Abstract
To investigate the causes of fetal death, focusing on maternal antiphospholipid syndrome diagnosis, and to follow the patients for changes in antiphospholipid antibodies, subsequent pregnancy outcomes, and thrombotic events. This is a prospective longitudinal cohort study that recruited patients who were hospitalized for fetal death at ≥ 10 weeks of gestation from three tertiary hospitals in China. Antiphospholipid syndrome was diagnosed according to the 2006 Sydney classification criteria. In total, 159 patients were recruited to the study; 3 were excluded and 144 of whom tested for aPLs. Among these, 126 (87.5%) were available for diagnostic analysis of antiphospholipid syndrome, 13 (10.3%) of which carried a diagnosis of antiphospholipid syndrome. Meanwhile, 136 of 156 patients had fetal samples for which copy number variation sequencing was completed, and 12 (8.8%) of which carried a diagnosis of fetal chromosomal abnormalities. During later follow-up, among the 13 patients with antiphospholipid syndrome, seven were persistently positive serostatus of antiphospholipid antibodies, four exhibited fluctuation, and one had negative conversion; four patients with subsequent pregnancies received guideline-based therapy and had term livebirths. None of the participants experienced thrombotic events. Maternal antiphospholipid syndrome was found to be one of the important causes of fetal death, contributing 10.3% of cases of fetal death at ≥ 10 weeks of gestation, slight ahead of fetal chromosomal abnormalities. Follow-up indicated that the serostatus of antiphospholipid antibodies may fluctuate significantly in some patients with antiphospholipid syndrome.
Clinical trial registration:
As this study was an observational study, we did not register it as a clinical trial.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10238-025-01607-0.
Keywords: Fetal death, Cause, Antiphospholipid antibody, Subsequent pregnancy, Classification criterion, Chromosomal abnormality
Introduction
Fetal death has devastating effects on families. These effects are exacerbated by the uncertainty surrounding the cause of death and recurrent risk for future pregnancies [1]. Identifying the cause of fetal death is essential for managing future pregnancies [2]. Previous studies reported that fetal chromosomal abnormalities (FCA) was an important cause of fetal death [3, 4]. However, those studies did not comprehensively investigate other causes of fetal death [5].
Antiphospholipid syndrome (APS) is an autoimmune disorder that can cause fetal death. According to the 2006 Sydney classification criteria, one of the features of APS is one or more unexplained fetal deaths at ≥ 10 gestational weeks (GW)[6] However, few studies have specifically focused on APS in patients with fetal death at ≥ 10 GW. Because of the different diagnostic terminologies for disease classification, most studies on APS and pregnancy loss do not discriminate between embryonic loss before the 10th GW and fetal loss after the 10th GW, and most studies on the causes of fetal death have focused on stillbirths after the 20th GW. Moreover, the epidemiology of APS remains understudied [7] and the proportion of APS-related fetal deaths in patients with fetal death is unknown.
APS is characterized by recurrent thrombosis and pregnancy morbidity, which can result in life-threatening emergencies and serious pregnancy complications, such as pulmonary embolism, myocardial infarction, and preeclampsia[8, 9]. Identifying APS early, these serious events can be effectively prevented by taking anticoagulants chronically and using low-dose aspirin (LDA) combined with low molecular weight heparin (LMWH) during pregnancy[10]. However, APS can be easily delayed or missed in patients with fetal death owing to the clinical non-specific presentation of APS, the numerous etiologies of fetal death and absence of confirmatory aPLs testing.
Hence, we conducted this etiological investigation targeting a population experiencing fetal death after the 10th GW, with an emphasis on maternal APS, while following patients for changes in aPLs, subsequent pregnancy outcomes, and thrombotic events.
Methods
Study design and patient population
This was a prospective longitudinal cohort study that investigated the causes of fetal death. The study was conducted at three tertiary hospitals in Xining, a city in Northwest China. The study was approved by the Medical Science Research Ethics Committee of Qinghai University School of Medicine (2021–017). Written informed consent was obtained from each participant in accordance with the Declaration of Helsinki.
Pregnant patients at ≥ 10 GW who were hospitalized for absence of a fetal heartbeat on ultrasonography were recruited and numbered in chronological order. GW was calculated based on the last menstrual period and first-trimester ultrasound reports (crown-rump length). Cases in which fetal death occurred before the 10th GW, after the 40th GW, or during labor were excluded.
Etiological investigative procedure for fetal death
Professional obstetricians collected detailed clinical histories and relevant information. The approximate GW of occurrence of fetal death and the period of intrauterine retention of dead fetus were extrapolated based on the fetal size measured by ultrasound at admission and the records from prenatal visits. Routine clinical laboratory tests were conducted (including complete blood tests, liver and renal function tests, coagulation parameters, urinalysis, hepatitis B and C, HIV, syphilis serology, neisseria gonorrhoeae and chlamydia trachomatis DNA load). After termination of the pregnancy, gross fetal observation and placental histopathology were performed following clinical procedures. Fetal chromosomes were analyzed using copy number variation sequencing (CNV‐Seq). Placental samples were stored at -80 °C for further investigations if needed. After discharge, the patients’ progress was tracked and further reviews were recommended as needed.
Testing methods for antiphospholipid antibodies
Initial testing for aPLs
Initial testing for aPLs was performed at local laboratories following respective practices during their hospitalization, including testing for lupus anticoagulant (LA), anticardiolipin antibody (aCL) and anti-β 2 glycoprotein 1 (aβ2GP1) (IgG and IgM).
Retesting for aPLs
Patients who were positive in the initial testing were retested for aPLs. To control for differences among detection methods used from the different laboratories, the patients were recommended that the aPL retesting were at the Laboratory Center of Qinghai Provincial People’s Hospital after 12 weeks. LA was detected using diluted Russel’s viper venom time (Siemens; Marburg, Germany). The screening test was considered positive if the clotting time exceeded 44 s; the LA ratio (screen/confirm) above 1.2 was regarded as positive (based on the reference value provided by the manufacturer). aCL and aβ2GP1 were detected using enzyme-linked immunosorbent assay (EUROIMMUN Medizinische Labordiagnostika AG, Lübeck, Germany). IgG and/or IgM isotypes of aCL in serum with titers > 40 IU/mL were considered as medium or high titer positive, and IgG and/or IgM isotypes of aβ2GP1 in serum with titers > 20 RU/mL were established as > the 99th percentile in titer (provided by the manufacturers). Additionally, antinuclear antibodies spectrum (ANAs) was tested to identify overlap with other autoimmune conditions. Patients who were negative in the initial testing were advised to retest aPLs at local hospital before the next pregnancies.
Diagnosis and follow-up of patients
Post-discharge management was not limited by the study protocol. After a comprehensive assessment by obstetricians, the results for the investigative etiologies communicated to the patient as a reference for their subsequent management. The patients were followed up for at least 3 months via outpatient visits, telephone, and the WeChat App. Diagnosis of APS was confirmed by rheumatologists according to the 2006 Sydney criteria after completion of the retesting for aPLs. The classification criteria for APS of domain in fetal death are summarized in Supplemental Table 1. The variations for CNV-Seq at > 5 Mb were defined as FCA and considered to have caused the fetal death, which did not include those chromosomal deletions or duplications at < 5 Mb.
In patients willing to continue, the follow-up period was extended to three years to obtain information on the changes in aPL titer, subsequent pregnancy outcomes, and thrombotic events. Negative conversion of aPLs was defined as two consecutive negative results for at least 12 months. The pregnancy outcomes included livebirth at ≥ 34 weeks, livebirth < 34 weeks, fetal death at ≥ 10 weeks and miscarriage at < 10 weeks but did not include biochemical pregnancy. The thrombotic events included deep venous thrombosis, pulmonary embolism, stroke and myocardial infarction in which there is any clinical evidence but it is not obligatory for histological confirmation. Participants were considered lost to follow-up if study staff were unable to contact them after at least three attempts within six months. Those lost to follow-up were censored from the time of last follow-up.
Statistical analysis
The current cohort of patients was recruited from July 1, 2021 to August 15, 2024. The cutoff date for follow-up was November 30, 2024. Demographics of the patients are summarized with descriptive statistics. Mean and standard deviation were used for normally distributed data and median and range for non-normally distributed data. The results are summarized as absolute numbers and percentages. Chi-squared test was used to compare proportions between APS subgroup and FCA subgroup, p < 0.05 was considered statistically significant. The diagnostic performance of each type of aPLs was assessed by receiver operating characteristic (ROC) curves. The area under the curve (AUC) indicates the performance of the type of aPLs. Statistical Package for Social Science software (version 25) was used for statistical analysis.
Results
Study population
A total of 159 patients were enrolled, of whom 3 (1.9%) were excluded because the fetal death was extrapolated to be < 10 GW. Of 156 patients in the current cohort, 49 were primigravida; of the remaining 107, 63 had experienced one or more prior pregnancy losses. None of the patients had experienced a thrombotic event before enrollment. The details for each patient are listed in Supplemental Table 2.
Table 2.
aPL values and clinical details of the 13 patients with APS
| Study number†† | aPLs | Testing interval | Pregnancy state | Clinical interventions before testing | NPO | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| LA | aβ2GP-1/IU | aCL/IU | ||||||||
| IgG | IgM | IgG | IgM | |||||||
| 001 | IT | 1.58* | 0.24 | 3.42 | 3.73 | 4.51 | Null | Fetal death | No | Term livebirths |
| RT | 1.49* | 0.81 | 10.52 | 3.2 | 1.65 | 13 weeks | Nonpregnant | No | ||
| 1.25* | NA | 8 months | Nonpregnant | LDA | ||||||
| 1.21* | 2.84 | 0.38 | 1.79 | 4.92 | 5 months | Pregnancy-F | LDA + LMWH | |||
| 1.11 | 1.12 | 6.16 | 10.14 | 3.8 | 3 months | Pregnancy-S | LDA + LMWH | |||
| 1.10 | 0.27 | 4.44 | 0.88 | 4.20 | 6 months | Postpartum | No | |||
| 020 | IT | 0.97 | 13.76 | 76.06* | 4.38 | 5.71 | Null | Fetal death | No | Term livebirths |
| RT | NA | 16.51 | 84.66* | 7.90 | 17.25 | 12 weeks | Nonpregnant | No | ||
| NA | 12.74 | 87.57* | 9.02 | 8.54 | 3 months | Pregnancy-F | LDA + LMWH | |||
| 1.05 | 91.19* | 10.78 | 22.38 | 3.68 | 11 months | Postpartum | No | |||
| 1.18 | 39.03* | 130.63* | 6.87 | 45.71* | 5 months | Nonpregnant | LDA | |||
| 1.29* | 70.34* | 77.26* | 15.33 | 16.50 | 5 months | Nonpregnant | LDA + HCQ | |||
| 027 | IT | 1.56* | 6.84 | 49.09* | 4.38 | 2.15 | Null | Fetal death | No | Term livebirths |
| RT | 1.33* | 4.33 | 41.74* | 7.89 | 4.81 | 3 months | Nonpregnant | TDF | ||
| 1.45* | 3.20 | 52.08* | 0.12 | 5.10 | 7 months | Pregnancy-S | TDF + LDA + LMWH | |||
| 1.25* | 2.75 | 21.62* | 1.35 | 2.83 | 4 months | Postpartum | TDF + LMWH | |||
| 1.19 | 4.47 | 10.26 | 0.40 | 1.98 | 4 months | Nonpregnant | TDF | |||
| 1.07 | 4.94 | 21.13* | 1.26 | 6.93 | 12 months | Nonpregnant | TDF | |||
| 036 | IT | 1.11 | 1.94 | 42.02* | 1.02 | 5.92 | Null | Fetal death | LDA + LMWH | Nonpregnant |
| RT | 1.12 | 2.35 | 93.19* | 2.14 | 4.39 | 13 weeks | Nonpregnant | No | ||
| 1.12 | 2.29 | 26.70* | 9.07 | 3.61 | 7 months | Nonpregnant | No | |||
| 1.02 | 0.00 | 22.36* | 0.01 | 2.85 | 14 months | Nonpregnant | No | |||
| 055 | IT | 0.92 | 1.63 | 109.8* | 5.22 | 38.43 | Null | Fetal death | No | Nonpregnant |
| RT | 1.09 | 3.91 | 191.08* | 2.33 | 57.86* | 11 months | Nonpregnant | No | ||
| 1.11 | 1.64 | 180.33* | 3.51 | 58.81* | 4 | Nonpregnant | LDA | |||
| 0.76 | 4.98 | 87.30* | 2.8 | 50.49* | 7 months | Nonpregnant | LDA + HCQ + PNS | |||
| 058 | IT | 1.27* | 2.58 | 36.09* | 1.15 | 46.41* | Null | Fetal death | No | Ongoing |
| RT | 0.99 | 3.88 | 13.10 | 2.11 | 35.35 | 12 weeks | Nonpregnant | No | ||
| 1.21* | 3.17 | 36.65* | 2.48 | 22.03 | 5 weeks | Nonpregnant | No | |||
| 1.01 | 4.69 | 107.38* | 2.5 | 65.39* | 15 months | Pregnancy-F | LDA + HCQ | |||
| 1.13 | 1.93 | 52.83* | 1.57 | 20.15 | 6 months | Pregnancy-T | LMWH + HCQ | |||
| Study number†† | aPLs | Testing interval | Pregnancy state | Clinical interventions | NPO | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| LA | aβ2GP-1/IU | aCL/IU | ||||||||
| IgG | IgM | IgG | IgM | |||||||
| 062 | IT | 1.26* | weakly positive § | Negative § | Null | Fetal death | No | Nonpregnant | Nonpregnant | |
| RT | 1.03 | 5.88 | 37.53* | 3.79 | 3.45 | 12 weeks | Nonpregnant | No | ||
| No | 10.01 | 38.85* | 3.86 | 3.97 | 7 weeks | Nonpregnant | No | |||
| 0.98 | 3.82 | 10.76 | 0.19 | 2.44 | 6 months | Nonpregnant | LDA | |||
| 0.97 | 6.48 | 27.89* | 0.41 | 3.72 | 6 months | Nonpregnant | No | |||
| 063 | IT | 1.19 | 2.04 | 51.8* | 0.08 | 0.11 | Null | Fetal death | No | |
| RT | 1.23* | 3.36 | 38.86* | 5.20 | 5.24 | 12 weeks | Nonpregnant | No | ||
| 1.06 | 4.51 | 30.74* | 2.97 | 6.77 | 5 months | Nonpregnant | No | |||
| 1.04 | 0.07 | 17.24 | 1.06 | 5.19 | 13 weeks | Nonpregnant | LDA | |||
| 1.16 | 5.15 | 81.38* | 2.78 | 50.26* | 6 months | Nonpregnant | LDA + HCQ | |||
| 066 | IT | No | 0.10 | 63.00* | 0.30 | 0.70 | Null | Fetal death | No | NA |
| RT | 0.93 | 2.12 | 30.52* | 2.95 | 4.97 | 12 weeks | Nonpregnant | No | ||
| 080† | IT | 1.11 | 5.03 | 111.50* | 1.79 | 3.74 | Null | Fetal death | LMWH | Term livebirths |
| RT | NA | NA | 71.02* | No | No | 12 weeks | Nonpregnant | No | ||
| 0.98 | 4.31 | 60.87* | 3.17 | 16.33 | 14 weeks | Pregnancy-F | No | |||
| 0.97 | 1.16 | 7.02 | 1.85 | 2.54 | 3 months | Pregnancy-S | LDA + LMWH + HCQ | |||
| 1.08 | 0.00 | 30.93* | 2.67 | 21.41 | 3 months | Pregnancy-T | LMWH + HCQ | |||
| 1.00 | 0.84 | 116.18* | 1.14 | 23.54 | 5 months | Postpartum | HCQ | |||
| 092 | IT | 1.21* | Positive § | Negative § | Null | Fetal death | No | Nonpregnant | Nonpregnant | |
| RT | 1.00 | 2.03 | 77.86* | 0.94 | 9.44 | 4 months | Nonpregnant | No | ||
| 1.11 | 2.64 | 127.5* | 0.11 | 43.84* | 5 months | Nonpregnant | LDA | |||
| 117 ‡ | IT | 1.40 | Positive § | Negative § | Null | Fetal death | No | Ongoing | Ongoing | |
| RT | 1.12 | 1.23 | 76.94* | 1.35 | 25.69 | 4 weeks | Nonpregnant | No | ||
| 1.15 | 1.62 | 50.14* | 1.20 | 17.40 | 4 months | Nonpregnant | LDA + HCQ + MPS | |||
| 1.13 | 1.24 | 51.02 | 1.60 | 23.25 | 5 months | Pregnancy-F | LDA + LMWH + HCQ | |||
| 136 | IT | 1.03 | Positive § | Negative § | Null | Fetal death | No | Nonpregnant | Nonpregnant | |
| RT | 0.93 | 0.00 | 63.81* | 1.37 | 0.77 | 5 months | Nonpregnant | No | ||
aPLs; antiphospholipid antibodies, LA; lupus anticoagulant, aCL; anticardiolipin antibody, aβ2GP1; anti-β2 glycoprotein-1 antibodies, NPO; next pregnancy outcomes, IT; Initial testing, RT; Repeat testing, Pregnancy-F; first-trimester, Pregnancy-S; second trimester, Pregnancy-T; third trimester, LDA; low-dose aspirin, LMWH; low molecular weight heparin, HCQ; hydroxychloroquine, TDF; tenofovir disoproxil fumarate, PNS; prednisone acetate, MPS; methylprednisolone
†† the numbered in chronological order; *and the bold positive results fulfilling the 2006 Sydney criteria; † combined rheumatoid arthritis; ‡ combined Sjögren's syndrome; § the testing method was qualitative
Results of the etiological investigation
Of the total cohort, 144 patients had completed the initial testing for aPL, 60 (41.7%) of which tested positive or weakly positive. Forty-two (70%) of the 60 positive or weakly positive had the repeat testing for aPL after 12 weeks. LA values were missing in 12 (8.3%) of the 144 initial testing for aPL due to LA testing not being performed. The missing LA were imputed using a mean plus random noise imputation. Eighteen patients were excluded due to a lack of confirmation for aPL, and 126 were included in the final diagnostic analysis of APS.
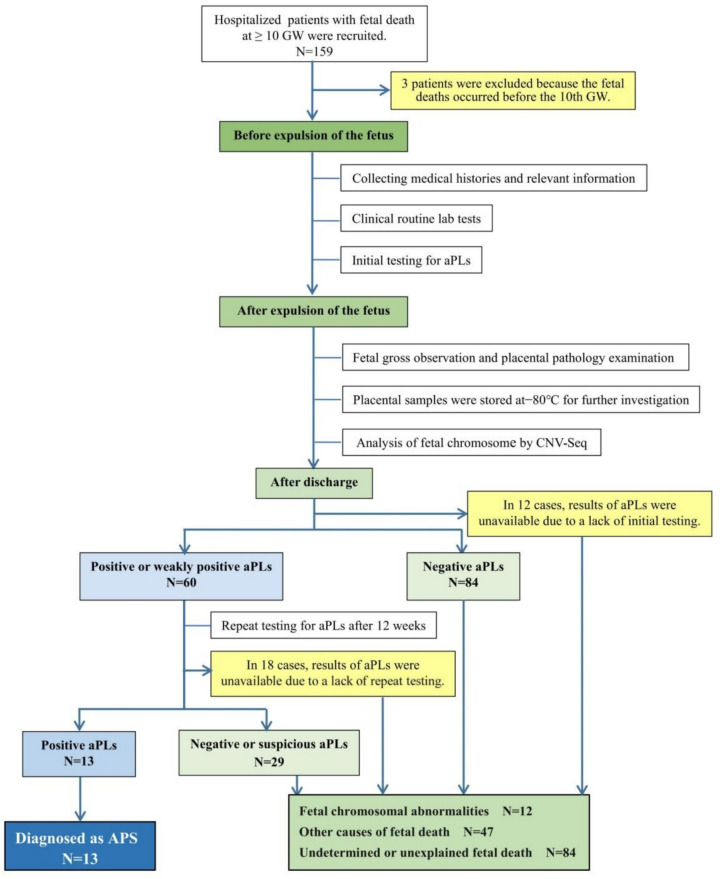
According to the 2006 Sydney classification criteria, 13 (10.3%) were diagnosed with APS. Two patients diagnosed with APS had an overlap with other autoimmune conditions (one had rheumatoid arthritis and the other had Sjögren's syndrome). Figure 1 presents the diagnostic flowchart for APS. Meanwhile, 136 fetal samples had completed CNV-Seq analysis, 12 (8.8%) of which carried FCA, presented in Supplemental Table 2, column 10. Table 1 presents the baseline characteristics for the total population and the two subgroups. Of those non-APS patients, 47 patients were categorized as other-cause fetal deaths, in addition to the 12 FCA. The remaining 84 had unexplained fetal deaths.
Fig. 1.
Diagnostic flowchart for APS. APS, antiphospholipid syndrome, GW, gestational weeks; aPLs, antiphospholipid antibodies; CNVs, copy number variations; NGS, next generation sequencing; ANAs, antinuclear antibodies
Table 1.
Baseline characteristics for the total population and the two subgroups
| Age (years) | Approximate GW-O | Previous pregnancy losses | ||||||
|---|---|---|---|---|---|---|---|---|
| 10–15 weeks |
16–19 weeks | 20–33 weeks | 34–40 weeks | 1 time | 2 times | ≥ 3 times | ||
| The total number of patients N = 156 | 29.5 (17–45) | 62 (40.4%) | 23 (14.1%) | 51 (32.7%) | 20 (12.8%) | 48 (30.8%) | 10 (6.4%) | 5 (3.2%) |
| The maternal APS N = 13 | 27.5 (20–37) | 5 (38.5%) | 3 (23.0%) | 5 (38.5%) | 0 (0%) | 4 (30.7%) | 1 (7.7%) | 0 (0%) |
| The fetal chromosomal abnormalities N = 12 | 33.7 (21–45) | 8 (66.7%) | 0 (0%) | 3 (25%) | 1 (8.3%) | 2 (16.7%) | 2 (16.7%) | 0 (0%) |
Age are given as mean (range); other data are given as absolute numbers (percentages)
GW-O; gestational week for occurrence of the fetal death, APS; antiphospholipid syndrome
Statistical analysis
Chi square test showed that although the 10.3% of the proportion of maternal APS was higher than the 8.8% of FCA, there was no statistical difference (χ2 = 0.04, p = 0.84). Next, diagnostic performance of each type of aPLs was analyzed retrospectively. Thirty non-APS patients with quantitative ELISA results were chosen as the controls to evaluate the diagnostic performance for each type of aPLs ( see Supplementary Table 3). ROC curve analysis revealed that aβ2GP-1 IgM exhibited high diagnostic performance for the diagnosis of APS in patients experienced fetal death (AUC, 0.918 [95% CI, 0.789–1.000]). The other types of aPls showed relatively low diagnostic performance for APS (AUC, 0.5–0.7) (see Supplementary Fig. 1).
Follow-up results
Of the 156 patients, 16 (10.3%) were lost to follow-up within 3 months due to the unavailability by contact, and 9 (5.8%) were lost to extended follow-up after 3 months due to the unwillingness of participants. The median follow-up duration was 17 months with a minimum of 3 months and a maximum of 36 months. Of the 13 patients with APS, seven were persistently positive for aPLs with one developing aβ2GP-1 from alone IgM to both IgM and IgG; four had fluctuating aPL status; one patient who was positive for at least 12 months had conversion to negative aPLs, and one was lost to extended follow-up. Four APS patients with subsequent pregnancy received therapy according to guidelines and had term livebirths. Details on the 13 APS patients are listed in Table 2. The gestational stage or clinical interventions when aPLs were retested are presented in column 8 and 9 of Table 2. Of the 131 patients without APS, in the next pregnancies, 28 had livebirths at ≥ 34 weeks and 8 miscarried at < 10 GW (see column 12 of Supplementary Table 2). None of the 156 patients experienced thrombotic events.
Evaluation using the 2023 ACR/EULAR criteria
When the American College of Rheumatology and European League Against Rheumatism (ACR/EULAR) criteria [11] were used in our cohort, none of the patients fully met the classification conditions for APS. Of the 13 patients meeting the 2006 Sydney criteria, eight patients did not meet the score neither in clinical nor laboratory domain based on the new criteria; for the remaining five patients, three did not meet the clinical score and two did not meet the laboratory score. Supplemental Table 4 lists the detailed evaluation using the 2023 ACR/EULAR criteria in 13 patients with APS. The classification criteria for fetal death in the 2023 ACR/EULAR criteria are summarized in Supplemental Table 5.
Discussion
In this cohort, we included 156 patients with fetal death at ≥ 10 GW. Twelve patients did not complete initial aPL testing, and 18 patients did not complete repeat aPL testing due to loss to follow-up or distance constraints. One hundred and twenty-six patients were therefore included in the diagnostic analysis of APS. Maternal APS was diagnosed per the 2006 Sydney classification criteria in 10.3% (13/126) of the cohort. Similar proportion was noted in a case–control study that had positive tests for aPLs (aCL or aβ2GPI) in 9.6% of stillbirths [12]. However, that study included patients without LA testing and did not include those who were fetal death at 10–20 GW. In out cohort, the proportion of APS was slightly higher than the 8.8% (12/136) of FCA although the difference was not statistically significant. The diagnosis of FCA did not include those chromosomal deletion or duplication at < 5 Mb because there is no convincing evidence that those microdeletion / microduplication are associated with fetal death although the variants classified as pathogenic according to American College of Medical Genetics (ACMG) criteria.
aPLs are the crucial diagnostic marker of APS. In our study, aβ2GP-1 IgM exhibited high diagnostic performance for the diagnosis of APS in patients experienced fetal death (AUC, 0.918 [95% CI, 0.79–1.00]). This result is consistent with the result that isolated aPLs IgM positivity is associated with pregnancy morbidity[13]. One study has reported that aβ2GP-1 isotypes are associated with the lowest live birth rate and highest incidence of stillbirth, compared with the presence of aCL or LA alone[14]. Thus, aPL testing should be emphasized more and the positive aβ2GP-1 IgM should be given attention in women who experienced fetal death.
During follow-up, of 13 patients with APS, seven were persistently positive for aPLs, four exhibited fluctuation, and one had negative conversion. Some scholars have reported that the rate of aPL positivity decreases during follow-up in primary APS, estimating that seroconversion occurs in between 8.9 and 59% of patients over time, and hydroxychloroquine has been identified as the most effective pharmacological agent to reduce aPL titers [15]. Another study found that aPL titers decreased modestly during pregnancy among patients who were positive [16]. We could not conduct an analysis based on GW or interventions due to the small number of patients in these subgroups. But from real-world individual observations, we noticed that the fluctuations of aPLs (solid-phase assays) were independent of the gestational stage and clinical interventions (Table 2, column 8 and 9). The factors contributing to the fluctuation of aPLs were unknown. Notably, clinicians need to further monitor individuals who were suspicious for APS but have only one time negative aPL result.
Four patients with subsequent pregnancies received therapy according to guidelines and delivered at term. None of the enrolled patients experienced a thrombotic event. A retrospective study found that aPLs at low titers and fulfilling the 2006 Sydney criteria were associated with pregnancy morbidity and that treatment by LDA plus LMWH appeared to improve outcomes [17]. Furthermore, there is evidence that isolated IgM was rare in thrombotic APS, but more frequent in obstetric APS [13]. Our results, with a preponderance of positive aβ2GP-1 IgM and relatively infrequent positive LA, supported these conclusions.
Our study was initiated in 2021. In 2023, new classification criteria for APS, the ACR/EULAR criteria were published. When the ACR/EULAR criteria were used in our cohort, none of the patients fully met the classification conditions for APS. An international study reported that the percentage of obstetric APS dropped from 26.9% per Sydney criteria to 3.2% per ACR/EULAR criteria [18]. This observation coupled with our results indicate that the new criteria may have an extremely low sensitivity to obstetric APS. The ACR/EULAR criteria assign a low weight to fetal death in the absence of preeclampsia and/or placental insufficiency [19]. In our cohort, half of APS patients were observed before 20 weeks of gestation. Experience holds that preeclampsia and placental insufficiency cannot manifest before 20 weeks of pregnancy [5]. Clearly, although the new 2023 ACR/EULAR classification criteria for APS were established, they should not be adhere dogmatically in patients experienced fetal death.
The strengths of our study include the prospective design, relatively comprehensive etiological investigation, longitudinal follow-up data for aPL serostatus changes over time and pregnancy outcomes post-diagnosis, which can guide clinical expectations and inform future research. In this study, the GW was carefully assessed to be close to the time of occurrence of fetal death rather than that of recognition. Our findings from real clinical settings have provided insight into APS diagnosis and management in cases of fetal death, which can be useful for clinicians treating similar cases.
Our study has several limitations. First, it was the absence of a control cohort with viable pregnancies for comparison, which prevented us from establishing a stronger association between aPLs and fetal death. Second, LA detection in our study did not use two tests with different assaying principles, which may have decreased the sensitivity of LA, contributing to the inconsistency between our current finding and a previous report that LA is the best predictor of adverse pregnancy outcomes [20]. Additionally, the generalizability of our results may be limited due to the relatively homogeneous study population and a relatively small sample size.
In conclusion, maternal APS was found to be one of the important causes of fetal death, contributing 10.3% of cases of fetal death at ≥ 10 weeks of gestation, slight ahead of fetal chromosomal abnormalities. Follow-up indicated that the aPL serostatus may fluctuate significantly in some patients with APS.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Editage (www.editage.com) for English language editing.
Author contributions
A.X., Z.J., and C.L. are co-first authors and are listed in order of their contribution to the study. A.X. contributed to study conceptualization and design, clinical evaluations, the interpretation of results and writing the paper. A.X., Z.J., C.L., C.L., X.Z., S.J., D.L., Y.X. and L.X. contributed to the clinical information and the clinical execution of the study. G.L. analyzed the data with A.X.. X.W. critically revised the manuscript for important content. All authors have approved the final version of the manuscript for publication. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
The Natural Science Foundation of Qinghai Province (2024-ZJ-923) and "Kunlun Elite—High level Innovation and Entrepreneurship Talents" program in Qinghai Province have funded the costs of the fetal chromosomal sequencing analyses and the data collection for this study. The data collection and publication of this study was supported financially by the Natural Science Foundation of Qinghai Province (2024-ZJ-923) and "Kunlun Elite—High level Innovation and Entrepreneurship Talents" program in Qinghai Province.
Data availability
Data are provided within the manuscript or supplementary information files.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The study was approved by the Medical Science Research Ethics Committee of Qinghai University School of Medicine (2021–017).
Informed consent
Written informed consent was obtained from each participant in accordance with the Declaration of Helsinki.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Anxia Xie, Ziwei Jin, and Changping Li are shared first authorship.
References
- 1.Byrne AB, Arts P, Ha TT, et al. Genomic autopsy to identify underlying causes of pregnancy loss and perinatal death. Nat Med. 2023;29:180–9. 10.1038/s41591-022-02142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie A, Cui Y, Luo G, et al. Determining the cause of intrauterine fetal death in monochorionic twins: a case report. Front Med (Lausanne). 2023;9:1055275. 10.3389/fmed.2022.1055275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khare M, Howarth E, Sadler J, Healey K, Konje JC. A comparison of prenatal versus postnatal karyotyping for the investigation of intrauterine fetal death after the first trimester of pregnancy. Prenat Diagn. 2005;25:1192–5. 10.1002/pd.1295. [DOI] [PubMed] [Google Scholar]
- 4.Howarth ES, Konje JC, Healey KA, Duckett DP, Scudamore IW, Taylor DJ. Invasive testing for the karyotyping of mid-trimester intrauterine fetal death (IUFD): a pilot study. Prenat Diagn. 2002;22:453–5. 10.1002/pd.339. [DOI] [PubMed] [Google Scholar]
- 5.Branch DW, Lim MY. How I diagnose and treat antiphospholipid syndrome in pregnancy. Blood. 2024;143:757–68. 10.1182/blood.2023020727. [DOI] [PubMed] [Google Scholar]
- 6.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295–306. 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 7.Schreiber K, Sciascia S, de Groot PG, et al. Antiphospholipid syndrome. Nat Rev Dis Primers. 2018;4:1–20. 10.1038/nrdp.2017.104. [DOI] [PubMed] [Google Scholar]
- 8.Espinosa G, Cervera R. Antiphospholipid syndrome: frequency, main causes and risk factors of mortality. Nat Rev Rheumatol. 2010;6:296–300. 10.1038/nrrheum.2010.47. [DOI] [PubMed] [Google Scholar]
- 9.De Carolis S, Tabacco S, Rizzo F, et al. Antiphospholipid syndrome: an update on risk factors for pregnancy outcome. Autoimmun Rev. 2018;17:956–66. 10.1016/j.autrev.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med. 2018;378:2010–21. 10.1056/NEJMra1705454. [DOI] [PubMed] [Google Scholar]
- 11.Barbhaiya M, Zuily S, Naden R, et al. 2023 ACR/EULAR antiphospholipid syndrome classification criteria. Ann Rheum Dis. 2023;82:1258–70. 10.1136/ard-2023-224609. [DOI] [PubMed] [Google Scholar]
- 12.Silver RM, Parker CB, Reddy UM, et al. Antiphospholipid antibodies in stillbirth. Obstet Gynecol. 2013;122:641–57. 10.1097/AOG.0b013e3182a1060e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chayoua W, Kelchtermans H, Gris JC, Moore GW, Musiał J, Wahl D, et al. The (non-)sense of detecting anti-cardiolipin and anti-β2glycoprotein I IgM antibodies in the antiphospholipid syndrome. J Thromb Haemost. 2020;18:169–79. 10.1111/jth.14633. [DOI] [PubMed] [Google Scholar]
- 14.Saccone G, Berghella V, Maruotti GM, et al. Antiphospholipid antibody profile based obstetric outcomes of primary antiphospholipid syndrome: the pregnants study. Am J Obstet Gynecol. 2017;216:521–5. 10.1016/j.ajog.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 15.Chighizola CB, Willis R, Maioli G, et al. Deciphering the clinical significance of longitudinal antiphospholipid antibody titers. Autoimmun Rev. 2024;23: 103510. 10.1016/j.autrev.2023.103510. [DOI] [PubMed] [Google Scholar]
- 16.Yelnik CM, Porter TF, Branch DW, et al. Brief report: changes in antiphospholipid antibody titers during pregnancy: effects on pregnancy outcomes. Arthritis Rheumatol. 2016;68:1964–9. 10.1002/art.39668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medina G, Briones-García E, Cruz-Domínguez MP, Flórez-Durante OI, Jara LJ. Antiphospholipid antibodies disappearance in primary antiphospholipid syndrome: thrombosis recurrence. Autoimmun Rev. 2017;16:352–4. 10.1016/j.autrev.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Foddai SG, Radin M, Cecchi I, et al. ACR/EULAR classification criteria in existing research cohorts: an international study. Rheumatology (Oxford). 2024;30:58. 10.1093/rheumatology/keae058. [DOI] [PubMed] [Google Scholar]
- 19.Miro-Mur FA, Alijotas-Reig J, Anunciacion-Llunell A, Marques-Soares J, Enrique Esteve-Valverde E, Jose P-G. Correspondence on ‘2023 ACR/EULAR antiphospholipid syndrome classification criteria.’ Ann Rheum Dis. 2024;83: e2. 10.1136/ard-2023-225042. [DOI] [PubMed] [Google Scholar]
- 20.Lockshin MD. Pregnancy and antiphospholipid syndrome. Am J Reprod Immunol. 2013;69:585–7. 10.1111/aji.12071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are provided within the manuscript or supplementary information files.