Abstract
Transient global or forebrain ischemia induced experimentally in animals can cause selective, delayed neuronal death of hippocampal CA1 pyramidal neurons. A striking feature is a delayed rise in intracellular free Zn2+ in CA1 neurons just before the onset of histologically detectable cell death. Here we show that α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors (AMPARs) at Schaffer collateral to CA1 synapses in postischemic hippocampus exhibit properties of Ca2+/Zn2+-permeable, Glu receptor 2 (GluR2)-lacking AMPARs before the rise in Zn2+ and cell death. At 42 h after ischemia, AMPA excitatory postsynaptic currents exhibited pronounced inward rectification and marked sensitivity to 1-naphthyl acetyl spermine (Naspm), a selective channel blocker of GluR2-lacking AMPARs. In control hippocampus, AMPA excitatory postsynaptic currents were electrically linear and relatively insensitive to Naspm. Naspm injected intrahippocampally at 9-40 h after insult greatly reduced the late rise in intracellular free Zn2+ in postischemic CA1 neurons and afforded partial protection against ischemia-induced cell death. These results implicate GluR2-lacking AMPA receptors in the ischemia-induced rise in free Zn2+ and death of CA1 neurons, although a direct action at the time of the rise in Zn2+ is unproven. This receptor subtype appears to be an important therapeutic target for intervention in ischemia-induced neuronal death in humans.
Keywords: glutamate, 1-naphthyl acetyl spermine, neurodegeneration
Transient global or forebrain ischemia arising because of cardiac arrest, near drowning, or open heart surgery, affects 150,000 Americans each year. Global ischemia in humans or induced experimentally in animals causes selective and delayed neuronal death and in many cases delayed onset of neurological deficits (for review, see refs. 1 and 2). Pyramidal neurons in the hippocampal CA1 are particularly vulnerable. Histological evidence of degeneration is not observed until 2-3 d after ischemia in rats or 3-4 d in gerbils (3-6). Excessive stimulation of glutamate receptors (excitotoxicity) is a fundamental mechanism underlying ischemia-induced neuronal death. Although initial studies focused on NMDA-type glutamate receptors (GluRs; NMDA receptors) as a critical mediator in focal ischemic injury, subsequent studies support a more central role for α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid (AMPA)-type glutamate receptors in the hippocampal injury associated with global ischemia (7-9).
AMPA receptors (AMPARs) are tetrameric assemblies of subunits GluR1-GluR4 (or GluRA-GluRD) and are encoded by separate genes, which are differentially expressed throughout the CNS (for review, see ref. 10). AMPARs assembled from GluR1, GluR3, and GluR4 subunits (lacking the GluR2 subunit) are permeable to Ca2+ and Zn2+ (11-13) and are blocked by intracellular polyamines in a voltage-dependent manner, giving rise to their characteristic inwardly rectifying current-voltage (I-V) relations (14, 15). The presence of the edited GluR2 subunit [GluR2(R)] in recombinant AMPARs greatly reduces Ca2+/Zn2+ permeability (11-13), voltage-dependent block by polyamines (14-16), and single-channel conductance (17) due to the presence of an Arg residue in the pore-lining region. The presence of GluR2(R) also influences AMPAR assembly, forward trafficking from the endoplasmic reticulum, recycling, and targeting to synaptic sites (16, 18-20). Principal neurons of the hippocampus express primarily GluR2-containing AMPARs (21); thus, an acute change in the level of GluR2 expression could have significant consequences for neuronal survival. The relative expression of GluR2 in neurons is not static but is regulated in a cell-specific manner during development (22) and is altered after seizures (23, 24), ischemic insult (25, 26), or treatment with antipsychotics, drugs of abuse, or corticosteroids (for review, see ref. 27).
Considerable evidence supports a role for GluR2-lacking Ca2+/Zn2+-permeable AMPARs in global ischemia-induced neuronal death (for review, see ref. 27). Global ischemia triggers down-regulation of GluR2 gene expression and protein abundance (25, 28) and enhances AMPAR-mediated Ca2+ influx in vulnerable CA1 pyramidal neurons before the onset of neuronal death (26). Ischemia induces prolonged, Ca2+-dependent AMPA excitatory postsynaptic currents (EPSCs) at CA1 synapses, which are inwardly rectifying (29) and sensitive to the Ca2+-permeable AMPARs channel blockers, Joro spider toxin, and 1-naphthyl acetyl spermine (Naspm) (30, 31). These findings provide molecular and functional evidence for enhanced expression of GluR2-lacking, Ca2+/Zn2+-permeable receptors at CA1 synapses of postischemic brain and predict enhanced vulnerability of CA1 neurons to ambient glutamate. Consistent with this finding, knockdown of the GluR2 gene by administration of antisense oligonucleotides, even in the absence of an ischemic insult, causes death of pyramidal neurons (32), and overexpression of Ca2+-permeable GluR2(Q) channels in vivo promotes ischemia-induced death of normally resistant CA3 pyramidal cells and dentate gyrus granule cells (29). Moreover, overexpression of Ca2+-impermeable GluR2(R) channels protects CA1 neurons against ischemia-induced neuronal death (29).
The present study was undertaken to determine the functional properties of synaptic AMPARs in postischemic hippocampus and to examine a possible causal role for GluR2-lacking AMPARs in the late rise of intracellular Zn2+ and neuronal death. Here we show that AMPA EPSCs at Schaffer collateral to CA1 synapses of postischemic hippocampus exhibit properties characteristic of GluR2-lacking AMPARs before histologically detectable neuronal death. Moreover, a blocker selective for GluR2-lacking AMPARs substantially reduces Zn2+ accumulation and postischemic death of CA1 neurons, consistent with a role for these receptors in ischemia-induced delayed cell death.
Materials and Methods
Global Ischemia and Naspm Administration. Male Sprague-Dawley rats (150-200 g; Charles River Laboratories) were subjected to transient global ischemia or sham operation by four-vessel occlusion as described in ref. 33. Detailed procedures are described in Supporting Materials and Methods, which is published as supporting information on the PNAS web site. At indicated times after surgery, animals were anesthetized with halothane as above, restrained in a stereotaxic apparatus, and injected intrahippocampally with Naspm or saline (see Supporting Materials and Methods).
RT-PCR. To assess restrictive-element-1-silencing transcription factor (REST) and GluR2 mRNA abundance, animals were killed at the indicated times after surgery and the hippocampal CA1 was removed. RNA isolation, reverse transcription, and DNA amplification were performed according to the manufacturer's instructions (Invitrogen). Primers are described in Supporting Materials and Methods.
Electrophysiology, Histological Analysis, and N-6-(Methoxy-8-Quinolyl)-para-Toluenesulfonamide (TSQ) and Acid Fuchsin Staining. Electrophysiological recordings were performed from CA1 pyramidal neurons in acute hippocampal slices as described in ref. 34. Neuronal cell loss was assessed as described in ref. 33. To visualize free Zn2+, brain sections were labeled with the Zn2+-selective fluorescent dye TSQ (35). Adjacent sections were labeled with acid fuchsin. Details can be found in Supporting Materials and Methods.
Results
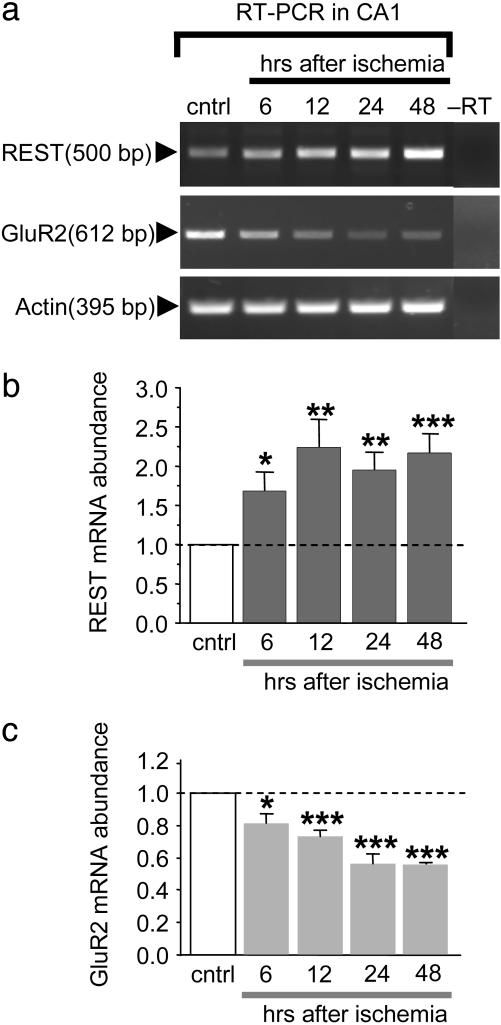
Global Ischemia Suppresses GluR2 mRNA Expression in Hippocampal CA1. Global ischemia activates the REST/neuron-restrictive silencer factor selectively in CA1 (33). REST is a 9-zinc-finger transcription factor that suppresses neuron-specific target genes (23). A well characterized target of REST is the AMPAR subunit GluR2, which is suppressed in CA1 before neuronal death (33). We first assessed the time course of REST activation and GluR2 suppression in CA1 by RT-PCR. In control CA1, REST mRNA abundance was low. Ischemia triggered an increase in REST mRNA expression in CA1, evident as late as 48 h [increase to 1.68 ± 0.24 times control at 6 h (n = 5; P < 0.05 vs. control), 2.24 ± 0.36 times control at 12 h (n = 5; P < 0.01), 1.95 ± 0.23 times control at 24 h (n = 5; P < 0.01), and 2.18 ± 0.23 times control at 24 h (n = 5; P < 0.001)] (Fig. 1 a and b). In control CA1, GluR2 mRNA expression was pronounced. Ischemia induced a significant reduction in GluR2 mRNA at 6 and 12 h after ischemia and a greater reduction at 24 and 48 h, times before cell death [reduced to 0.82 ± 0.06 times control at 6 h (n = 5; P < 0.05 vs. control), 0.73 ± 0.04 times control at 12 h (n = 5; P < 0.001), 0.56 ± 0.06 times control at 24 h (n = 5; P < 0.001), and 0.55 ± 0.01 times control at 48 h (n = 5; P < 0.001)] (Fig. 1 a and c). In contrast, actin mRNA was unchanged at all times examined (Fig. 1a). These findings are consistent with findings that REST represses GluR2 gene expression (33).
Fig. 1.
Global ischemia induces REST and suppresses GluR2 mRNA expression in hippocampal CA1. RT-PCR products amplified from DNase-treated RNA from the hippocampal CA1 of control animals at 48 h after sham operation and experimental animals at 6, 12, 24, and 48 h after global ischemia. (a) Representative agarose gel electrophoresis of RT-PCR products obtained with primers specific to REST, GluR2, and actin mRNAs. The REST mRNA signal was low in control CA1 and increased by 6 h after ischemia; it reached a plateau at 12 h that was maintained at least until 48 h. The GluR2 mRNA signal was prominent in control CA1 and was decreased by 6 h after ischemia; it was further decreased at 12 and 24 h and remained decreased until at least 48 h. No changes were detected in actin mRNA expression. (b and c) Quantitation of REST (b) and GluR2 (c) mRNA abundance after global ischemia (n = 5 per time point). Band densities for REST and GluR2 were normalized to the corresponding band density for actin and expressed as the ratio of the band density for the experimental sample to the band density of the corresponding control sample. Bars represent means ± SEMs. Statistical significance was assessed by Student's unpaired t test of each band density vs. its control (*, P < 0.05; **, P < 0.01; ***, P < 0.001). cntrl, control.
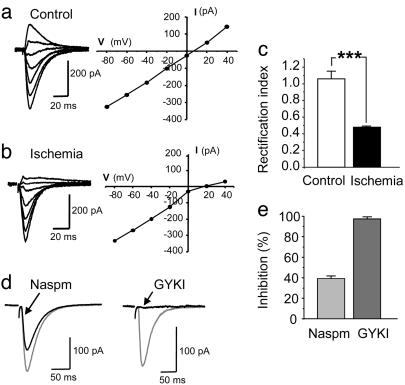
Synaptic Currents in Postischemic CA1 Neurons Exhibit Properties of GluR2-Lacking AMPARs. Ischemia-induced suppression of GluR2 mRNA expression in CA1 would be expected to alter AMPAR subunit composition and properties at CA1 synapses. To examine AMPAR EPSCs in postischemic neurons, we subjected rats to sham operation or global ischemia and prepared acute slices at 40-44 h after reperfusion. GluR2-lacking AMPARs are blocked by internal polyamines, which results in a characteristic inwardly rectifying I-V relation (14-16, 36-38); moreover, EPSCs of GluR2-lacking receptors tend to decay more rapidly than those of GluR2-containing receptors (39). We measured AMPA EPSCs evoked by Schaffer collateral stimulation in the presence of 25 μM d-2-amino-5-phosphonovaleric acid and 100 μM picrotoxin to block NMDA and GABA postsynaptic currents and included 1 mM spermine in the recording pipette to prevent loss of rectification due to diffusion of polyamines out of the cell into the pipette. In control slices, the I-V relation of the peak AMPA EPSC was linear from -60 to +40 mV (Fig. 2a), whereas, in postischemic slices, the AMPA EPSCs at CA1 synapses exhibited rectification. The amplitude was reduced at positive membrane potentials compared with that at negative potentials (Fig. 2b). The rectification index (RI), defined as (EPSC amplitude at +40 mV/EPSC amplitude at -60 mV) × 1.5 to correct for the difference in driving force, varied between 0.9 and 1.5 for CA1 pyramidal neurons in control slices (mean RI = 1.06 ± 0.09; n = 6). The RI was markedly reduced in slices from postischemic animals (range: 0.44-0.5; average = 0.48 ± 0.01; n = 6; P < 0.001 vs. control) (Fig. 2c). In addition, the decay time constant of the EPSCs was significantly faster in postischemic neurons (τ = 10.0 ± 0.3 ms; n = 4) than in control neurons (τ = 18.1 ± 1.2 ms; n = 4; P < 0.01 control vs. postischemic). The inwardly rectifying I-V relations and faster decay time constants indicate that, in postischemic CA1 neurons, a substantial fraction of the AMPA EPSC is mediated by GluR2-lacking AMPARs.
Fig. 2.
Synaptic currents in postischemic CA1 neurons exhibit properties of GluR2-lacking AMPARs. (a and b) Representative AMPA EPSCs and I-V relations of the peak responses at Schaffer collateral synapses on CA1 pyramidal cells recorded in acute hippocampal slices. The external solution contained 25 μM d-2-amino-5-phosphonovaleric acid and 100 μM picrotoxin to block NMDA and GABAA receptors, respectively. (a) AMPA EPSCs recorded in a neuron from a control animal exhibited a linear I-V relation. (b) AMPA EPSCs recorded from postischemic neurons 42 h after reperfusion exhibited reduced current at positive potentials (Left) and an inwardly rectifying I-V relation (1 mM permine was included in pipette to maintain rectification) (Right). (c) The rectification index [(EPSC amplitude at +40 mV/EPSC amplitude at -60 mV) × 1.5] was significantly reduced in neurons from postischemic animals (P < 0.001, n = 6 per group). (d) AMPA EPSCs in postischemic neurons were reduced but not blocked by Naspm (250 μM) (Left), an antagonist selective for GluR2-lacking AMPARs, and nearly completely blocked by the general AMPAR antagonist GYKI-53655 (50 μM) (Right). The neurons were voltage-clamped at -60 mV. (e) Percentage inhibition of AMPA EPSCs by Naspm (n = 6) and GYKI-53655 (n = 4) in hippocampal slices from postischemic animals. The effect of Naspm on AMPA EPSCs in postischemic neurons was significantly different from that of GYKI-53655 (P < 0.001).
As a further test for GluR2-lacking AMPARs, we recorded AMPA EPSCs in the absence and presence of Naspm (250 μM), a synthetic analog of Joro spider toxin and a relatively selective channel blocker of GluR2-lacking AMPARs (40). In AMPARs containing the edited GluR2 subunit, the positively charged Arg in the pore-forming region reduces the block by Naspm (41). In postischemic slices, bath application of Naspm (250 μM) inhibited AMPA EPSCs at Schaffer collateral synapses by 38.5 ± 2.9% of the initial amplitude (holding potential, -60 mV; n = 6) (Fig. 2 d and e). Subsequent bath application of GYKI-53655 (50 μM), a broad-spectrum AMPAR antagonist, almost completely blocked the EPSCs (by 96.7 ± 1.6%; n = 4) (Fig. 2 d and e), indicating that the currents remaining after Naspm were mediated by AMPARs. In sham-operated slices, bath application of 250 μM Naspm induced a small reduction of AMPA EPSCs (by 12.2 ± 5.1%; n = 4; data not shown); this reduction was significantly smaller than that observed after ischemia (P < 0.01 control vs. after ischemia). Together, these findings indicate enhanced expression of GluR2-lacking AMPARs in CA1 pyramidal neurons after ischemia.
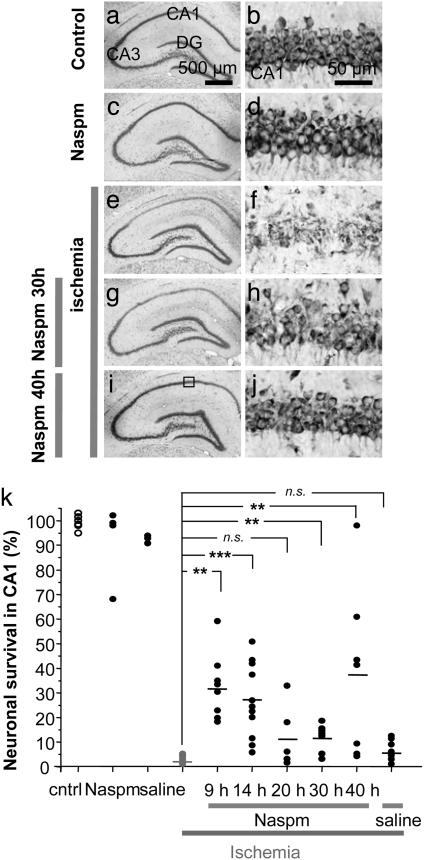
Naspm Protects Against Ischemia-Induced Death of CA1 Neurons in Vivo. To test whether ion fluxes through Ca2+/Zn2+-permeable AMPARs mediate ischemia-induced cell death, we examined whether Naspm protects CA1 neurons when administered at various times after ischemia. We subjected rats to global ischemia or sham operation and injected Naspm directly into the hippocampus at 9, 14, 20, 30, or 40 h after ischemia or at 30 or 40 h after sham operation and assessed neuronal death histologically (representative sections in Fig. 3 a-j; summary data in Fig. 3k). Cell counts were obtained from animals killed 5 d after ischemia or sham operation, and the values were normalized to counts in sham-operated animals in the same group. Global ischemia induced delayed and selective death of pyramidal cells in CA1, evident at 5 d (Fig. 3 e and f), consistent with previous studies (for review, see ref. 27). Neuronal death in CA1 was extensive, with few remaining neurons (2.8 ± 0.5% of control; n = 8 for controls, n = 10 for ischemia). However, administration of Naspm (10 mM in 5 μl injected intrahippocampally) at 9, 14, 20, 30, or 40 h after ischemic insult afforded partial but significant protection against ischemia-induced death of CA1 neurons (Fig. 3 g-j illustrates the greatest protection for 30 and 40 h). Cell counts showed an increase of neuronal survival in CA1 to 32.4 ± 4.7% of control at 9 h (n = 8, P < 0.01 vs. ischemia), 27.4 ± 3.9% of control at 14 h (n = 13, P < 0.001 vs. ischemia), 11.2 ± 4.9% at 20 h (n = 6, 0.1 > P > 0.05 vs. ischemia), 11.8 ± 1.8% of control at 30 h (n = 8; P < 0.01 vs. ischemia), and 37.4 ± 13% of control at 40 h (n = 7; P < 0.01 vs. ischemia). In a separate group of control animals, a single injection of saline was administered between 9 and 40 h after ischemia and may have caused a small increase in cell survival in CA1, to 5.8 ± 1.2% of control (n = 10, P > 0.05). Neither Naspm nor saline produced a deleterious action in the CA1 of sham-operated (control) animals (Fig. 3 c, d, and k). These data suggest a causal role for GluR2-lacking AMPARs in postischemic CA1 cell death. The protection by Naspm at 9-20 h precedes a decrease in GluR2 protein (33); however, some expression of GluR2-lacking AMPARs occurs in control hippocampus (42), and the protective action may have been mediated by block of these receptors. It is highly unlikely that early Naspm would remain at a blocking concentration until the time of the rise in cytoplasmic free Zn2+, which is not until at least 48 h after injection. Furthermore, the residual Naspm from the earlier injections would be less than that from later injections, although the protection by the earlier injections was greater.
Fig. 3.
Block of GluR2-lacking AMPARs protects CA1 neurons from postischemic neurodegeneration. (a-j) Toluidine blue-stained coronal brain sections at the level of the dorsal hippocampus from control (a and b) and experimental animals subjected to Naspm injection (c and d), global ischemia (e and f), or to global ischemia, followed by Naspm at 30 or 40 h (g-j) and killed 5 d after reperfusion. The sections are from maximally protected animals. (k) Summary data for control (n = 8) and Naspm injections given at 9 h (n = 8), 14 h (n = 13), 20 h (n = 6), 30 h (n = 8), or 40 h (n = 7) after ischemia. Injection of Naspm at all times except 20 h after ischemia significantly protected CA1 neurons; saline was ineffective (n = 10). Statistical significance was assessed by a nonparametric Kruskal-Wallis test (*, P < 0.05; **, P < 0.01; ***, P < 0.001). (Scale bars: a, c, e, and g, 500 μm; b, d, f, and h, 50 μm.)
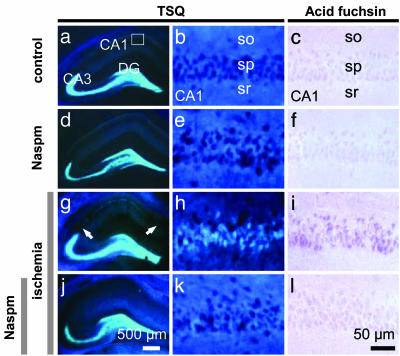
Naspm Blocks the Delayed Rise in Free Zn2+ After Global Ischemia in the Vulnerable CA1 Neurons. Zn2+ at high concentrations is implicated in neuronal death associated with global ischemia, seizures, traumatic brain injury, and other brain disorders (43-46). Global ischemia elicits a delayed rise in Zn2+ in selectively vulnerable CA1 neurons, evident at late times after insult but before onset of morphologically apparent cell death (46-48). The late rise in Zn2+ may occur by influx through GluR2-lacking AMPARs (49) and/or by release of Zn2+ from intracellular stores (50, 51). To address the question of Zn2+ and/or Ca2+ influx, we examined the impact of Naspm on the late rise in intracellular Zn2+ in CA1 neurons. In sham-operated control hippocampus, TSQ labeling revealed intense fluorescence in the axons of dentate granule neurons in the hilus and stratum lucidum of CA3, which contains the mossy fiber tracts, as well as faint fluorescence in the stratum radiatum and stratum oriens of CA1 and CA3 (Fig. 4a). Zn2+ fluorescence was not detectable in the CA1 pyramidal cell layer (Fig. 4b, sp). In adjacent sections, there was minimal staining, with acid fuchsin indicating little acidophilia and early neurodegeneration (Fig. 4c). In contrast, at 72 h after ischemia, Zn2+ fluorescence was pronounced in the cell bodies of CA1 pyramidal neurons (Fig. 4 g and h). Acid fuchsin staining was also intense in CA1 neurons (Fig. 4i), linking neuronal degeneration and Zn2+ fluorescence. Naspm injected intrahippocampally at 40 h after induction of global ischemia markedly attenuated the rise in Zn2+ (Fig. 4 j and k) and appearance of acidophilia at 72 h (Fig. 4l) in five of six animals (of the five animals, two exhibited no acidophilia, in one ≈10% of the cells were acidophilic, and in two ≈30% of the cells were acidophilic). In separate control experiments, administration of Naspm to sham-operated (control) animals did not detectably alter Zn2+ fluorescence (Fig. 4 d and e) or acid fuchsin staining (Fig. 4f) in CA1. Prevention of the late rise in Zn2+ by Naspm is consistent with increased neuron survival at 5 d, but the time resolution does not permit us to conclude that Naspm is acting directly to block Zn2+ through GluR2-lacking AMPARs at the later time. It remains possible that Ca2+ uptake at earlier times leads to the later death and rise in Zn2+.
Fig. 4.
Block of GluR2-lacking AMPARs can prevent the late rise in TSQ-reactive Zn2+ in postischemic CA1 neurons. Coronal sections stained with TSQ showing Zn2+ fluorescence (Left and Center), and adjacent sections were stained with acid fuchsin (Right). Sections from animals subjected to sham operation (a-c), to sham operation followed by Naspm injection (d-f), to global ischemia (g-i), or to global ischemia followed by Naspm (j-l). (a-c) In control hippocampus, TSQ labeling revealed intense fluorescence in axon terminals of dentate granule (DG) neurons in the hilus and stratum lucidum of CA3 and faint fluorescence in the stratum radiatum (sr) and stratum oriens (so) of CA1 and CA3 (a and b); acid fuchsin staining was minimal (c). (d-f) Injection of Naspm at 40 h after sham operation did not detectably alter the Zn2+ fluorescence (d and e) or acidophilia (f) assessed at 72 h after surgery. (g-i) Global ischemia induced a pronounced increase in Zn2+ fluorescence in the cell bodies of CA1 stratum pyramidale (sp) at 72 h (g, arrows, and h) and pronounced acid fuchsin staining (i). (j-l) Injection of Naspm at 40 h after ischemia markedly reduced the ischemia-induced rise in Zn2+ fluorescence (j and k) and acid fuchsin staining (l) in CA1. (n = 5 per group). (Scale bars: Left, 500 μm; Right and Center, 50 μm.)
Discussion
Transient forebrain or global ischemia arising as a consequence of cardiac arrest, cardiac surgery, or near drowning or ischemia that is experimentally induced elicits selective, delayed neuronal death. The most sensitive neurons in terms of insult duration are the hippocampal CA1 pyramidal cells (and scattered hilar interneurons), the loss of which is associated with neurological deficits (2). A striking event is a late rise in cytoplasmic free Zn2+ in cell bodies of the CA1 neurons just before onset of histologically detectable cell death (46-48). Here we show by RT-PCR that global ischemia promotes up-regulation of the transcriptional repressor REST/neuron-restrictive silencer factor and down-regulation of the AMPAR subunit GluR2 in CA1 as early as 6 h after ischemia. The present study is consistent with previous studies involving in situ hybridization (33) and extends those studies to an earlier time point. We also show that AMPA EPSCs at Schaffer collateral to CA1 pyramidal cell synapses exhibit properties diagnostic of Ca2+/Zn2+ permeable, GluR2-lacking AMPARs. At 40-44 h after ischemia, I-V relations of AMPA EPSCs exhibit pronounced inward rectification, faster decay than control, and enhanced sensitivity to the subtype-selective antagonist Naspm. In contrast, AMPA EPSCs in control slices exhibit electrical linearity, slower decay, and less sensitivity to Naspm. These findings provide functional evidence that global ischemia induces a change in the subunit composition of synaptic AMPARs in postischemic CA1. The findings of an intermediate rectification index compared with those of GluR2-lacking and GluR2-containing AMPARs (10, 16) and partial blockade of AMPA currents by Naspm are consistent with the finding of partial reduction in GluR2 mRNA and protein expression after global ischemia (25, 28) and suggest the presence of a combination of GluR2-containing and GluR2-lacking AMPARs at postischemic CA1 synapses. Our findings differ somewhat from the findings of Liu et al. (29) that hippocampal synapses exhibit a very low rectification index (0.21 ± 0.034) at 12 h after ischemia, which is indicative of an essentially pure population of GluR2-lacking AMPARs. These differences could arise because of differences in the duration of ischemia and/or the age of animals used in the two studies. Whereas we used 10-min global ischemia in relatively young (P21-P28) rats, Liu et al. used 5-min global ischemia in older (P90) rats. Further experiments will be required to resolve these differences.
We further show that Naspm injected intrahippocampally at 9-40 h after ischemia affords partial protection against global ischemia-induced neuronal death evaluated at 5 d after ischemia. Moreover, Naspm injected at 40 h largely prevents the late rise in Zn2+ in CA1 neurons evaluated at 72 h after ischemia. Our finding that Naspm blocks the late rise in Zn2+ is consistent with findings that the Zn2+ chelator CaEDTA protects CA1 neurons when administered intraventricularly as late as 60 h after ischemia in vivo (46). Naspm blocks Zn2+ accumulation and affords protection in an acute slice model of oxygen-glucose deprivation in vitro (49). However, in this preparation, Zn2+ accumulation and cell death are more rapid, occurring by 4 h after oxygen-glucose deprivation, and CA3 is as sensitive as CA1. Our finding that late injection of Naspm protects CA1 neurons against ischemia-induced death is also consistent with the finding that overexpression of GluR2(R) AMPAR subunits protects CA1 neurons against global ischemia (29); both treatments would reduce Ca2+ and Zn2+ permeation through AMPARs, although by different mechanisms.
The Timing Issue. The rise in Zn2+ to toxic levels and onset of histologically detectable death of CA1 neurons in response to transient global ischemia in vivo does not occur until >48 h after the insult. In contrast, the pharmacological effective life time of Naspm in vivo, although unknown, is likely to be on the order of several hours; thus, the duration of direct action of Naspm injected at 9 h or even 40 h is unlikely to last until the rise in Zn2+ and death of CA1 neurons. [The duration of action of CaEDTA injected into the lateral ventricle appears to be ≈1 h (52).] Furthermore, the less effective protection afforded by Naspm at 20-30 h relative to that at 9 or 14 h after ischemia indicates that early and late Naspm act by means of distinct mechanisms to protect postischemic CA1 neurons. How then does early Naspm prevent the late rise in neurotoxic Zn2+? Under control conditions, GluR2-lacking AMPARs are expressed at low density on distal dendrites of CA1/CA3 pyramidal neurons (53-55). One possibility is that Naspm injected at early times blocks these GluR2-lacking AMPARs, thus preventing influx of Ca2+ and/or Zn2+ as part of a death cascade that leads to the much later dramatic rise in Zn2+. The prevention of the late rise in Zn2+ by Naspm at 40 h is consistent with increased neuron survival at 5 d, but the time resolution does not permit us to conclude that even at 40 h Naspm is acting directly to block Zn2+ influx through GluR2-lacking AMPARs at the later time.
The Source of the Late-Appearing Zn2+. An important unresolved issue is the source of the free Zn2+ in CA1 neurons that appears long after ischemia. Early studies proposed that anoxic depolarization or hyperactivity caused Zn2+ to be released from presynaptic vesicles and translocated into postsynaptic neurons (47, 56). This notion was predicated in part on measurements of Zn2+ release from presynaptic terminals of the hippocampus (57). However, Zn2+ accumulates in degenerating CA1/CA3 pyramidal neurons of ZnT-3-null mice after kainate-elicited seizures despite the virtual absence of vesicular Zn2+ (50). These findings suggest that the accumulation of free Zn2+ in postischemic neurons of wild-type animals originates from sources other than synaptic vesicles as, for example, intracellular stores. Consistent with this mechanism, exposure of neurons in culture to oxidative stress promotes the release of Zn2+ from metallothioneins and other intracellular stores, an event that may be critical to the initiation of neuronal apoptosis (51).
Mechanisms by Which Late Zn2+ Promotes Neuronal Death. The molecular mechanisms by which Zn2+ mediates neuronal death have begun to be unraveled. Whereas Zn2+ at relatively low concentrations (≈20 μM) elicits neuronal death with the characteristics of apoptosis, Zn2+ at higher concentrations (50-100 μM) elicits neuronal death with the characteristics of necrosis (58). Mechanisms by which Zn2+ elicits neuronal death include the production of free radicals, loss of mitochondrial membrane potential, formation of the mitochondrial transition pore, release of cytochrome c, production of reactive oxygen species, and reduction in ATP (43-45, 59). Whereas Zn2+ at near physiological concentrations is necessary for the breakdown of the functional integrity of the mitochondrial outer membrane, caspase activation, and transcriptional events critical to cell death, late Zn2+ at high intracellular concentrations promotes the induction of p75NTR, p75NTR-associated death executor in neurons, DNA fragmentation, and apoptosis (46, 48).
Clinical Implications. Perhaps the most striking findings of the present study are that late Naspm administration largely prevents the late rise in Zn2+ and affords partial neuroprotection of CA1 neurons. These findings implicate GluR2-lacking AMPARs expressed at CA1 synapses at late times after ischemia in the late rise in Zn2+ and death of CA1 neurons. However, the time resolution does not permit the inference that Naspm is blocking uptake of the late-appearing cytosolic free Zn2+. In addition, the early opening of the therapeutic window suggests that blocking GluR2-lacking receptors, which are expressed at low density in hippocampal neurons under physiological conditions, can also lead to neuroprotection. The therapeutic window is open for a remarkably long period, like that for protection by Zn2+ chelation with CaEDTA (46). For CaEDTA, also, the source of late cytosolic free Zn2+ is ambiguous; by reducing extracellular Zn2+, CaEDTA may prevent Zn2+ influx or it may “suck” Zn2+ out of the cell that otherwise would accumulate from intracellular stores. Ca2+-permeable AMPARs are implicated in the neuronal death associated with other neurological insults and disorders, including seizures (23, 24, 60-62), kainic-induced excitotoxicity (44, 63, 64), spinal cord injury (44, 65), amyotrophic lateral sclerosis (66, 67), and Alzheimer's disease (68). The present study provides further compelling evidence for a causal role for GluR2-lacking AMPARs in delayed hippocampal injury and highlights GluR2-lacking AMPARs as an important therapeutic target for intervention in the delayed neuronal death associated with neuronal insults and neurological disorders.
Supplementary Material
Acknowledgments
We thank R. Regis for technical assistance and C. Hall and C. Wang for statistical analysis. This work was supported in part by National Institutes of Health Grants NS46742 and NS45693 (to R.S.Z.) and NS45287 (to M.V.L.B.) and by the F. M. Kirby Program in Neuroprotection and Repair. M.V.L.B. is the Sylvia and Robert S. Olnick Professor of Neuroscience.
Author contributions: K.-M.N., H.Y., P.E.C., R.S.Z., and M.V.L.B. designed research; K.-M.N., H.Y., T.M., and P.E.C. performed research; K.-M.N., H.Y., T.M., P.E.C., R.S.Z., and M.V.L.B. analyzed data; and R.S.Z. and M.V.L.B. wrote the paper.
Abbreviations: AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; AMPAR, AMPA receptor; EPSC, excitatory postsynaptic current; GluR, glutamate receptor; Naspm, 1-naphthyl acetyl spermine; REST, restrictive-element-1-silencing transcription factor; TSQ, N-6-(methoxy-8-quinolyl)-para-toluenesulfonamide.
References
- 1.Lo, E. H., Dalkara, T. & Moskowitz, M. A. (2003) Nat. Rev. Neurosci. 4, 399-415. [DOI] [PubMed] [Google Scholar]
- 2.Zukin, R. S., Jover, T., Yokota, H., Calderone, A., Simionescu, M., Lau, C. G. (2004) in Stroke Pathophysiology, Diagnosis, and Management, eds. Mohr, J. P., Choi, D. W., Grotta, J. C., Weir, B. & Wolf, P. A. (Churchill Livingstone, Philadelphia), pp. 829-854.
- 3.Kirino, T. (1982) Brain Res. 239, 57-69. [DOI] [PubMed] [Google Scholar]
- 4.Pulsinelli, W. A., Brierley, J. B. & Plum, F. (1982) Ann. Neurol. 11, 491-498. [DOI] [PubMed] [Google Scholar]
- 5.Rosenbaum, D. M., D'Amore, J., Llena, J., Rybak, S., Balkany, A. & Kessler, J. A. (1998) Ann. Neurol. 43, 654-660. [DOI] [PubMed] [Google Scholar]
- 6.Chen, J., Zhu, R. L., Nakayama, M., Kawaguchi, K., Jin, K., Stetler, R. A., Simon, R. P. & Graham, S. H. (1996) J. Neurochem. 67, 64-71. [DOI] [PubMed] [Google Scholar]
- 7.Pulsinelli, W., Sarokin, A. & Buchan, A. (1993) Prog. Brain Res. 96, 125-135. [DOI] [PubMed] [Google Scholar]
- 8.Sheardown, M. J., Suzdak, P. D. & Nordholm, L. (1993) Eur. J. Pharmacol. 236, 347-353. [DOI] [PubMed] [Google Scholar]
- 9.Buchan, A. M., Li, H., Cho, S. & Pulsinelli, W. A. (1991) Neurosci. Lett. 132, 255-258. [DOI] [PubMed] [Google Scholar]
- 10.Dingledine, R., Borges, K., Bowie, D. & Traynelis, S. F. (1999) Pharmacol. Rev. 51, 7-61. [PubMed] [Google Scholar]
- 11.Burnashev, N., Monyer, H., Seeburg, P. H. & Sakmann, B. (1992) Neuron 8, 189-198. [DOI] [PubMed] [Google Scholar]
- 12.Hollmann, M., Hartley, M. & Heinemann, S. (1991) Science 252, 851-853. [DOI] [PubMed] [Google Scholar]
- 13.Verdoorn, T. A., Burnashev, N., Monyer, H., Seeburg, P. H. & Sakmann, B. (1991) Science 252, 1715-1718. [DOI] [PubMed] [Google Scholar]
- 14.Bowie, D. & Mayer, M. L. (1995) Neuron 15, 453-462. [DOI] [PubMed] [Google Scholar]
- 15.Donevan, S. D. & Rogawski, M. A. (1995) Proc. Natl. Acad. Sci. USA 92, 9298-9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu, S. Q. & Cull-Candy, S. G. (2000) Nature 405, 454-458. [DOI] [PubMed] [Google Scholar]
- 17.Swanson, G. T., Kamboj, S. K. & Cull-Candy, S. G. (1997) J. Neurosci. 17, 58-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Passafaro, M., Piech, V. & Sheng, M. (2001) Nat. Neurosci. 4, 917-926. [DOI] [PubMed] [Google Scholar]
- 19.Greger, I. H., Khatri, L. & Ziff, E. B. (2002) Neuron 34, 759-772. [DOI] [PubMed] [Google Scholar]
- 20.Greger, I. H., Khatri, L., Kong, X. & Ziff, E. B. (2003) Neuron 40, 763-774. [DOI] [PubMed] [Google Scholar]
- 21.Wenthold, R. J., Petralia, R. S., Blahos, J., II, & Niedzielski, A. S. (1996) J. Neurosci. 16, 1982-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar, S. S., Bacci, A., Kharazia, V. & Huguenard, J. R. (2002) J. Neurosci. 22, 3005-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang, Y., Meyers, S. J. & Dingledine, R. (1999) Nat. Neurosci. 2, 867-872. [DOI] [PubMed] [Google Scholar]
- 24.Friedman, L. K. (1998) Hippocampus 8, 511-525. [DOI] [PubMed] [Google Scholar]
- 25.Pellegrini-Giampietro, D. E., Zukin, R. S., Bennett, M. V. L., Cho, S. & Pulsinelli, W. A. (1992) Proc. Natl. Acad. Sci. USA 89, 10499-10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorter, J. A., Petrozzino, J. J., Aronica, E. M., Rosenbaum, D. M., Opitz, T., Bennett, M. V. L., Connor, J. A. & Zukin, R. S. (1997) J. Neurosci. 17, 6179-6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka, H., Grooms, S. Y., Bennett, M. V. L. & Zukin, R. S. (2000) Brain Res. 886, 190-207. [DOI] [PubMed] [Google Scholar]
- 28.Opitz, T., Grooms, S. Y., Bennett, M. V. L. & Zukin, R. S. (2000) Proc. Natl. Acad. Sci. USA 97, 13360-13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, S., Lau, L., Wei, J., Zhu, D., Zou, S., Sun, H. S., Fu, Y., Liu, F. & Lu, Y. (2004) Neuron 43, 43-55. [DOI] [PubMed] [Google Scholar]
- 30.Tsubokawa, H., Oguro, K., Masuzawa, T. & Kawai, N. (1994) J. Neurophysiol. 71, 1190-1196. [DOI] [PubMed] [Google Scholar]
- 31.Tsubokawa, H., Oguro, K., Masuzawa, T., Nakaima, T. & Kawai, N. (1995) J. Neurophysiol. 74, 218-225. [DOI] [PubMed] [Google Scholar]
- 32.Oguro, K., Oguro, N., Kojima, T., Grooms, S. Y., Calderone, A., Zheng, X., Bennett, M. V. L. & Zukin, R. S. (1999) J. Neurosci. 19, 9218-9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calderone, A., Jover, T., Noh, K.-M., Tanaka, H., Yokota, H., Lin, Y., Grooms, S., Regis, R., Bennett, M. V. L. & Zukin, R. S. (2003) J. Neurosci. 23, 2112-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chevaleyre, V. & Castillo, P. E. (2002) Proc. Natl. Acad. Sci. USA 99, 9538-9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frederickson, C. J., Kasarskis, E. J., Ringo, D. & Frederickson, R. E. (1987) J. Neurosci. Methods 20, 91-103. [DOI] [PubMed] [Google Scholar]
- 36.Rozov, A. & Burnashev, N. (1999) Nature 401, 594-598. [DOI] [PubMed] [Google Scholar]
- 37.Kamboj, S. K., Swanson, G. T. & Cull-Candy, S. G. (1995) J. Physiol. (London) 486, 297-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koh, D. S., Burnashev, N. & Jonas, P. (1995) J. Physiol. (London) 486, 305-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geiger, J. R., Melcher, T., Koh, D. S., Sakmann, B., Seeburg, P. H., Jonas, P. & Monyer, H. (1995) Neuron 15, 193-204. [DOI] [PubMed] [Google Scholar]
- 40.Koike, M., Iino, M. & Ozawa, S. (1997) Neurosci. Res. 29, 27-36. [DOI] [PubMed] [Google Scholar]
- 41.Blaschke, M., Keller, B. U., Rivosecchi, R., Hollmann, M., Heinemann, S. & Konnerth, A. (1993) Proc. Natl. Acad. Sci. USA 90, 6528-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogoshi, F. & Weiss, J. H. (2003) J. Neurosci. 23, 10521-10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi, D. W. & Koh, J. Y. (1998) Annu. Rev. Neurosci. 21, 347-375. [DOI] [PubMed] [Google Scholar]
- 44.Weiss, J. H. & Sensi, S. L. (2000) Trends. Neurosci. 23, 365-371. [DOI] [PubMed] [Google Scholar]
- 45.Dineley, K. E., Votyakova, T. V. & Reynolds, I. J. (2003) J. Neurochem. 85, 563-570. [DOI] [PubMed] [Google Scholar]
- 46.Calderone, A., Jover, T., Mashiko, T., Noh, K. M., Tanaka, H., Bennett, M. V. L. & Zukin, R. S. (2004) J. Neurosci. 24, 9903-9913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koh, J. Y., Suh, S. W., Gwag, B. J., He, Y. Y., Hsu, C. Y. & Choi, D. W. (1996) Science 272, 1013-1016. [DOI] [PubMed] [Google Scholar]
- 48.Park, J. A., Lee, J. Y., Sato, T. A. & Koh, J. Y. (2000) J. Neurosci. 20, 9096-9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yin, H. Z., Sensi, S. L., Ogoshi, F. & Weiss, J. H. (2002) J. Neurosci. 22, 1273-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee, J. Y., Cole, T. B., Palmiter, R. D. & Koh, J. Y. (2000) J. Neurosci. 20, RC79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aizenman, E., Stout, A. K., Hartnett, K. A., Dineley, K. E., McLaughlin, B. & Reynolds, I. J. (2000) J. Neurochem. 75, 1878-1888. [DOI] [PubMed] [Google Scholar]
- 52.Frederickson, C. J., Suh, S. W., Koh, J. Y., Cha, Y. K., Thompson, R. B., LaBuda, C. J., Balaji, R. V. & Cuajungco, M. P. (2002) J. Histochem. Cytochem. 50, 1659-1662. [DOI] [PubMed] [Google Scholar]
- 53.Lerma, J., Morales, M., Ibarz, J. M. & Somohano, F. (1994) Eur. J. Neurosci. 6, 1080-1088. [DOI] [PubMed] [Google Scholar]
- 54.Toomim, C. S. & Millington, W. R. (1998) J. Comp. Neurol. 402, 141-154. [PubMed] [Google Scholar]
- 55.Yin, H. Z., Sensi, S. L., Carriedo, S. G. & Weiss, J. H. (1999) J. Comp. Neurol. 409, 250-260. [PubMed] [Google Scholar]
- 56.Tonder, N., Johansen, F. F., Frederickson, C. J., Zimmer, J. & Diemer, N. H. (1990) Neurosci. Lett. 109, 247-252. [DOI] [PubMed] [Google Scholar]
- 57.Li, Y., Hough, C. J., Suh, S. W., Sarvey, J. M. & Frederickson, C. J. (2001) J. Neurophysiol. 86, 2597-2604. [DOI] [PubMed] [Google Scholar]
- 58.Kim, Y. H., Kim, E. Y., Gwag, B. J., Sohn, S. & Koh, J. Y. (1999) Neuroscience 89, 175-182. [DOI] [PubMed] [Google Scholar]
- 59.Frederickson, C. J., Koh, J. Y. & Bush, A. I. (2005) Nat. Rev. Neurosci. 6, 449-462. [DOI] [PubMed] [Google Scholar]
- 60.Friedman, L. K., Pellegrini-Giampietro, D. E., Sperber, E. F., Bennett, M. V. L., Moshe, S. L. & Zukin, R. S. (1994) J. Neurosci. 14, 2697-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Friedman, L. K., Sperber, E. F., Moshe, S. L., Bennett, M. V. L. & Zukin, R. S. (1997) Dev. Neurosci. 19, 529-542. [DOI] [PubMed] [Google Scholar]
- 62.Huang, Y., Myers, S. J. & Dingledine, R. (1999) Nat. Neurosci. 2, 867-872. [DOI] [PubMed] [Google Scholar]
- 63.Lu, Y. M., Yin, H. Z., Chiang, J. & Weiss, J. H. (1996) J. Neurosci. 16, 5457-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carriedo, S. G., Yin, H. Z., Sensi, S. L. & Weiss, J. H. (1998) J. Neurosci. 18, 7727-7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grossman, S. D., Wolfe, B. B., Yasuda, R. P. & Wrathall, J. R. (1999) J. Neurosci. 19, 5711-5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kawahara, Y., Ito, K., Sun, H., Aizawa, H., Kanazawa, I. & Kwak, S. (2004) Nature 427, 801. [DOI] [PubMed] [Google Scholar]
- 67.Takuma, H., Kwak, S., Yoshizawa, T. & Kanazawa, I. (1999) Ann. Neurol. 46, 806-815. [DOI] [PubMed] [Google Scholar]
- 68.Ikonomovic, M. D., Mizukami, K., Davies, P., Hamilton, R., Sheffield, R. & Armstrong, D. M. (1997) J. Neuropathol. Exp. Neurol. 56, 1018-1027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.