Abstract
Because of a dearth of Cenozoic grass fossils, the timing of the taxonomic diversification of modern subclades within the grass family (Poaceae) and the rise to ecological dominance of open-habitat grasses remain obscure. Here, I present data from 99 Eocene to Miocene phytolith assemblages from the North American continental interior (Colorado, Nebraska, Wyoming, and Montana/Idaho), constituting the only high-resolution mid-Cenozoic record of grasses. Analyses of these assemblages show that open-habitat grasses had undergone considerable taxonomic diversification by the earliest Oligocene (34 million years ago) but that they did not become ecologically dominant in North America until 7–11 million years later (Late Oligocene or Early Miocene). This pattern of decoupling suggests that environmental changes (e.g., climate changes), rather than taxonomic radiations within Poaceae, provided the key opportunity for open-habitat grasses to expand in North America.
Keywords: grasslands, phytoliths, Great Plains
Grasses are today of immense importance, both ecologically, in the form of grass-dominated vegetation (e.g., steppes and savannas), and economically, as cereals and feed for domesticated animals. The evolution of grasses and grasslands played a fundamental role in the formation of modern ecosystems and has captured the attention of botanists, geologists, and paleontologists alike (1). Still, despite over a century of research, the evolutionary history of the grass clade is largely unknown. Pollen data from northern Gondwana may indicate a Late Cretaceous or Paleocene origin of Poaceae [70–55 Ma (million years ago) (2, 3)], and unequivocal graminoid reproductive structures from Early Eocene deposits reveal that crown-group grasses existed in North America from this time onward (4, 5). However, for most of the Cenozoic, the fossil record of grasses is extremely poor, providing little insight into taxonomic diversification patterns within the family (1). Abundant and diverse grass fossils (e.g., pollen, leaves, and reproductive structures) do not appear until the Middle to Late Miocene, pointing to a scenario of ongoing taxonomic diversification within Poaceae in tandem with a successive spread of grass-dominated vegetation during this time (1, 6). A roughly simultaneous taxonomic proliferation and rise to ecological dominance of the grass family long after its origin is thought to have been stimulated by changes in global and regional climates toward increased seasonal aridity during the Neogene (7–9).
Other lines of evidence are partly or fully at odds with this scenario. Molecular phylogenetic dating within the grass family, which is complicated by the non-clock-like behavior of the genes used (5, 10), has led to the suggestion that the main taxonomic diversification of modern grass subclades [e.g., Pooideae and PACCAD (Panicoideae, Arundinoideae, Chloridoideae, Centothecoideae, Aristidoideae, and Danthonioideae)] occurred sometime between 25 and 15 Ma (10). However, other molecular clock estimates indicate that these groups may have originated considerably earlier (>50 Ma) (5). The timing of the rise to ecological dominance of grasses is equally uncertain (1, 11). In North America, several lines of evidence provide notably older time estimates for the spread of grasslands [evolution of grazing ungulates: late Early Miocene (1, 12); phytolith data: Early Miocene (13); paleosol data: Early Oligocene (14, 15)], suggesting that the Middle Miocene date represents an underestimate owing to insufficient data rather than a true pattern. Based on stable carbon isotopes in horse teeth (16) and paleosols (17), it has been hypothesized that the earliest grasslands were composed mainly of grasses using a C3 photosynthetic pathway (pooids and certain PACCAD grasses) that today thrive in cooler and moister habitats. C4 grasses tolerant of warmer and more arid conditions, and of lower atmospheric CO2 (many PACCAD grasses), became dominant only in the Late Miocene (7–5 Ma) (1, 16, 17).
Here, I use a high-resolution Cenozoic record of bio-opal from vascular plants (phytoliths) along a latitudinal transect of the North American continental interior to enable a look at patterns of grass evolution and ecological change. Phytolith assemblages record information about vegetation type, such as degree of habitat openness (13, 18). In particular, phytoliths can be used to distinguish grass subclades (19, 20), which is of key importance in vegetation inference, but also provide an opportunity to track taxonomic diversification patterns within Poaceae.
Materials and Methods
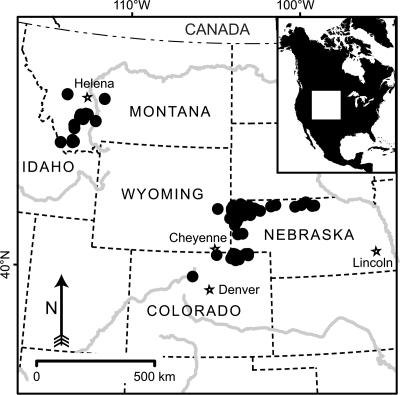
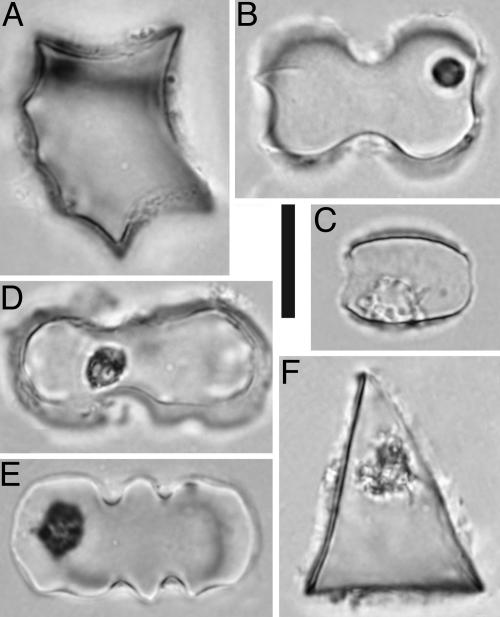
Eocene to early Late Miocene (≈40–9 Ma) sediment samples were collected from Montana, Idaho, Nebraska, Wyoming, and Colorado (Fig. 1). To ensure maximum age control, lithostratigraphical-type and reference sections and well known faunal localities were primarily chosen (see the supporting information, which is published on the PNAS web site). For each chronostratigraphical unit, all available facies were sampled to test for spatial variation in vegetation. Phytoliths and other biosilica (e.g., diatoms and sponge spicules) were extracted by using modified standard methods (13), resulting in 99 productive assemblages, of which 93 were sufficiently well preserved to allow quantitative analysis. Classification and analysis of phytoliths principally followed Strömberg (13); however, description and quantitative analysis of a more comprehensive reference collection of phytoliths from modern plants (170 taxa from 70 families of vascular plants) allowed a refinement of previous morphotype assignments. As a result, phytoliths typical of important subclades could be identified with enhanced precision and confidence (Fig. 2) compared with previous studies (13). The following classes of phytoliths were used in vegetation inference:
Fig. 1.
Geographic distribution of localities at which sediment that was productive for phytoliths was collected. (See the supporting information.) Symbols often represent several closely spaced localities.
Fig. 2.
Selected diagnostic GSSC morphotypes in phytolith assemblages from the northern and central Great Plains (Eocene to Miocene). (A) Chusquea-type rondel (Bambusoideae). (B) Inverted bilobate (PACCAD clade). (C) Saddle (Chloridoideae). (D) Stipa-type bilobate (Pooideae). (E) Crenate (Pooideae). (F) Tall, keel-shaped rondel from the Eocene–Oligocene of Montana (unknown affinity). (Scale bar, 10 μm.)
Forest indicator (FI) forms from (i) woody or herbaceous dicotyledons, conifers, and ferns, (ii) palms, and (iii) spiral gingers (Costaceae).
Grass silica short cells (GSSCs) (Fig. 2), exclusive to grasses, including forms typical of (i) closed-habitat grasses (bambusoid, ehrhartoid, and basal grasses) and (ii) the two clades of open-habitat grasses: pooids and PACCAD grasses (10, 21).
Phytoliths from wetland plants, including sedges.
Nondiagnostic and unclassified phytoliths.
Overall vegetation structure (openness) was determined through analysis of (i) the amount of FI vs. GSSC morphotypes and (ii) grass community composition (open-habitat vs. closed-habitat grasses) in each assemblage. The diversity and distribution in each sample of morphotypes within the FI class (forms typically produced by conifers vs. dicotyledons vs. palms, etc.) were studied to further characterize the type of vegetation as far as is presently possible with phytolith analysis (13). Each grass (or other plant) produces an array of phytolith morphotypes (multiplicity), which may, in addition, show morphological overlap with phytoliths produced by other grasses/plants (redundancy) (22). To interpret grass community composition, the GSSC assemblages were therefore compared statistically to modern grasses (hypothesis testing using bootstrap analysis; see the supporting information). The presence of rare open-habitat grasses in GSSC assemblages with a high percentage of closed-habitat morphotypes was determined based on consideration of the statistical analyses as well as identification of particular morphotypes with high diagnostic value (see the supporting information). The relative contribution of pooids vs. PACCAD grasses was similarly examined. In addition, GSSC morphotypes that provide more specific information about the grass taxa present (e.g., Chusquea-type GSSCs) (13, 20) were noted. Wetland phytoliths and nondiagnostics were excluded from the main vegetation analysis, but sedge morphotypes plus diatoms and sponge spicules provided information regarding proximity to water.
Results and Discussion
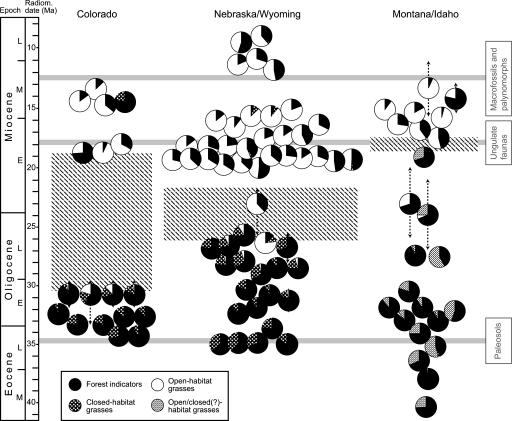
The phytolith assemblages of the central Great Plains (Colorado, Nebraska, and Wyoming) show a clear vegetation pattern independent of facies (Fig. 3). Late Eocene and Early Oligocene assemblages are dominated by diverse FI morphotypes, including primarily forms that are typically produced by deciduous and evergreen dicotyledons, as well as relatively abundant palm phytoliths. GSSC assemblages are indicative of bamboos with affinity to the extant genus Chusquea (Fig. 2 A), representing the earliest well dated evidence for Bambusoideae in the fossil record (23). These assemblages suggest the presence of closed forest with palms and a bamboo understory in the area throughout the Early Oligocene (13). Beginning in the Early Oligocene (≈32–30 Ma), low to moderate frequencies of typical pooid and PACCAD GSSCs (Fig. 2 B, D, and E) observed in some assemblages indicate that open-habitat grasses had evolved locally or immigrated into the area and persisted in the understory or in forest glades. This date for the local appearance of open-habitat grasses is comparable to previously described rare leaves and reproductive structures with affinity to the genus Stipa from Late Eocene (≈34 Ma) and Early Oligocene (≈33 Ma) deposits in Colorado (23–26).
Fig. 3.
Record of vegetation changes in the North American continental interior based on phytolith assemblages (represented as pie charts) compared with previous work. Areas with oblique hatched pattern represent timing of the spread of grass-dominated habitats according to phytoliths; the height of the areas reflects the degree of uncertainty in timing for each region due to missing data and problems with relative and absolute age assignment (marked as black dashed arrows bracketing phytolith assemblages). Inferred dates for the spread of grasslands from other lines of evidence [macrofossils and palynomorphs, ungulate faunas, and paleosols, respectively (1)] are marked as thick gray lines. “Open/closed(?)-habitat grasses” indicates grasses with unknown autecology but potentially related to pooids or PACCAD grasses [e.g., chionochloids (29)] (Fig. 2F).
Early Miocene assemblages show the same range of FI morphotypes as older samples, but contrast in being largely dominated by GSSC morphotypes. Among the GSSCs, diagnostic open-habitat types, mainly suggestive of stipoid pooids, dominate, although typical PACCAD forms are abundant (up to 41% of GSSCs) in certain samples. With few exceptions, closed-habitat types are rare or absent. These assemblages indicate a shift to relatively open habitats with a mixture of trees and mainly C3 pooid grasses, such as woodlands or savannas [as defined without consideration of climate (27)] in the area. More closed habitats were also present, pointing to spatial or temporal heterogeneity in vegetation. Because of the shortage of Late Oligocene to Early Miocene deposits in Colorado, this regional vegetation change is best documented in the Nebraska/Wyoming record, where it is constrained to the latest Oligocene or earliest Miocene (26.5–21.9 Ma). This date is markedly earlier than that inferred from previous paleobotanical and faunal studies and substantially later than that interpreted from paleosols (Fig. 3) (1, 13).
The phytolith record points to further changes in grass communities during the Miocene. The occurrence of several new morphotypes (e.g., various bilobate and cross forms) in modest abundances implies an increased taxonomic diversity of pooid and PACCAD grasses in the late Early Miocene and early Late Miocene (≈19–15.5 Ma), which is also in agreement with the macrofossil record (6). Low frequencies of true saddle GSSC morphotypes (Fig. 2C) at ≈19 Ma mark the earliest evidence for the Chloridoideae subclade (mainly C4) in the Great Plains; previously described chloridoid fossils date to ≈14 Ma (28). Nevertheless, a predominantly C3 grassland in the central Great Plains is consistent with isotopic studies of paleosols (17).
The record from the northern Rocky Mountains (Montana/Idaho) shows a similar pattern. Rare pooid grasses appear in Early Oligocene forests and expand substantially later into grass-dominated habitats with a ground cover of chiefly pooids. These woodlands or savannas show strong similarities to vegetation types in the central Great Plains. However, phytolith data also suggest biogeographical differences within the continental interior. For example, the change to pooid-dominated habitats seems to have occurred later in Montana/Idaho, by the late Early Miocene, although more data are needed to verify this pattern. Also, the grasses dominating Eocene and Oligocene grass communities (and, in some cases, vegetation overall) are not typical bamboos or basal grasses. Instead, their GSSCs resemble those produced by certain pooids and PACCAD grasses [e.g., “chionochloids” (29)] (Fig. 2F), implying that open habitats may have existed in the northern Rocky Mountains as early as the Late Eocene. However, the phylogenetic affinity, and thus the autecology, of these grasses is far from clear.
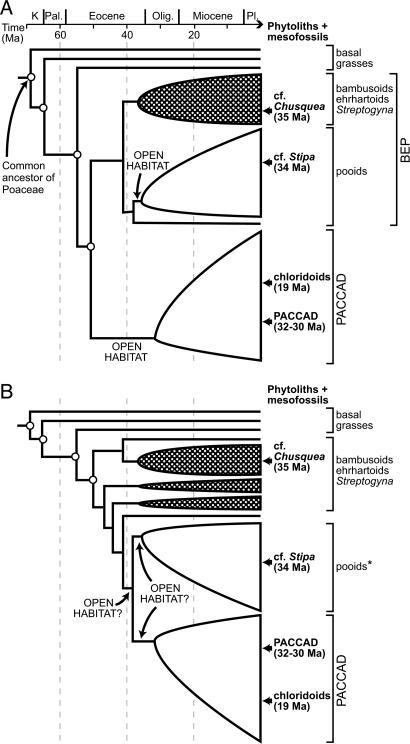
The phytolith record also constrains the timing for major cladogenetic events within Poaceae, such as the divergence of PACCAD grasses and the BEP (Bambusoideae, Erharthoideae, and Pooideae) clade (ref. 10 and Fig. 4A). The inferred presence in the Great Plains of Chusquea-like bambusoids by 35 Ma and Stipa-like pooids by 34 Ma indicates that the Pooideae and the (Bambusoideae + Erharthoideae + Streptogyna) clade, and, by inference, the BEP and PACCAD clades, had diverged by at least the Late Eocene (≈35 Ma). If alternative tree topologies (30–32) are used (Fig. 4B), the divergence of pooids and PACCAD grasses would still have occurred by 34 Ma. These dates are consistent with recent molecular clock estimates for the origin of these subclades (5). Diversification of chloridoids within the PACCAD clade by at least 19 Ma is also suggested by phytoliths.
Fig. 4.
Dating of taxonomic radiation within subclades of the grass family (Poaceae) by using fossil phytoliths (this study) and macrofossils (23–26). Approximate ages for basal nodes (marked with an open circle; 95% confidence intervals not shown) are provided by molecular clock analysis (5). (A) Phylogeny in which the BEP clade is sister to the PACCAD clade (10). (B) Phylogeny in which the Pooideae is sister to the PACCAD grasses (30). The shift to open habitats could have occurred once or twice. Open shapes, openhabitat grass clades; black shapes with white dots and all other terminal taxa, closed-habitat grasses; “OPEN HABITAT,” shift to open habitats; *, except Brachyelytrum.
Furthermore, because Chusquea and Stipa (and their close relatives) are nested well within Bambusoideae and Pooideae, respectively (10, 30, 31), some taxonomic diversification within these groups must have occurred before they were first recorded by phytoliths and macrofossils in the Great Plains (that is, by ≥34 Ma). The implication of this inference is that pooids had undergone a taxonomic radiation minimally 7–11 million years before they became ecologically important in North America. This pattern of delayed ecological expansion of open-habitat grasses contradicts the notion that the spread of grasslands occurred in parallel with taxonomic diversification on this continent (1, 6). The traditional notion also holds that the Late Eocene to Oligocene of the North American continental interior was characterized by open, but grass-free, ecosystems into which grasses later expanded (1, 33). The Eocene and Oligocene phytolith record, which suggests that open-habitat grasses occurred as rare or marginal members of forest communities, contrasts strongly with this scenario. Instead, the current study indicates that external factors likely triggered alterations in vegetation structure during the Late Oligocene or Early Miocene, allowing open-habitat grasses and other plants with traits appropriate for life in open habitats to spread at the expense of forest trees and closed-habitat grasses. However, the main taxonomic diversification of Poaceae must be attributed to something besides Neogene climate deterioration.
Among the several potential environmental influences on the ecological success of open-habitat grasses, the most commonly discussed is climate change (34). The Late Oligocene was characterized by a global warming trend (7), which was interrupted by a cooling event at the Oligocene–Miocene boundary that coincided with a period of depressed seasonality, manifested primarily through cooler summers (35). Thus, warmer climates and an instance of reduced seasonality on a global scale may have coincided with the earliest grass-dominated habitats rather than the increasing seasonal dryness generally assumed to be among the major drivers of grassland expansion (8, 9). Lowered CO2 levels during the Cenozoic are regularly used to elucidate the origin of C4 photosynthesis in grasses (36, 37) and the advent of C4 grasslands in the Late Miocene (7–5 Ma) (38), but their role in the spread of primarily C3 open-habitat grasses by the Early Miocene is less clear. It has recently been proposed that interaction between low CO2 levels and frequent fires may promote expansion of grasses (and other herbaceous plants) at the expense of forest trees under higher rainfall conditions (39). According to this model, an augmented occurrence of fire or other disturbances (e.g., herbivory) limits the abundance of trees, and a lowered CO2 level acts to suppress the postburn recovery growth rates of trees. To my knowledge, there is no fossil evidence of fire (charcoal) described from North America to test this hypothesis. However, sedimentary records from Africa and the Western Pacific that show a marked increase in the abundance of charred grass cuticle in the Late Miocene (≤10 Ma) (14, 40) have been used to argue an influence of fire on vegetation structure only after this time. In contrast, the role of herbivory should be investigated in more detail by using the rich and well described fossil record of North American mammals (41).
The pattern of delayed ecological expansion relative to taxonomic radiation of open-habitat grasses in North America can be explained in two ways. It may be that open-habitat grasses experienced parallel taxonomic and ecological expansion outside the North American continent and that the Early Oligocene pooids observed in the Great Plains represent grasses at the border of their ecological extension. It is often hypothesized that the evolution in South America of notoungulates with presumed adaptations for grazing implies the presence of grass-dominated habitats in the Early Oligocene (1, 42, 43). However, there is as yet no clear paleobotanical evidence for pooids or PACCAD grasses in South America until the Late Miocene (1), and recent biogeographic analyses are unable to resolve the ancestral distribution of the BEP + PACCAD clade (5).
Alternatively, substantial cladogenesis within the pooid and PACCAD clades occurred in North America in or before the Early Oligocene, and the observed offset between taxonomic radiation and ecological success represents a true pattern of evolution. Although there is evidence for crown-group grasses in North America by the Early Eocene (4, 5), current phytolith data do not provide strong evidence for speciation of open-habitat grasses in the Great Plains. However, this lack of evidence may relate to the low number of GSSCs of open-habitat grasses counted in Eocene samples, as well as limits in taxonomic resolution currently achieved through phytolith analysis. The hypothesis should be tested by detailed systematic work on phytoliths in tandem with sampling in other geographic regions and in older strata. If true, this pattern reiterates, at a lower taxonomic rank, the well known lag (≤30 Ma) between angiosperm taxonomic radiation and rise to ecological dominance during the Cretaceous (44, 45) and parallels patterns in other highly successful clades [e.g., ants, termites, and wasps (46)]. On a smaller scale, the presence of possible C4 grasses long before the worldwide expansion of C4-dominated ecosystems in the Late Miocene, documented herein (at ≈19 Ma) and elsewhere (17, 37), points to a similar ecological delay.
Supplementary Material
Acknowledgments
This research forms part of dissertation work at University of California, Berkeley. I thank my dissertation committee, D. R. Lindberg, N. C. Arens, C. M. D'Antonio, and D. R. Kaplan, for their support. H. E. LaGarry, L. A. LaGarry, R. M. Hunt, B. E. Bailey, R. Knudsen, B. Beasley, A. D. Barnosky, D. L. Hanneman, R. Nichols, A. R. Tabrum, D. Lofgren, D. L. Rasmussen, D. G. Kron, E. Evanoff, R. W. Graham, P. A. Holroyd, C. Christensen, R. H. Tedford, and C. Collins helped me obtain samples in the field and in museums (University of California, Berkeley; American Museum of Natural History, New York; University of Montana Museum of Paleontology, Missoula; and University of Nebraska State Museum, Lincoln). J. G. Jones, S. C. Mulholland, and D. R. Piperno taught me about phytolith laboratory methods and identification. Comments from E. M. Friis, C. Rydin, D. Cantrill, L. Werdelin, G. D. Wesley-Hunt, G. P. Wilson, and three anonymous reviewers improved the manuscript. This work was supported by grants from University of California, Berkeley, the Royal Academy of Science of Sweden, the Geological Society of America, the Paleontological Society of America, Sigma Xi, and the National Science Foundation (Dissertation Improvement Grant DEB-1-0104975), as well as a Swedish Research Council grant (to E. M. Friis and L. Werdelin). This is University of California Museum of Paleontology contribution 1897.
Author contributions: C.A.E.S. designed research, performed research, contributed new reagents/analytic tools, analyzed data, and wrote the paper.
Abbreviations: FI, forest indicator; GSSC, grass silica short cell; Ma, million years ago; PACCAD, Panicoideae, Arundinoideae, Chloridoideae, Centothecoideae, Aristidoideae, and Danthonioideae; BEP, Bambusoideae, Ehrhartoideae, and Pooideae.
References
- 1.Jacobs, B. F., Kingston, J. D. & Jacobs, L. L. (1999) Ann. Mo. Bot. Gard. 86, 590–643. [Google Scholar]
- 2.Linder, H. P. (1986) Kew Bull. 42, 297–318. [Google Scholar]
- 3.Herendeen, P. S. & Crane, P. R. (1995) in Monocotyledons: Systematics and Evolution, eds. Rudall, P. J., Cribb, P., Cutler, D. F. & Humphries, C. J. (Royal Botanic Gardens, Kew, Surrey, U.K.), pp. 1–21.
- 4.Crepet, W. L. & Feldman, G. D. (1991) Am. J. Bot. 78, 1010–1014. [Google Scholar]
- 5.Bremer, K. (2002) Evolution 56, 1374–1387. [DOI] [PubMed] [Google Scholar]
- 6.Thomasson, J. R. (1985) Ann. Mo. Bot. Gard. 72, 843–851. [Google Scholar]
- 7.Zachos, J., Pagani, M., Sloan, L., Thomas, E. & Billups, K. (2001) Science 292, 686–693. [DOI] [PubMed] [Google Scholar]
- 8.Webb, S. D. & Opdyke, N. D. (1995) in Effects of Past Global Change on Life, eds. Kennett, J. & Stanley, S. (Natl. Acad. Press, Washington, DC), pp. 184–208.
- 9.Wing, S. L. (1998) in Evolution of Tertiary Mammals in North America: Terrestrial Carnivores, Ungulates and Ungulatelike Mammals, eds. Janis, C. M., Scott, K. M. & Jacobs, L. L. (Cambridge Univ. Press, Cambridge, U.K.), Vol. 1, pp. 37–65. [Google Scholar]
- 10.Grass Phylogeny Working Group (2001) Ann. Mo. Bot. Gard. 88, 373–457. [Google Scholar]
- 11.Strömberg, C. A. E. (2002) Palaeogeogr. Palaeoclimatol. Palaeoecol. 177, 59–75. [Google Scholar]
- 12.MacFadden, B. J. (2000) in Evolution of Herbivory in Terrestrial Vertebrates: Perspectives from the Fossil Record, ed. Sues, H.-D. (Cambridge Univ. Press, New York), pp. 223–244.
- 13.Strömberg, C. A. E. (2004) Palaeogeogr. Palaeoclimatol. Palaeoecol. 207, 239–275. [Google Scholar]
- 14.Retallack, G. J. (2001) J. Geol. 109, 407–426. [Google Scholar]
- 15.Terry, D. O. (2001) Palaeogeogr. Palaeoclimatol. Palaeoecol. 168, 1–38. [Google Scholar]
- 16.Wang, Y., Cerling, T. E., MacFadden, B. J. & Bryant, J. D. (1994) Palaeogeogr. Palaeoclimatol. Palaeoecol. 107, 269–280. [Google Scholar]
- 17.Fox, D. L. & Koch, P. L. (2003) Geology 31, 809–812. [Google Scholar]
- 18.Alexandre, A., Meunier, J.-D., Lezine, A.-M., Vincens, A. & Schwartz, D. (1997) Palaeogeogr. Palaeoclimatol. Palaeoecol. 136, 213–229. [Google Scholar]
- 19.Twiss, P. C., Suess, E. & Smith, R. M. (1969) Soil Sci. Soc. Am. Proc. 33, 109–115. [Google Scholar]
- 20.Piperno, D. R. & Pearsall, D. M. (1998) Smithsonian Contrib. Bot. 85, 1–40. [Google Scholar]
- 21.Kellogg, E. A. (2001) Plant Physiol. 125, 1198–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piperno, D. R. (1988) Phytolith Analysis: An Archaeological and Geological Perspective (Academic, San Diego). [DOI] [PubMed]
- 23.Thomasson, J. R. (1987) in Grass Systematics and Evolution, eds. Soderstrom, T. R., Hilu, K. W., Campbell, C. S. & Barkworth, M. E. (Smithsonian Institution, Washington, DC), pp. 159–167.
- 24.MacGinitie, H. D. (1953) Publ. Carnegie Inst. Washington 599, 1–198. [Google Scholar]
- 25.Galbreath, E. C. (1974) Trans. Ill. State Acad. Sci. 67, 366–368. [Google Scholar]
- 26.Evanoff, E., Gregory-Wodzicki, K. M. & Johnson, K. R., eds. (2001) Fossil Flora and Stratigraphy of the Florissant Formation, Colorado (Denver Museum of Nature and Science, Denver), Series 4, No. 1, 218 pp.
- 27.Dansereau, P. (1957) Biogeography: An Ecological Perspective (Ronald, New York).
- 28.Dugas, D. P. & Retallack, G. J. (1993) J. Paleontol. 67, 113–128. [Google Scholar]
- 29.Kondo, R., Childs, C. & Atkinson, I. (1994) Opal Phytoliths of New Zealand (Manaaki Whenua, Canterbury, New Zealand).
- 30.Soreng, R. J. & Davis, J. L. (1998) Bot. Rev. 64, 1–85. [Google Scholar]
- 31.Salamin, N., Hodkinson, T. R. & Savolainen, V. (2002) Syst. Biol. 51, 136–150. [DOI] [PubMed] [Google Scholar]
- 32.Hsiao, C., Jacobs, S. W. L., Chatterton, N. J. & Asay, K. H. (1998) Aust. Syst. Bot. 11, 667–688. [Google Scholar]
- 33.Leopold, E. B., Liu, G. & Clay-Poole, S. (1992) in Eocene–Oligocene Climatic and Biotic Evolution, eds. Prothero, D. R. & Berggren, W. A. (Princeton Univ. Press, Princeton), pp. 399–420.
- 34.Willis, K. J. & McElwain, J. C. (2002) The Evolution Of Plants (Oxford Univ. Press, New York).
- 35.Zachos, J. C., Shackleton, N. J., Revenaugh, J. S., Palike, H. & Flower, B. P. (2001) Science 292, 274–278. [DOI] [PubMed] [Google Scholar]
- 36.Keeley, J. E. & Rundel, P. W. (2005) Ecol. Lett. 8, 683–690. [Google Scholar]
- 37.Sage, R. F. (2004) New Phytol. 161, 341–370. [DOI] [PubMed] [Google Scholar]
- 38.Cerling, T. E., Wang, Y. & Quade, J. (1993) Nature 361, 344–345. [Google Scholar]
- 39.Bond, W. J., Midgley, G. F. & Woodward, F. I. (2003) Glob. Change Biol. 9, 937–982. [Google Scholar]
- 40.Morley, R. J. & Richards, K. (1993) Rev. Palaeobot. Palynol. 77, 119–127. [Google Scholar]
- 41.Janis, C. M., Damuth, J. & Theodor, J. M. (2004) Palaeogeogr. Palaeoclimatol. Palaeoecol. 207, 371–398. [Google Scholar]
- 42.Kay, R. F., Madden, R. H., Vucetich, M. G., Carlini, A. A., Mazzoni, M. M., Re, G. H., Heizler, M. & Sandeman, H. (1999) Proc. Natl. Acad. Sci. USA 96, 13235–13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stebbins, G. L. (1981) Ann. Mo. Bot. Gard. 68, 75–86. [Google Scholar]
- 44.Wing, S. L., Hickey, L. J. & Swisher, C. C. I. (1993) Nature 363, 342–344. [Google Scholar]
- 45.Lupia, R., Lidgard, S. & Crane, P. R. (1999) Paleobiology 25, 305–340. [Google Scholar]
- 46.Grimaldi, D. & Agosti, D. (2000) Proc. Natl. Acad. Sci. USA 97, 13678–13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.