtures were shaken, incubated at 4°C for 1 h, and centrifuged for 20 min at 1,500 × g (5°C). The supernatant fluid was collected and further diluted in saline.
Immunohistochemistry. Anti-TRPA1 antisera were raised against a C-terminal peptide (CVLNAVKTKTHCSISHPDI; AnaSpec, San Jose, CA) and affinity-purified with a Sulfolink (Pierce) column. Rat dorsal root ganglia (DRG) and mesenteric artery were fixed in PBS containing 4% formaldehyde, cryoprotected in 15% sucrose, mounted with OCT compound, and sectioned (10 μm) on a cryostat. DRG sections and whole mount arterial preparations were washed with PBS (pH 7.6) containing 0.2% Triton X-100 and 0.1% BSA for 2 h, incubated with the primary Ab (anti-TRPA1 1:1,000 for DRG and 1:250 for mesenteric arteries, goat anti-TRPV1 C-term 1:1,000, Santa Cruz Biotechnology, and guinea pig anti-CGRP 1:8,000, Euro-Diagnostica, Malmö, Sweden). For diaminobenzidine staining, tissues were incubated with biotinylated secondary Ab and developed with ABC-reagent (Vectastain kit, Vector Laboratories). For immunofluorescence, tissues were incubated with fluorophore-linked secondary Ab Alexa Fluor 594/568 or Alexa Fluor 488. Images were acquired with a Olympus Bx60F-3 microscope and DP50 camera. Confocal microscopy on whole-mount preparations was performed with a Multiprobe 2001 confocal laser scanning microscope (Molecular Dynamics).
Results
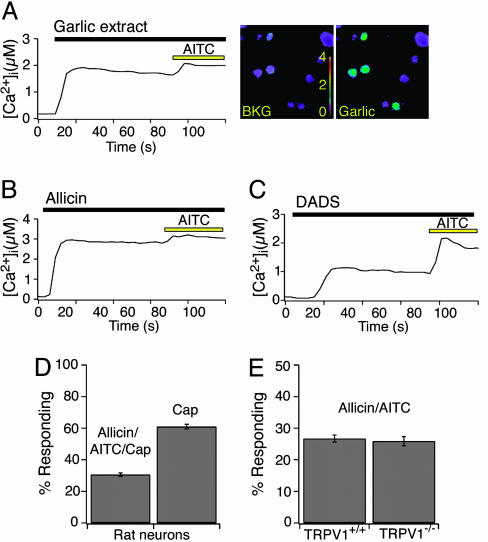
Garlic Extracts and Pungent Garlic Derivatives Excite Primary Sensory Neurons. To determine whether garlic derivatives have direct effects on primary sensory neurons, we used ratiometric calcium imaging to ask whether fresh garlic extracts or purified garlic derivatives excite dissociated neurons from rodent trigeminal ganglia. We found that ≈30% of cultured neurons showed significant increases in intracellular-free calcium after application of garlic extract, purified allicin, or DADS (Fig. 2 A-C). All responses were eliminated by coapplication of the nonselective TRP channel blocker ruthenium red (data not shown). These compounds activated all mustard oil (AITC)-responsive neurons, which represent a subset (≈50%) of capsaicin-responsive cells (Fig. 2D). Thus, garlic derivatives and mustard oil activate and TRPV1-deficient mice were indistinguishable in their sensitivity to AITC, garlic extract, allicin, or DADS (Fig. 2E).
Fig. 2.
Garlic extracts and derivatives excite a subset of primary sensory neurons responses of dissociated rat trigeminal neurons to garlic extracts and derivatives as measured by fura-2 ratiometric imaging. (A) Graph displays intracellular calcium responses to garlic extracts (1:10,000 dilution) as a function of time (Left). Pseudocolor images of fura-2-loaded sensory neurons before and after application of garlic extract [scale bar indicates the intracellular calcium concentration in μM (Right)]. (B) Calcium responses of sensory neurons to 40 μM allicin, followed by subsequent application of 100 μM AITC. (C) Calcium responses of sensory neurons to 200 μM DADS, followed by subsequent application of 100 μM AITC. All graphs represent an average of 100 responsive cells. (D) Allicin/AITC activate a subset of capsaicin-responsive cells. Percentage of rat neurons exhibiting an agonist-evoked rise in intracellular calcium to 100 μM AITC, 100 μM allicin, or 1 μM capsaicin. All AITC-responsive cells were also responsive to both allicin and capsaicin. (E) Allicin evokes the similar calcium responses in neurons isolated from TRPV1+/+ and TRPV-/- mice. Percentage of TRPV1+/+ and TRPV-/- neurons exhibiting an agonist-evoked rise in intracellular calcium to 100 μM AITC and 100 μM allicin. Rat and mouse sensory neurons were insensitive to DAS (data not shown).
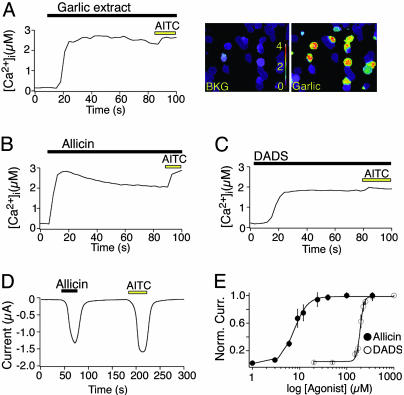
Garlic Extracts and Pungent Garlic Derivatives Activate TRPA1. Allicin, DADS, and AITC are organosulfur compounds that contain allyl groups and reactive sulfur atoms (Fig. 1). Intrigued by these structural similarities and the complete cellular overlap of the native responses, we asked whether garlic extracts, allicin, or DADS activate the cloned mustard oil-activated channel, TRPA1 (12, 21). Indeed, robust increases in intracellular calcium were observed in TRPA1-expressing HEK293t cells on exposure to each of these agents, whereas no response was seen in vector-transfected controls (Fig. 3 A-C). Voltage-clamp recordings from TRPA1-expressing Xenopus oocytes showed that allicin produced robust membrane currents with a potency (EC50 = 7.5 ± 0.4 μM) and efficacy similar to that of AITC (Fig. 3 D and E) (12). DADS was an equally efficacious but significantly less potent (EC50 = 192 ± 3 μM) TRPA1 agonist (Fig. 3E).
Fig. 3.
Garlic extracts and pungent garlic derivatives activate TRPA1 in transfected HEK293t cells and Xenopus oocytes. Garlic extract (1:10,000 dilution) (A), 40 μM allicin (B), and 200 μM DADS (C) activate calcium influx into human TRPA1-expressing HEK293t cells, as measured by fura-2/AM imaging. Subsequent exposure to 100 μM AITC elicited a slight increase in intracellular calcium in all allicin/DADS-responsive cells. Cells transfected with rat TRPA1, but not pcDNA3 alone, were also responsive to garlic extract, allicin, and DADS (data not shown). Traces represent an average of 100 responsive cells. (D) Representative trace from an oocyte expressing human TRPA1 recorded in ND-96. Allicin (3 μM, 40 s) and AITC (200 μM, 40 s) evoke large, reversible inward currents at -80 mV. No responses were observed in control injected oocytes (data not shown). (E) Dose-response curves for activation of human TRPA1 in oocytes by 20-sec application of allicin (•) or DADS (○). Currents were normalized to maximal response evoked by 100 μM allicin or 1 mM DADS. Half-maximal activates (EC50) occurred at 7.5±0.34 μM and 192 ± 2.6 μM, for allicin and DADS, respectively.
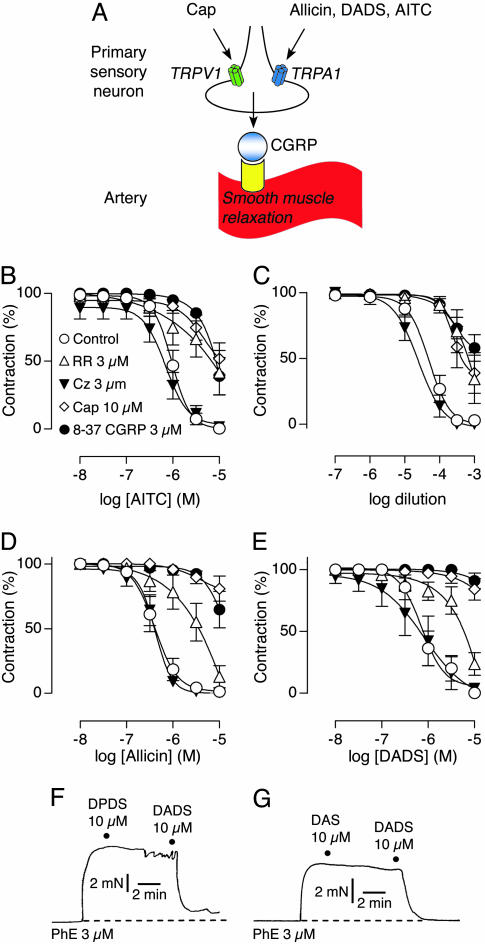
Garlic Compounds Act on Sensory Nerve Fibers to Produce Vasodilation. Activation of capsaicin receptors on perivascular nerve endings induces vasodilation of mesenteric arterial segments through the release of CGRP from these fibers (Fig. 4A) (18, 22). Treatment of phenylepinephrine-constricted arterial segments with TRPA1 agonists, such as Δ9-tetrahydrocannabinol or cannabinol, produces vasorelaxation through the same mechanism (20). As expected, we found that AITC induced a concentration-dependent relaxation of mesenteric arterial segments in vitro (pEC50 of 6.0 ± 0.1). Pretreatment with capsaicin inhibited this effect, presumably reflecting functional desensitization of TRPA1-expressing peptidergic fibers (Fig. 4B). Moreover, co-incubation with the CGRP receptor antagonist, 8-37 CGRP, or the TRP channel blocker, ruthenium red, attenuated AITC-evoked relaxation (Fig. 4B). In contrast, the TRPV1 antagonist, capsazepine, had no effect, ruling out a direct action of AITC on TRPV1. Taken together, these results are consistent with a mechanism in which isothiocyanates produce vasodilation by activating TRPA1 channels on capsaicin-sensitive, peptidergic nerve fibers that innervate vascular smooth muscle.
Fig. 4.
Vascular relaxation induced by AITC, garlic extract, allicin, and DADS. (A) Schematic diagram of vascular relaxation mediated by activation of sensory neurons. Inflammatory mediators, such as capsaicin and allicin, activate TRP channels (TRPV1 and TRPA1, respectively), on sensory neurons in blood vessels. TRP channel activation triggers local release of neuropeptides, such as the potent vasodilator CGRP. Concentration-response curves for AITC (B), garlic extracts (C), allicin (D), and DADS (E). Vasorelaxation was recorded in phenylephrine (PhE)-contracted mesenteric arterial segments in the presence of ruthenium red (3 μM, ▵), capsazepine (3 μM, ▾), 8-37 CGRP (3 μM, •), or vehicle (○), or after pretreatment with capsaicin (10 μM for 30 min; ⋄). Dipropyl disulfide (DPDS) (F) and DAS (G) failed to induce relaxation. DADS was applied as positive control. Force of vessel contraction is plotted as a function of time. Dashed line indicates basal tension before addition of drugs. Data are expressed as mean ± SEM (n = 6-8 in B and D, 5-6 in C, 5-8 in E, and 4-5 in F and G).
Garlic extract and its derivatives have hypotensive properties and can induce vasodilation in vivo and in vitro (6, 23-25). We therefore asked whether these compounds evoke vasodilation through a TRPA1-dependent mechanism. Indeed, garlic extracts produced complete relaxation of mesenteric arterial segments with pharmacological properties identical to those elicited by AITC (Fig. 4C). Moreover, both allicin and DADS induced concentration-dependent and complete relaxation (pEC50 = 6.4 ± 0.1 and pEC50 = 6.0 ± 0.1, respectively) and displayed the same pharmacological properties as AITC and garlic extracts, suggesting that these agents mediate vasorelaxation induced by garlic extracts (Fig. 4 D and E). We also tested the vasorelaxation effects of dipropyl disulfide and DAS, analogs of DADS in which the allyl groups are replaced by propyl groups or are connected by a single sulfur atom, respectively (Fig. 1). Neither of these compounds produced significant vasodilation of arterial segments (Fig. 4 F and G) or activated native or cloned TRPA1 (data not shown), suggesting that both the allyl groups and labile sulfur bonds are crucial for efficient induction of the response.
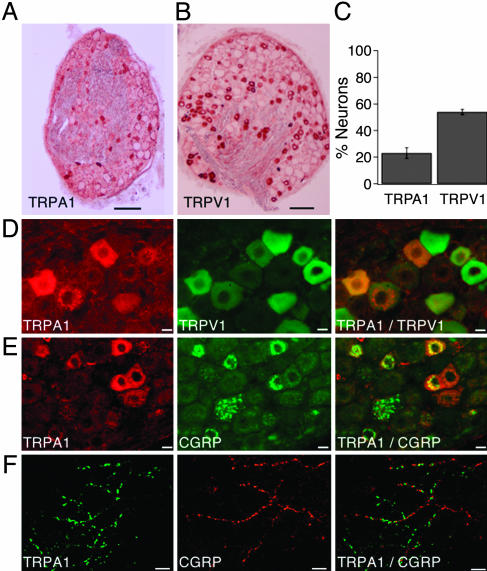
TRPA1 Is Expressed by a Subpopulation of CGRP-Containing Sensory Neurons. In situ hybridization and immunofluorescence studies have shown that TRPA1 is expressed by ≈20% of small-diameter sensory neurons in trigeminal ganglia or DRG (12, 16). Using an Ab that recognizes the C terminus of rat TRPA1, we observed immunoreactivity in 22 ± 4% (n = 7) of DRG neurons (Fig. 5 A and C), consistent with present and previous anatomical and functional studies (12, 16). Capsaicin receptor (TRPV1) immunoreactivity was found in 54 ± 4% (n = 7) of DRG neurons (Fig. 5 B-D). Colocalization studies showed that all TRPA1-positive neurons also expressed TRPV1 (Fig. 5D). Additionally, many TRPA1-expressing neurons were immunoreactive for CGRP (Fig. 5E). A similar pattern of TRPA1 immunoreactivity was observed on perivascular nerve fibers in mesenteric artery (Fig. 5F). Taken together, these histological results are consistent with our physiological data indicating that TRPA1 activates a subset of capsaicin-sensitive, peptidergic sensory neurons.
Fig. 5.
TRPA1 immunoreactivity in sensory neurons and vascular nerve fibers coincides with the presence of CGRP and TRPV1. (A) Immunohistochemical staining of a rat DRG section with anti-TRPA1 Ab. The Ab stains cells of smaller diameter. (B) Immunohistochemical staining of a rat DRG section with anti-TRPV1 Ab. (C) Percentage of TRPA1- and TRPV1-positive cells in rat DRG sections. (D) Localization of TRPA1 in TRPV1-expressing cells. Staining of TRPA1 (Left, red) and TRPV1 (Center, green) in the same rat DRG section. The overlay (Right) indicates localization of TRPA1 in a subset of TRPV1-positive cells. (E) Partial overlap of TRPA1 and CGRP expression in DRG neurons. The same rat DRG section has been stained with TRPA1 (Left, red) and CGRP (Center, green). The overlay (Right) shows partial coexpression of TRPA1 and CGRP in the same neurons (yellow). (F) Expression of TRPA1 in adventitial nerve fibers in rat mesenteric artery. Confocal images of whole mount preparations are shown. TRPA1 immunoreactivity is indicated in green (Left). The same preparation was stained with anti-CGRP Ab (Center, red). The overlay (Right) indicates partial overlap between TRPA1 and CGRP on the same fibers (yellow). [Scale bars: 250 μm (A and B), 10μm (D and E), and 2.5 μm (F).]
Discussion
Plants have evolved ingenious defensive strategies to ward off herbivorous predators. In many cases this is achieved through the production of chemical agents that produce irritation and inflammation (26, 27). Our findings support an emerging theme whereby irritants such as capsaicin, isothiocyanates, and thiosulfinates produce their psychophysical effects by targeting excitatory TRP channels on primary afferent nerve fibers of the pain pathway. Moreover, the ability of these agents to produce neurogenic inflammation and vasodilation can be attributed to the fact that their molecular sites of action (TRPV1 and TRPA1) are expressed on neurons whose activation leads to the peripheral release of CGRP, substance P, and other inflammatory neurotransmitters.
Garlic has for centuries been appreciated for its culinary and medicinal properties, which likely reflect its effects on olfactory, gustatory, and somatosensory systems. Here we have focused on understanding the molecular basis of garlic's somatosensory actions, which contribute to its pungent and vasodilatory actions. Namely, we have shown that allicin and DADS, two of the main pungent ingredients of garlic, directly activate TRPA1 on sensory neurons of the trigeminal ganglia and DRG.
Several lines of evidence suggest that TRPA1 is the major, if not sole site of isothiocyanate and garlic derivative action on nociceptive sensory neurons. First, our functional studies show complete overlap between AITC and allicin/DADS sensitive neurons; that is, no cells were found to respond to one agonist but not the other. Second, the cellular pattern of TRPA1 expression based on histochemical analyses is consistent with functional imaging studies. Third, calcium responses, membrane currents, and vasodilatory actions are inhibited by ruthenium red, a blocker of TRPA1 (and TRPV1) channels on sensory neurons. In contrast, capsazepine, a TRPV1-specific blocker had no effect on responses to AITC, allicin, or DADS. At the same time, no differences were observed in either the magnitude or prevalence of allicin/DADS-evoked responses in sensory neurons from WT or TRPV1-deficient mice. This result suggests that TRPV1 does not contribute significantly to the pungent effects of these compounds in vivo.
Animal studies indicate that garlic extracts and garlic derivatives may have beneficial cardiovascular effects. For example, garlic has been found to reduce hypertension and to lower serum cholesterol levels (29, 30). Our data indicate that garlic extracts and garlic derivatives can, indeed, mediate vasodilation through activation of TRPA1 channels on sensory neurons in vitro. It remains to be established whether this mechanism contributes to the systemic hypotensive activity of garlic in vivo. Moreover, whether garlic has beneficial effects on human cardiovascular health remains controversial (6). Future genetic and pharmacological studies will help to resolve these issues.
Note. During the preparation of this manuscript, another study (28) reported that garlic extract and allicin activate TRPA1 and, to a lesser extent, TRPV1. These results are generally in agreement with our data. However, as discussed above, we found no significant effect of garlic extract and derivatives on native TRPV1.
Acknowledgments
We thank R. Nicoll and E. Lumpkin for discussion and comments on the manuscript, and J. Poblete and members of the D.J. laboratory for advice and assistance. This work was supported by postdoctoral fellowships (to D.M.B. and A.H.) and grants from the National Institutes of Health (to D.J.), the Swedish Research Council, the Swedish Strategic Foundation, the School in Pharmaceutical Sciences (FLAK), and the Medical Faculty (ALF) at Lund University.
Author contributions: D.M.B., A.H., E.D.H., P.M., H.E.A., D.J., S.-E.J., and P.M.Z. designed research; D.M.B., P.M., A.H., H.E.A., and S.-E.J. performed research; O.S. and S.-E.J. contributed new reagents/analytical tools; D.M.B., P.M., E.D.H., D.J., and P.M.Z. analyzed data; and D.M.B., D.J., and S.-E.J. wrote the paper.
Abbreviations: AITC, allyl isothiocyanate; DADS, diallyl disulfide; DAS, diallyl sulfide; CGRP, calcitonin gene-related peptide; DRG, dorsal root ganglia.
References
- 1.McGee, H. (2004) On Food and Cooking: The Science and Lore of the Kitchen (Scribner, New York).
- 2.Stoll, A. & Seebeck, E. (1947) Experientia 3, 114-115. [DOI] [PubMed] [Google Scholar]
- 3.Jones, M. G., Hughes, J., Tregova, A., Milne, J., Tomsett, A. B. & Collin, H. A. (2004) J. Exp. Bot. 55, 1903-1918. [DOI] [PubMed] [Google Scholar]
- 4.Block, E. (1992) Angew. Chem. Int. Ed. 31, 1135-1178. [Google Scholar]
- 5.Fahey, J. W., Zalcmann, A. T. & Talalay, P. (2001) Phytochemistry 56, 5-51. [DOI] [PubMed] [Google Scholar]
- 6.Brace, L. D. (2002) J. Cardiovasc. Nurs. 16, 33-49. [DOI] [PubMed] [Google Scholar]
- 7.Burgess, J. F. (1952) Can. Med. Assoc. J. 66, 275. [PMC free article] [PubMed] [Google Scholar]
- 8.Parish, R. A., McIntire, S. & Heimbach, D. M. (1987) Pediatr. Emerg. Care 3, 258-260. [PubMed] [Google Scholar]
- 9.Joseph, P. K., Rao, K. R. & Sundaresh, C. S. (1989) Indian J. Exp. Biol. 27, 977-979. [PubMed] [Google Scholar]
- 10.Jancso, N., Jancso-Gabor, A. & Szolcsanyi, J. (1967) Br. J. Pharmacol. 31, 138-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caterina, M. J. & Julius, D. (2001) Annu. Rev. Neurosci. 24, 487-517. [DOI] [PubMed] [Google Scholar]
- 12.Jordt, S. E., Bautista, D. M., Chuang, H. H., McKemy, D. D., Zygmunt, P. M., Hogestatt, E. D., Meng, I. D. & Julius, D. (2004) Nature 427, 260-265. [DOI] [PubMed] [Google Scholar]
- 13.Wang, H. & Woolf, C. J. (2005) Neuron 46, 9-12. [DOI] [PubMed] [Google Scholar]
- 14.Tominaga, M., Caterina, M. J., Malmberg, A. B., Rosen, T. A., Gilbert, H., Skinner, K., Raumann, B. E., Basbaum, A. I. & Julius, D. (1998) Neuron 21, 531-543. [DOI] [PubMed] [Google Scholar]
- 15.Story, G. M., Peier, A. M., Reeve, A. J., Eid, S. R., Mosbacher, J., Hricik, T. R., Earley, T. J., Hergarden, A. C., Andersson, D. A., Hwang, S. W., et al. (2003) Cell 112, 819-829. [DOI] [PubMed] [Google Scholar]
- 16.Nagata, K., Duggan, A., Kumar, G. & Garcia-Anoveros, J. (2005) J. Neurosci. 25, 4052-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gamse, R., Holzer, P. & Lembeck, F. (1980) Br. J. Pharmacol. 68, 207-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zygmunt, P. M., Petersson, J., Andersson, D. A., Chuang, H., Sørgård, M., Di Marzo, V., Julius, D. & Högestätt, E. D. (1999) Nature 400, 452-457. [DOI] [PubMed] [Google Scholar]
- 19.Julius, D. & Basbaum, A. I. (2001) Nature 413, 203-210. [DOI] [PubMed] [Google Scholar]
- 20.Zygmunt, P. M., Andersson, D. A. & Hogestatt, E. D. (2002) J. Neurosci. 22, 4720-4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bandell, M., Story, G. M., Hwang, S. W., Viswanath, V., Eid, S. R., Petrus, M. J., Earley, T. J. & Patapoutian, A. (2004) Neuron 41, 849-857. [DOI] [PubMed] [Google Scholar]
- 22.Kawasaki, H., Takasaki, K., Saito, A. & Goto, K. (1988) Nature 335, 164-167. [DOI] [PubMed] [Google Scholar]
- 23.Wilburn, A. J., King, D. S., Glisson, J., Rockhold, R. W. & Wofford, M. R. (2004) J. Clin. Hypertens. (Greenwich) 6, 242-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozturk, Y., Aydin, S., Kosar, M. & Baser, K. H. (1994) J. Ethnopharmacol. 44, 109-116. [DOI] [PubMed] [Google Scholar]
- 25.Ganado, P., Sanz, M., Padilla, E. & Tejerina, T. (2004) J. Pharmacol. Sci. 94, 434-442. [DOI] [PubMed] [Google Scholar]
- 26.Tewksbury, J. J. & Nabhan, G. P. (2001) Nature 412, 403-404. [DOI] [PubMed] [Google Scholar]
- 27.Jordt, S. E. & Julius, D. (2002) Cell 108, 421-430. [DOI] [PubMed] [Google Scholar]
- 28.Macpherson, L. J., Geierstanger, B. H., Viswanath, V., Bandell, M., Eid, S. R., Hwang, S. & Patapoutian, A. (2005) Curr. Biol. 15, 929-934. [DOI] [PubMed] [Google Scholar]
- 29.Fallon, M. B., Abrams, G. A., Abdel-Razek, T. T., Dai, J., Chen, S. J., Chen, Y. F., Luo, B., Oparil, S. & Ku, D. D. (1998) Am. J. Physiol. 275, L283-L287. [DOI] [PubMed] [Google Scholar]
- 30.Ali, M., Al-Qattan, K. K., Al-Enezi, F., Khanafer, R. M. & Mustafa, T. (2000) Prostaglandins Leukotrienes Essent. Fatty Acids 62, 253-259. [DOI] [PubMed] [Google Scholar]