Abstract
Pillar[n]arenes have broad applications in biological medicine, materials science, and supramolecular gels. Notably, enantiopure pillar[5]arenes are valued for their roles in enantioselective host–guest recognition, chiral sensing, asymmetric catalysis, and related fields. Current methods for obtaining chiral pillar[n]arenes rely heavily on resolution agents or chiral HPLC resolution. However, the synthesis of these compounds via asymmetric catalysis remains challenging. In this study, we develop an asymmetric extended side-arm Suzuki–Miyaura cross-coupling strategy to construct inherently chiral pillar[5]arenes with excellent yields and high enantioselectivities using a palladium catalyst and a Sadphos ligand. The reaction scope extends beyond arylboronic acids to encompass 2-arylvinylboronic acids and other multi-OTf-substituted substrates, all efficiently producing the desired products. Further exploration of the synthetic applications, along with photophysical and chiroptical analyses, confirm the potential of these chiral pillar[5]arenes for diverse applications across multiple disciplines.
Subject terms: Homogeneous catalysis, Asymmetric catalysis, Asymmetric synthesis
Enantiopure pillar[5]arenes are valued for their roles in host–guest recognition and chiral sensing, but the current synthesis of these compounds via asymmetric catalysis remains challenging. Here, the authors develop an asymmetric extended side-arm Suzuki–Miyaura cross-coupling strategy to construct inherently chiral pillar[5]arenes.
Introduction
Advances in the preparation of cyclic host molecules, including cyclodextrins1,2, cucurbit[n]urils3,4, crown ethers5,6, calix[n]arenes7, and pillar[n]arenes8–15, have significantly propelled progress in supramolecular chemistry. A key characteristic of these cyclic host molecules is their ability to recognize guest molecules within their cavities16. This distinctive property facilitates their utilization in various applications, such as optically responsive materials, self-assembly systems, host‒guest systems, supramolecular polymers, and molecular motors17–24. Pillar[n]arene, with its symmetrical pillar architecture, has become notable in the realm of macrocyclic arenes. Since Ogoshi’s pioneering report on pillar[n]arenes in 200825, these structures have emerged as some of the most valued macrocyclic arenes over the past decade. Compared with other traditional macrocyclic hosts, pillar[n]arenes offer several notable advantages26. Firstly, owing to their rigid, electron-rich cavity, they are easy to synthesize and more easily functionalized, making them excellent candidates for constructing molecular aggregates through host‒guest complexation. Moreover, chiral pillar[n]arenes are uniquely suited for applications in asymmetric catalysis, circularly polarized luminescence chiroptical materials, chiral host–guest conjugate, chiral supramolecular polymer, and homochiral metal-organic framework (Fig. 1a)27–32.
Fig. 1. Chiral pillar[n]arenes: background and proposal.
a Chemical structure of inherently chiral pillar[5]arene. b Dynamically inherent chirality of pillar[5]arenes. c The traditional strategies for asymmetric Suzuki–Miyaura cross-couplings. d This design: palladium-catalyzed Suzuki‒Miyaura cross-coupling reactions.
In pillar[n]arene structures, the rotation of dialkoxy benzene units around the methylene bridges gives rise to inherent chirality (Fig. 1b)33, and the dialkoxy benzene units within pillar[n]arene structures can perform a flipping motion along the annulus, termed “oxygen through the annulus rotations” flipping. Although this flipping mechanism has been extensively documented in other macrocyclic arenes, such as calixarenes, its occurrence in pillar[n]arene compounds results in interconversion between the pS and pR conformers34. This interconversion introduces a unique characteristic that can be suppressed by changing the solvent or temperature, or by introducing an achiral guest molecule35. The diameter of the cavity in pillar[n]arenes is adjustable, with pillar[5]arene having an approximate diameter of 4.7 Å. The incorporation of bulky substituents is beneficial for isolating enantiomers from racemic mixtures because of the ability of the bulky substituents to restrict rotation. Traditionally, chiral pillar[n]arenes have been synthesized by initially incorporating bulky substituents, followed by the separation of enantiomers using chiral high-performance liquid chromatography (HPLC) or resolving agents to isolate racemic mixtures36,37. However, the catalytic asymmetric synthesis of inherently chiral pillar[n]arenes remains a significant challenge. Recently, Wang and co-workers reported the enantioselective synthesis of chiral alkyne-substituted pillar[5]arenes via asymmetric Sonogashira cross-coupling38. Notably, Wang’s method requires high palladium catalyst loadings (30 mol%) and ligands (60%), and it is limited to the synthesis of C₂-symmetric chiral pillar[5]arenes.
The Suzuki–Miyaura coupling, which involves organoboron compounds and (pseudo)halides, is a pivotal carbon–carbon bond forming reaction in drug discovery. This method is particularly renowned for its efficiency in forming C(sp2)–C(sp2) bonds. While the Suzuki–Miyaura cross-coupling is well established, its asymmetric variant remains underdeveloped39–45. Despite notable progress, the asymmetric Suzuki–Miyaura reaction continues to pose significant challenges. Three main strategies have been documented in the literature, including direct cross-couplings of two aryl units46–57, dynamic kinetic asymmetric transformation58–66, and desymmetrization (Fig. 1c)67–71. However, the synthesis of chiral macrocycles presents additional difficulties due to the diversity of substituents and complex molecular conformations. This highlights the need for innovative strategies to achieve the efficient synthesis of chiral macrocycles72. Building on our recent advances in the stereoselective synthesis of inherent chirality73–75, we developed an asymmetric extended side-arm Suzuki–Miyaura cross-coupling strategy to synthesize chiral pillar[5]arene molecules by iteratively extending the substituents of achiral pillar[5]arenes (Fig. 1d). Unlike the desymmetrization strategy, the product remains achiral after the initial cross-coupling step, as the small substituents can freely rotate around the single bonds. However, further extension of the side arms increases the steric bulk on both sides of the pillar[5]arene, restricting rotation and inducing chirality. A total of 49 structurally diverse C2- and D5-symmetric pillar[5]arenes, including 6-membered aryl, 5-membered heteroaryl, and alkenyl-substituted variants, were synthesized with excellent yields and outstanding enantioselectivities.
Results
Optimization studies
Our initial research was aimed at enhancing the critical enantioselective control process involved the use of pillar[5]arene-based bifunctional triflate 1a and 4-phenylbenzene boronic acid 2a as representative substrates for optimization (Table 1). First, we examined the common chiral ligands (see the Supplementary Information Table S1 for details) under palladium-catalyzed cross-coupling reaction conditions (10 mol% Pd(OAc)2, 12 mol% ligand, 5.0 equiv. K3PO4 in TBME at 90 °C for 12 h). The corresponding chiral pillar[5]arene product 3a was successfully synthesized using chiral ligands, such as (R)-BINAP L1, phosphinic amide L2, and BI-DIME L3, achieving satisfactory enantioselectivities (40% to 47% ee). However, product 3a was afforded in 85% yield but remained racemic with the bulky ligand (R)-DTBM-Segphos L4. Notably, we attempted to use the sulfonamide phosphine ligand (Sadphos) L5, developed by the Zhang group, which has been demonstrated to be highly effective in palladium-catalyzed asymmetric systems76–78. Motivated by these encouraging outcomes, we shifted our focus to screening other Sadphos family ligands, L6 and L7, in an effort to further optimize their efficiency and stereoselectivity. To our delight, PC-Phos L7 exhibited outstanding efficiency (80% yield) and moderate stereocontrol (84% ee)79–81. Its diastereomer L8 demonstrated a low yield and enantioselectivity. Additionally, the use of L9 resulted in a significant decrease in yield and enantioselectivity. To increase enantioselectivity, various conditions were tested using L7. For instance, we evaluated different bases, including K2HPO4, Na2CO3, K2CO3, and Cs2CO3 (Table 1, Entries 1–4). While the majority of these bases resulted in the formation of 3a with reduced reactivity, and Cs₂CO₃ notably improved the enantioselectivity to 86%, although it slightly decreased the yield (Entry 4). Subsequently, the use of toluene as the solvent did not produce the desired product (Entry 5). Further investigation with various ether solvents showed that 1,4-dioxane improved the enantiomeric excess to 88%, but a trace yield was obtained (Entries 6–8). We were pleased to find that screening various palladium catalysts (Entries 9–12) and revealed that [Pd(allyl)Cl]₂ provided product 3a in 82% yield with 90% enantiomeric excess (Entry 12). Significantly, reducing the reaction temperature to 70 °C increased the enantioselectivity to 91% while maintaining excellent yield (Entry 13). Through extensive ligand modifications, we found that increasing the bulkiness of TY-Phos further with the use of L10 led to improved yield and enantioselectivity (Entry 14). Ultimately, by prolonging the reaction time to 24 h, we obtained product 3a in 85% isolated yield and 96% enantiomeric excess (Entry 15). The absolute configuration of 3a was confirmed as a pR conformer via X-ray crystallography.
Table 1.
Optimization of the reaction conditions
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | [M] (mol%) | Ligand | Base | T (°C) | Solvent | Time (h) | Yield of 3a (%)a | Ee of 3a (%)b |
| 1 | Pd(OAc)2 (10) | L7 | K2HPO4 | 90 | TBME | 12 | 10 | 86 |
| 2 | Pd(OAc)2 (10) | L7 | Na2CO3 | 90 | TBME | 12 | trace | - |
| 3 | Pd(OAc)2 (10) | L7 | K2CO3 | 90 | TBME | 12 | 25 | 62 |
| 4 | Pd(OAc)2 (10) | L7 | Cs2CO3 | 90 | TBME | 12 | 70 | 86 |
| 5 | Pd(OAc)2 (10) | L7 | Cs2CO3 | 90 | Toluene | 12 | nr | - |
| 6 | Pd(OAc)2 (10) | L7 | Cs2CO3 | 90 | THF | 12 | 15 | 80 |
| 7 | Pd(OAc)2 (10) | L7 | Cs2CO3 | 90 | 1,4-dioxane | 12 | trace | 88 |
| 8 | Pd(OAc)2 (10) | L7 | Cs2CO3 | 90 | Et2O | 12 | 10 | 37 |
| 9 | Pd(TFA)2 (10) | L7 | Cs2CO3 | 90 | TBME | 12 | 72 | 88 |
| 10 | Pd2(dba)3 (5) | L7 | Cs2CO3 | 90 | TBME | 12 | 65 | 88 |
| 11 | Pd(acac)2 (10) | L7 | Cs2CO3 | 90 | TBME | 12 | 35 | 81 |
| 12 | [Pd(allyl)Cl]2 (5) | L7 | Cs2CO3 | 90 | TBME | 12 | 82 | 90 |
| 13 | [Pd(allyl)Cl]2 (5) | L7 | Cs2CO3 | 70 | TBME | 12 | 73 | 91 |
| 14 | [Pd(allyl)Cl]2 (5) | L10 | Cs2CO3 | 70 | TBME | 12 | 75 | 96 |
| 15 | [Pd(allyl)Cl]2 (5) | L10 | Cs2CO3 | 70 | TBME | 24 | 88(85)c | 96 |
Unless otherwise specified, the reaction conditions were as follows: 1a (0.10 mmol), 2a (0.50 mmol), 5–10 mol% [Pd], 12 mol% ligand, and 5.0 equiv. of base in 3.0 mL of solvent at 70–90 °C for 12–24 h under nitrogen. The bold part represents the chiral ligand.
aDetermined by 1H-NMR analysis.
bDetermined by chiral HPLC analysis.
cIsolated yield.
Scope of the reaction
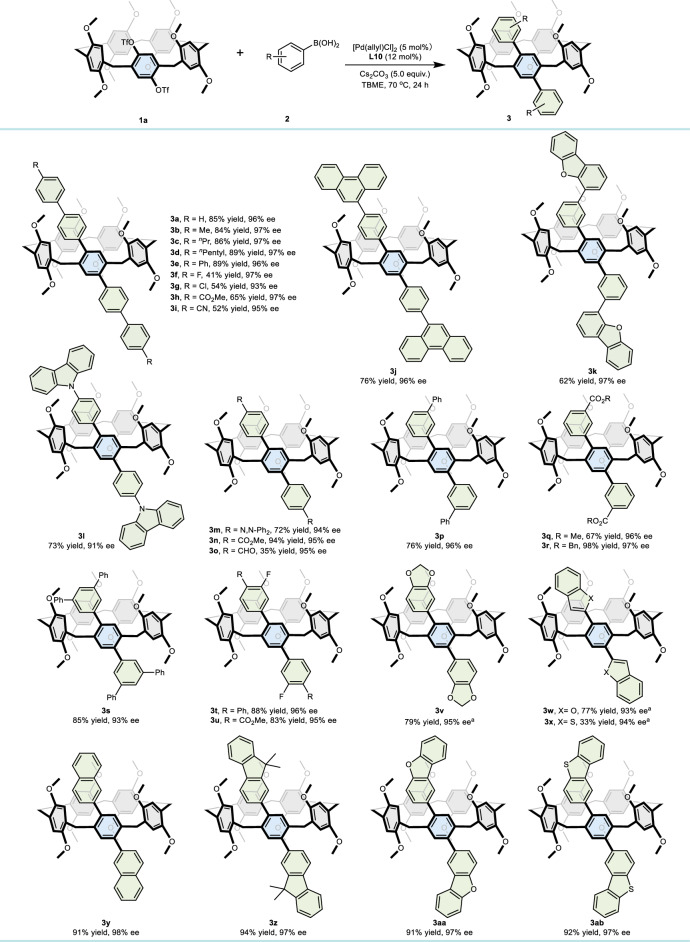
The scope of the enantioselective palladium-catalyzed Suzuki‒Miyaura cross-coupling process was investigated, and the results are presented in Table 2. A range of 4-phenylbenzene boronic acid and its para-substituted derivatives were investigated, which included various electronic properties and steric influences. The results (3a–3i) demonstrated excellent yields and remarkable enantioselectivity control. Substrates featuring a strongly coordinating cyano group also yielded favorable results (3i), demonstrating both high efficiency (52%) and enantioselectivity (95% ee). Subsequently, substrates incorporating various large ring groups, including phenanthrene (3j), dibenzofuran (3k), and carbazole (3l), afforded moderate to exceptional yields (62–76%) along with high enantioselectivities (91–97% ee). Moreover, substrates substituted with small steric groups, such as N,N-diphenyl (3m), CO2Me (3n), and CHO (3o), demonstrated high enantiocontrol. However, due to minimal steric hindrance, phenylboronic acid and ortho-substituted phenylboronic acids, such as 2-tolylboronic acid and 2-biphenylboronic acid, were unable to hinder the rotation of pillar[5]arene, failing to induce chiral control. Interestingly, meta-substituted phenylboronic acids, specifically those bearing phenyl and ester groups, demonstrated uniform compatibility with the standard conditions, resulting in the desired products 3p–3r with outstanding yields and enantioselectivities between 96% and 97%. The tolerance towards a wide range of di-substituted substrates (3s–3v) bearing diverse groups remained unaffected by the electronic nature of the substituents. Additionally, 2-benzofuran (3w), 2-benzothiophen (3x), and 2-naphthyl (3y) boronic acids were successfully employed in this methodology. However, pyridin-4-ylboronic acid did not react in the system, likely due to the strong coordinating ability of the pyridine group. Furthermore, 9,9-dimethylfluorene, dibenzofuran, and dibenzothiophene boronic acids were converted into the respective products 3z–3ab, with excellent yields and high enantioselectivities of 97%.
Table 2.
Scope of the reactions of arylboronic acids with OTf-pillar[5]arene 1a
Reaction conditions: 1a (0.10 mmol), 2 (0.50 mmol), 5 mol% [Pd(allyl)Cl]2, 12 mol% L10, 5.0 equiv. of Cs2CO3 in 3.0 mL of TBME at 70 °C for 24 h under nitrogen; isolated yield by silica gel chromatography; ee values were determined by chiral HPLC.
a72 h.
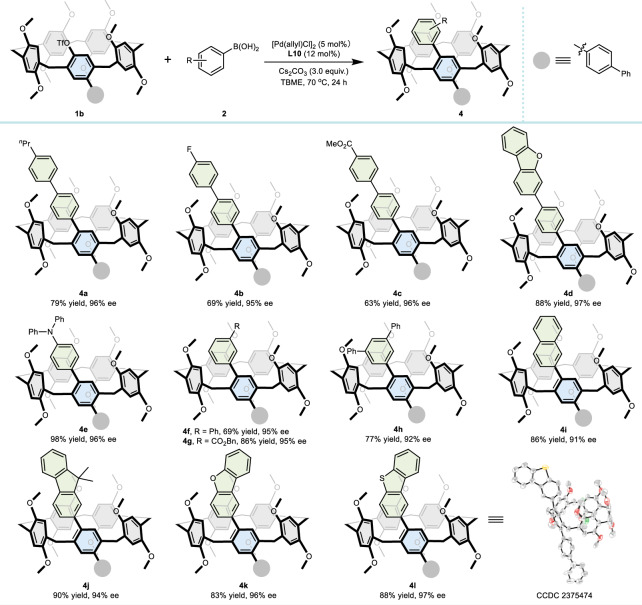
The reaction involved a stepwise asymmetric coupling process, wherein the first-step coupling product 1b formed in the initial step rapidly underwent racemization. Therefore, we obtained two different aryl-substituted chiral pillar[5]arenes by reacting 1b with arylboronic acids 2, as shown in Table 3. Initially, 4-phenylbenzene boronic acid derivatives, including those with para-substituents such as n-propyl, fluorine, CO2Me, and dibenzofuran, demonstrated good compatibility with the reaction conditions. The resulting products 4a–4d were obtained with enantiomeric excesses ranging from 95% to 97%. The functional groups, including N,N-diphenyl (4o), phenyl (4f and 4h), and ester (4g), all worked well. Finally, substrates with aromatic and heteroaromatic ring substitutions (4i–4l) yielded functionalized chiral pillar[5]arenes with excellent enantioselectivities.
Table 3.
Scope of the reactions of arylboronic acids with OTf-pillar[5]arene 1b
Reaction conditions: 1b (0.10 mmol), 2 (0.30 mmol), 5 mol% [Pd(allyl)Cl]2, 12 mol% L10, 3.0 equiv. of Cs2CO3 in 3.0 mL of TBME at 70 °C for 24 h under nitrogen; isolated yield by silica gel chromatography; ee values were determined by chiral HPLC.
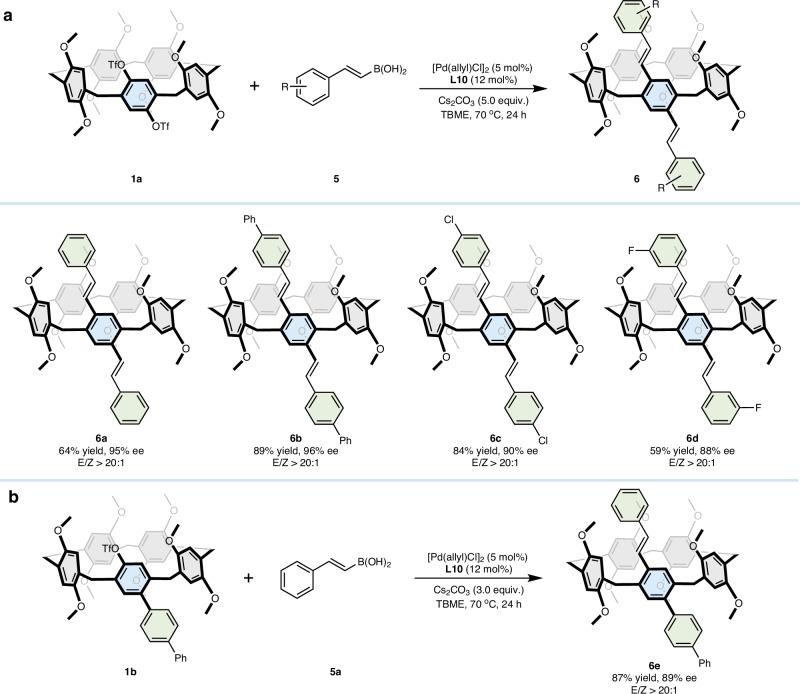
2-Arylvinylboronic acid is a commonly utilized coupling reagent in palladium-catalyzed Suzuki‒Miyaura coupling reactions. We explored the use of 2-phenylvinylboronic acid 5a in our developed system to evaluate its potential to generate Heck-type chiral pillar[5]arene (Fig. 2a). To our satisfaction, the product 6a was obtained in a yield of 64% with an enantiomeric excess of 95% and E/Z > 20:1. In addition, the reaction demonstrated broad functional group tolerance, including phenyl (6b), chlorine (6c), and fluorine (6d) substituents. However, alkynyl boronic reagents, such as 2-phenyl-1-ethynylboronic acid pinacol ester and potassium phenylethynyltrifluoroborate, did not lead to the formation of the corresponding products. Furthermore, we efficiently and enantioselectively synthesized product 6e by employing 1b in reactions with 5a in 87% yield with 89% ee (Fig. 2b), which possesses one side that is arylated and the other side that is alkenylated.
Fig. 2. Investigation of 1a and 1b with 2-phenylvinylboronic acids 5.
a Reaction of 1a and 2-arylvinylboronic acids 5 results in products 6a–6d under standard conditions. b Reaction of 1b and 2-phenylvinylboronic acid 5a results in product 6e under standard conditions.
Synthetic applications
To showcase the synthetic versatility of our method, we conducted several additional challenging substrates and performed transformations, as illustrated in Fig. 3. The ethyl-substituted compound 1c derived from 1,4-bis(ethoxy)pillar[5]arene reacted smoothly to produce 7a in 60% yield with 91% ee (Fig. 3a). Next, we attempted to react the more complex 4OTf-substituted substrate 1d with 2a, achieving high efficiency and producing the nearly enantiomerically pure product 7b (Fig. 3b). Most importantly, the fully aryl-substituted product 7c with high enantiocontrol could be obtained from 10OTf-pillar[5]arene 1e by simply prolonging the reaction time (Fig. 3c). Molecular universal joints (MUJs) represent a crucial class of bicyclic pillar[5]arenes that demonstrate chirality inversion in response to variations in temperature and solvent conditions82–84. Typically, enantiomers are resolved via chiral-phase HPLC. By employing our method, chiral MUJs 9a could be synthesized from substrate 3q through a process involving hydrolysis and subsequent cyclization (Fig. 3d). Additionally, we conducted a gram-scale experiment, where the product 3a was obtained in excellent yield with no loss of enantioselectivity, further demonstrating the practicality and scalability of our method (see Supplementary Information for more details).
Fig. 3. Extended applications of the palladium-catalyzed Suzuki‒Miyaura cross-coupling reaction.
a The reaction of 1c and 2a results in product 7a. b The reaction of 1d and 2a or PhB(OH)2 results in products 7b and 7c. c The reaction of 1e and 2a results in product 7d. d Application to the synthesis of bicyclic diester compound 9a.
Photophysical and optical property investigations
On the basis of the inherently chiral pillar[5]arenes prepared, we investigated the photophysical characteristics (see the Supplementary Information Figs. S1–S5 for details) of 3a, 3i, and 7b (Fig. 4). The ultraviolet-visible (UV-vis) absorption spectra revealed prominent absorption peaks at 305 nm, with additional smaller peaks observed at ~365 nm (Fig. 4a). Upon excitation at the maximum wavelength, fluorescence emission peaks were observed at 420 nm for 3a, 369 nm for 3i, and 412 nm for 7b (Fig. 4b). Notably, as the electronic properties of the substrate increased, there was a distinct red shift in the maximum emission wavelength in the fluorescence spectra. The circular dichroism (CD) spectra of the pR-3a and pS-3a enantiomers exhibited pronounced Cotton effects, revealing a distinct mirror image relationship between the pR and pS forms (Fig. 4c). Additionally, the circularly polarized luminescence (CPL) spectra were recorded for pR-3a, pS-3a, pR-3i, and pR-7b, confirming their CPL activity (Fig. 4d). The luminescence dissymmetry factors |glum| were quantified (Fig. 4e, f). Notably, compound pR-7b exhibited a |glum|value of 0.01673, highlighting its potential for CPL applications. These findings distinctly highlight the considerable promise of chiral pillar[5]arenes in advancing the development of chiral organic luminescent materials and CP-OLEDs85,86.
Fig. 4. Photophysical and optical property investigations.
a Absorption spectra of 3a, 3i, and 7b in DCM (1.0 × 10−5 M). b Emission spectra of 3a, 3i, and 7b in DCM (1.0 × 10−5 M). c CD spectra of pR-3a and pS-3a in DCM (1.0 × 10−5 M) at room temperature. d CPL spectra of pR-3a, pS-3a, pR-3i, and pR-7b in DCM (1.0 × 10−5 M) at room temperature and excited at 305 nm. e glum values–wavelength curve for pR-3a, pS-3a, pR-3i, and pR-7b. f Structures of 7b and glum values for 3a, 3i, and 7b.
Mechanistic investigations
To investigate the stereoselectivity-determining steps of the reaction, we initially performed stepwise control experiments (Fig. 5a). The ligand had no significant impact in the first step, while chiral control was observed in the second step. This result supports the validity of our proposed asymmetric extended side-arm Suzuki–Miyaura cross-coupling strategy. Based on these results and previous studies, we propose the following reaction mechanism (Fig. 5b). The Pd0 species undergoes oxidative addition to the C‒O bond of pillar[5]arene-based bifunctional triflate 1a, forming PdII complex I. This complex I then undergoes transmetallation with arylboronic acid 2 to generate intermediate III, which undergoes reductive elimination to deliver product 1a’ and regenerate Pd⁰. In the second step of the coupling reaction, a similar catalytic cycle occurs. We proposed an asymmetric induction model for intermediate III, where the aryl group of L10 is positioned near the palladium metal center (Fig. 5b)81,87. The electronic properties and the significant steric hindrance of this aryl group play a crucial role in controlling the stereoselectivity of the reaction. In contrast, with ligand L8, where the aryl group is positioned farther from the palladium center, the control over stereoselectivity is less effective. Notably, through intermediate III, we observed that the steric hindrance of the ligand is critical in determining the stereoselectivity. When considering an alternative pS-int transition state, the steric repulsion between the ligand’s aryl group and the substrate significantly reduces stereoselectivity, making this transition state less favorable. In this palladium-catalyzed Suzuki–Miyaura cross-coupling reaction, the stereochemical outcome is determined during the oxidative addition of the chiral-ligated palladium complex to the triflate38. However, the subsequent transmetallation and reductive elimination steps are also influenced by the steric and electronic properties of the arylboronic acid, which affect the stereoselective control of the reaction88–90.
Fig. 5. Mechanistic investigations.
a Investigation of stereoselectivity control by two-step reaction. b Proposed reaction mechanism.
In conclusion, we developed an enantioselective palladium-catalyzed Suzuki‒Miyaura cross-coupling methodology for the construction of inherently chiral pillar[5]arenes. The pivotal step in continuous enantioselective cross-coupling was effectively accomplished via a chiral Sadphos ligand. This versatile and practical palladium-catalyzed method accommodates a broad spectrum of arylboronic acids and 2-arylvinylboronic acids, facilitating the efficient synthesis of structurally diverse enantioenriched chiral pillar[5]arenes with exceptional enantiocontrol. Additionally, preliminary studies on the photophysical and optical properties of these chiral pillar[5]arenes highlight their potential for use in material science and self-assembly systems.
Methods
General procedure for the synthesis of chiral pillar[5]arenes 3 and 6a–6d
Under nitrogen atmosphere, to a mixture of 1 (0.1 mmol, 1.0 equiv.), 2 or 5 (0.5 mmol, 5.0 equiv.), [Pd(allyl)Cl]2 (5–10 mol%), L10 (12–24 mol%), and Cs2CO3 (0.5 mmol, 5.0 equiv.), followed by the addition of TBME (3.0 mL) in a sealed vial and the reaction was stirred at 70 °C for 24 h. After completion of the reaction, the solvent was removed under vacuum and the crude product was purified directly by column chromatography to afford the desired product 3 or 6a–6d.
General procedure for the synthesis of chiral pillar[5]arenes 4 and 6e
Under nitrogen atmosphere, to a mixture of 1b (0.1 mmol, 1.0 equiv.), 2 or 5 (0.3 mmol, 3.0 equiv.), [Pd(allyl)Cl]2 (5 mol%), L10 (12 mol%), and Cs2CO3 (0.3 mmol, 3.0 equiv.), followed by the addition of TBME (3.0 mL) in a sealed vial and the reaction was stirred at 70 °C for 24 h. After completion of the reaction, the solvent was removed under vacuum and the crude product was purified directly by column chromatography to afford the desired product 4 or 6e.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This work was supported by the financial support from the Taishan Scholar Youth Expert Program in Shandong Province (tsqn 201909096 R.L.), National Natural Science Foundation of China (22371152 R.L.), National Natural Science Foundation of Shandong (ZR2023JQ006 R.L., ZR2024QB091 L.X.) and Qingdao Natural Science Foundation (23-2-1-244-zyyd-jch R.L.).
Author contributions
T.L., C.S., Y.T., and Y.J. performed the experiments. All authors contributed to the analysis of the experimental results. R.L. and L.X. conceived the study, supervised the project, and wrote the paper.
Peer review
Peer review information
Nature Communications thanks Leyong Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data generated in this study are provided in the Supplementary Information file. The experimental procedures, data of NMR, and HRMS have been deposited in the Supplementary Information file. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre under deposition numbers CCDC 2373070 (3a) and 2375474 (4l) (Supplementary Data 1). Copies of the data can be obtained free of charge via www.ccdc.cam.ac.uk/getstructures. All other data supporting the findings of the study, including experimental procedures and compound characterization, are available within the paper and its Supplementary Information, or from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Ting-Rui Luan, Che Sun.
Contributor Information
Long-Long Xi, Email: xill@qdu.edu.cn.
Ren-Rong Liu, Email: renrongliu@qdu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-57461-x.
References
- 1.Crini, G. Review: a history of cyclodextrins. Chem. Rev.114, 10940–10975 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Alsbaiee, A. et al. Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nature529, 190–194 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Murray, J., Kim, K., Ogoshi, T., Yao, W. & Gibb, B. C. The aqueous supramolecular chemistry of cucurbit[n]urils, pillar[n]arenes and deep-cavity cavitands. Chem. Soc. Rev.46, 2479–2496 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nie, H., Wei, Z., Ni, X.-L. & Liu, Y. Assembly and applications of macrocyclic-confinement-derived supramolecular organic luminescent emissions from cucurbiturils. Chem. Rev.122, 9032–9077 (2022). [DOI] [PubMed] [Google Scholar]
- 5.Zheng, B., Wang, F., Dong, S. & Huang, F. Supramolecular polymers constructed by crown ether-based molecular recognition. Chem. Soc. Rev.41, 1621–1636 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Liu, Z., Nalluri, S. K. M. & Stoddart, J. F. Surveying macrocyclic chemistry: from flexible crown ethers to rigid cyclophanes. Chem. Soc. Rev.46, 2459–2478 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Kumar, R. et al. Revisiting fluorescent calixarenes: from molecular sensors to smart materials. Chem. Rev.119, 9657–9721 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Strutt, N. L., Zhang, H., Schneebeli, S. T. & Stoddart, J. F. Functionalizing pillar[n]arenes. Acc. Chem. Res.47, 2631–2642 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Ogoshi, T., Yamagishi, T. & Nakamoto, Y. Pillar-shaped macrocyclic hosts pillar[n]arenes: new key players for supramolecular chemistry. Chem. Rev.116, 7937–8002 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Zhang, H., Liu, Z. & Zhao, Y. Pillararene-based self-assembled amphiphiles. Chem. Soc. Rev.47, 5491–5528 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Song, N., Kakuta, T., Yamagishi, T. A., Yang, Y.-W. & Ogoshi, T. Molecular-scale porous materials based on pillar[n]arenes. Chem. 4, 2029–2053 (2018). [Google Scholar]
- 12.Kato, K. et al. Noncovalently bound and mechanically interlocked systems using pillar[n]arenes. Chem. Soc. Rev.51, 3648–3687 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Tain, Y. et al. Synthesis of covalent organic pillars as molecular nanotubes with precise length, diameter and chirality. Nat. Synth.2, 395–402 (2023). [Google Scholar]
- 14.Chen, L., Nixon, R. & De Bo, G. Force-controlled release of small molecules with a rotaxane actuator. Nature628, 320–325 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuo, D.-H., Shi, T.-H., Ohtani, S. & Ogoshi, T. Responsive pillar[n]arene materials. Responsive Mater.2, e20230024 (2024). [Google Scholar]
- 16.Schreiber, C. L. & Smith, B. D. Molecular conjugation using non-covalent click chemistry. Nat. Rev. Chem.3, 393–400 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dsouza, R. N., Pischel, U. & Nau, W. M. Fluorescent dyes and their supramolecular host/guest complexes with macrocycles in aqueous solution. Chem. Rev.111, 7941–7980 (2011). [DOI] [PubMed] [Google Scholar]
- 18.De Bo, G. et al. An artificial molecular machine that builds an asymmetric catalyst. Nat. Nanotechnol.13, 381–385 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Mako, T. L., Racicot, J. M. & Levine, M. Supramolecular luminescent sensors. Chem. Rev.119, 322–477 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Amano, S., Fielden, S. D. P. & Leigh, D. A. A catalysis-driven artificial molecular pump. Nature594, 529–534 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Li, Z. & Yang, Y.-W. Macrocycle-based porous organic polymers for separation, sensing, and catalysis. Adv. Mater.34, 2107401 (2022). [DOI] [PubMed] [Google Scholar]
- 22.Zhu, H., Li, Q., Zhu, W. & Huang, F. Pillararenes as versatile building blocks for fluorescent materials. Acc. Mater. Res.3, 658–668 (2022). [Google Scholar]
- 23.Zhang, L. et al. An electric molecular motor. Nature613, 280–286 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu, H. et al. Applications of macrocycle-based solid-state host–guest chemistry. Nat. Rev. Chem.7, 768–782 (2023). [DOI] [PubMed] [Google Scholar]
- 25.Ogoshi, T., Kanai, S., Fujinami, S., Yamagishi, T. & Nakamoto, Y. para-bridged symmetrical pillar[5]arenes: their lewis acid catalyzed synthesis and host–guest property. J. Am. Chem. Soc.130, 5022–5023 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Wada, K. & Ogoshi, T. Functionalization of pillar[n]arenes towards optically responsive systems via host–guest interactions. Mater. Chem. Front.8, 1212–1229 (2024). [Google Scholar]
- 27.Strutt, N. L., Zhang, H. & Stoddart, J. F. Enantiopure pillar[5]arene active domains within a homochiral metal–organic framework. Chem. Commun.50, 7455–7458 (2015). 2014. [DOI] [PubMed] [Google Scholar]
- 28.Yang, J., Zeng, Q. & Wang, L. Electrochemical polymerization induced chirality fixation of crystalline pillararene-based polymer and its application in interfacial chiral sensing. Anal. Chem.93, 9965–9969 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Chen, J.-F. et al. Planar chiral organoboranes with thermoresponsive emission and circularly polarized luminescence: integration of pillar[5]arenes with boron chemistry. Angew. Chem. Int. Ed.59, 11267–11272 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Ogoshi, T., Furuta, T. & Yamagishi, T. Chiral supramolecular polymers consisting of planar-chiral pillar[5]arene enantiomers. Chem. Commun.52, 10775–10778 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Nagata, Y. et al. A planar-chiral pillar[5]arene-based monophosphine ligand with induced chirality at the biaryl axis. Synlett29, 2167–2170 (2018). [Google Scholar]
- 32.Sun, Y. et al. Unimolecular chiral stepping inversion machine. J. Am. Chem. Soc.145, 16711–16717 (2023). [DOI] [PubMed] [Google Scholar]
- 33.Fa, S., Kakuta, T., Yamagishi, T. & Ogoshi, T. Conformation and planar chirality of pillar[n]arenes. Chem. Lett.48, 1278–1287 (2019). [Google Scholar]
- 34.Zheng, H. et al. Cation controlled rotation in anionic pillar[5]arenes and its application for fluorescence switch. Nat. Commun.14, 590 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, E. et al. Pseudo[1]catenane-type pillar[5]thiacrown whose planar chiral inversion is triggered by metal cation and controlled by anion. J. Am. Chem. Soc.140, 9669–9677 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Strutt, N. L. et al. Incorporation of an A1/A2-difunctionalized pillar[5]arene into a metal−organic framework. J. Am. Chem. Soc.134, 17436–17439 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Kato, K., Fa, S. & Ogoshi, T. Alignment and dynamic inversion of planar chirality in pillar[n]arenes. Angew. Chem. Int. Ed.62, e202308316 (2023). [DOI] [PubMed] [Google Scholar]
- 38.Zhou, X.-H. et al. Catalytic enantioselective synthesis of planar chiral pillar[5]arenes via asymmetric sonogashira coupling. Angew. Chem. Int. Ed. 10.1002/anie.202415190 (2024). [DOI] [PubMed]
- 39.Baudoin, O. The asymmetric Suzuki coupling route to axially chiral biaryls. Eur. J. Org. Chem.2005, 4223–4229 (2005). [Google Scholar]
- 40.Zhang, D. & Wang, Q. Palladium catalyzed asymmetric Suzuki−Miyaura coupling reactions to axially chiral biaryl compounds: chiral ligands and recent advances. Coord. Chem. Rev.286, 1–16 (2015). [Google Scholar]
- 41.Li, C., Chen, D. & Tang, W. Addressing the challenges in Suzuki−Miyaura cross-couplings by ligand design. Synlett27, 2183–2200 (2016). [Google Scholar]
- 42.Goetzke, F. W., Dijk, L. V. & Fletcher, S. P. Catalytic asymmetric Suzuki−Miyaura couplings.PATAI’S Chem. Funct. Groups Am. Cancer Soc.2019, 1–54 (2019). [Google Scholar]
- 43.Hedouin, G., Hazra, S., Gallou, F. & Handa, S. The catalytic formation of atropisomers and stereocenters via symmetric Suzu-ki−Miyaura couplings. ACS Catal.12, 4918–4937 (2022). [Google Scholar]
- 44.Carmona, J. A., Rodríguez-Franco, C., Fernández, R., Hornillos, V. & Lassaletta, J. M. Atroposelective transformation of axially chiral (hetero)biaryls. from desymmetrization to modern resolution strategies. Chem. Soc. Rev.50, 2968–2983 (2021). [DOI] [PubMed] [Google Scholar]
- 45.Cheng, J. K., Xiang, S.-H., Li, S., Ye, L. & Tan, B. Recent advances in catalytic asymmetric construction of atropisomers. Chem. Rev.121, 4805–4902 (2021). [DOI] [PubMed] [Google Scholar]
- 46.Cammidge, A. N. & Crépy, K. V. L. The first asymmetric Suzuki cross-coupling reaction. Chem. Commun.18, 1723–1724 (2000). [Google Scholar]
- 47.Yin, J. & Buchwald, S. L. A catalytic asymmetric Suzuki coupling for the synthesis of axially chiral biaryl compounds. J. Am. Chem. Soc.122, 12051–12052 (2000). [Google Scholar]
- 48.Bermejo, A., Ros, A., Fernández, R. & Lassaletta, J. M. C2-symmetric bis-hydrazones as ligands in the asymmetric Suzuki−Miyaura cross-coupling. J. Am. Chem. Soc.130, 15798–15799 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Xu, G., Fu, W., Liu, G., Senanayake, C. H. & Tang, W. Efficient syntheses of korupensamines A, B and michellamine B by asymmetric Suzuki−Miyaura coupling reactions. J. Am. Chem. Soc.136, 570–573 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Pan, C., Zhu, Z., Zhang, M. & Gu, Z. Palladium-catalyzed enantioselective synthesis of 2-aryl cyclohex-2-enone atropisomers: platform molecules for the divergent synthesis of axially chiral biaryl compounds. Angew. Chem. Int. Ed.56, 4777–4781 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Yang, H., Sun, J., Gu, W. & Tang, W. Enantioselective cross-coupling for axially chiral tetra-ortho-substituted biaryls and asymmetric synthesis of gossypol. J. Am. Chem. Soc.142, 8036–8043 (2020). [DOI] [PubMed] [Google Scholar]
- 52.Shen, D., Xu, Y. & Shi, S.-L. A bulky chiral N-heterocyclic carbene palladium catalyst enables highly enantioselective Suzuki−Miyaura cross-coupling reactions for the synthesis of biaryl atropisomers. J. Am. Chem. Soc.141, 14938–14945 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Liu, Z.-S. et al. Construction of axial chirality via palladium/chiral norbornene cooperative catalysis. Nat. Catal.3, 727–733 (2020). [Google Scholar]
- 54.Qiu, S.-Q. et al. Asymmetric construction of aryl-alkene axis by palladium-catalyzed Suzuki–Miyaura coupling reaction. Angew. Chem. Int. Ed.61, e202211211 (2022). [DOI] [PubMed] [Google Scholar]
- 55.Gan, K. B., Zhong, R.-L., Zhang, Z.-W. & Kwong, F. Y. Atropisomeric phosphine ligands bearing C-N axial chirality: applications in enantioselective Suzuki-Miyaura cross-coupling towards the assembly of tetra-ortho-substituted biaryls. J. Am. Chem. Soc.144, 14864–14873 (2022). [DOI] [PubMed] [Google Scholar]
- 56.Pearce-Higgins, R. et al. An enantioselective Suzuki-Miyaura coupling to form axially chiral biphenols. J. Am. Chem. Soc.144, 15026–15032 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang, Y., Wu, C., Xing, J. & Dou, X. Developing biarylhemiboronic esters for biaryl atropisomer synthesis via dynamic kinetic atroposelective Suzuki–Miyaura cross-coupling. J. Am. Chem. Soc.146, 6283–6293 (2024). [DOI] [PubMed] [Google Scholar]
- 58.Willis, M. C., Powell, L. H. W., Claverie, C. K. & Watson, S. J. Enantioselective Suzuki reactions: catalytic asymmetric synthesis of compounds containing quaternary carbon centers. Angew. Chem. Int. Ed.43, 1249–1251 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Lu, Z., Wilsily, A. & Fu, G. C. Stereoconvergent amine-directed alkyl−alkyl Suzuki reactions of unactivated secondary alkyl chlorides. J. Am. Chem. Soc.133, 8154–8157 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun, C., Potter, B. & Morken, J. P. A catalytic enantiotopic group-selective Suzuki reaction for the construction of chiral organoboronates. J. Am. Chem. Soc.136, 6534–6537 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Potter, B., Szymaniak, A. A., Edelstein, E. K. & Morken, J. P. Nonracemic allylic boronates through enantiotopic-group-selective cross-coupling of geminal bis(boronates) and vinyl halides. J. Am. Chem. Soc.136, 17918–17921 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ros, A. et al. Dynamic kinetic cross-coupling strategy for the asymmetric synthesis of axially chiral heterobiaryls. J. Am. Chem. Soc.135, 15730–15733 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Jiang, X. & Gandelman, M. Enantioselective Suzuki cross-couplings of unactivated 1-fluoro-1-haloalkanes: synthesis of chiral β-, γ-, δ-, and ε-fluoroalkanes. J. Am. Chem. Soc.137, 2542–2547 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Sidera, M. & Fletcher, S. P. Rhodium-catalysed asymmetric allylic arylation of racemic halides with arylboronic acids. Nat. Chem.7, 935–939 (2015). [DOI] [PubMed] [Google Scholar]
- 65.Jiang, S.-P. et al. Copper-catalyzed enantioconvergent radical Suzuki−Miyaura C-(Sp3)−C(Sp2) cross-coupling. J. Am. Chem. Soc.142, 19652–19659 (2020). [DOI] [PubMed] [Google Scholar]
- 66.van Dijk, L. et al. Mechanistic investigation of Rh(I)-catalysed asymmetric Suzuki−Miyaura coupling with racemic allyl halides. Nat. Catal.4, 284–292 (2021). [Google Scholar]
- 67.Lou, Y., Wei, J., Li, M. & Zhu, Y. Distal ionic substrate−catalyst interactions enable long-range stereocontrol: access to remote quaternary stereocenters through a desymmetrizing Suzuki−Miyaura reaction. J. Am. Chem. Soc.144, 123–129 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li, M., Chia, X. L., Tian, C. & Zhu, Y. Mechanically planar chiral rotaxanes through catalytic desymmetrization. Chem8, 2843–2855 (2022). [Google Scholar]
- 69.Wei, J., Candon, V. & Zhu, Y. Amino acid-derived ionic chiral catalysts enable desymmetrizing cross-coupling to remote acyclic quaternary stereocenters. J. Am. Chem. Soc.145, 16796–16811 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li, M., Ho, C. K. S., On, I. K. W., Gandon, V. & Zhu, Y. Inherently chiral resorcinarene cavitands through ionic catalyst-controlled cross-coupling. Chem10.1016/j.chempr.2024.06.012 (2024).
- 71.Ren, L.-Q. et al. Modular enantioselective assembly of multi-substituted boron-stereogenic BODIPYs. Nat. Chem. 10.1038/s41557-024-01649-z (2024). [DOI] [PubMed]
- 72.Gagnon, C., Godin, É., Minozzi, C., Sosoe, J. & Collins, S. K. Biocatalytic synthesis of planar chiral macrocycles. Science367, 917–921 (2020). [DOI] [PubMed] [Google Scholar]
- 73.Li, J.-H. et al. Organocatalytic enantioselective synthesis of seven-membered ring with inherent chirality. Angew. Chem. Int. Ed.63, e202319289 (2024). [DOI] [PubMed] [Google Scholar]
- 74.Zhang, H. et al. Palladium-catalyzed asymmetric carbene coupling en route to inherently chiral heptagon-containing polyarenes. Nat. Commun.15, 3353 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang, Y.-K. et al. Organocatalytic enantioselective synthesis of inherently chiral calix[4]arenes. Angew. Chem. Int. Ed.63, e202407752 (2024). [DOI] [PubMed] [Google Scholar]
- 76.Zhang, Z.-M. et al. A new type of chiral sulfinamide monophosphine ligands: stereo-divergent synthesis and application in enantioselective gold(I)-catalyzed cycloaddition reactions. Angew. Chem. Int. Ed.53, 4350–4354 (2014). [DOI] [PubMed] [Google Scholar]
- 77.Qi, S. et al. Ligand-enabled palladium-catalysed enantioselective synthesis of α-quaternary amino and glycolic acids derivatives. Nat. Synth.3, 357–367 (2024). [Google Scholar]
- 78.Li, W. & Zhang, J. Sadphos as adaptive ligands in asymmetric palladium catalysis. Acc. Chem. Res.57, 489–513 (2024). [DOI] [PubMed] [Google Scholar]
- 79.Wang, L., Chen, M., Zhang, P.-C., Li, W. & Zhang, J. Palladium/PC-Phos-catalyzed enantioselective arylation of general sulfenate anions: scope and synthetic applications. J. Am. Chem. Soc.140, 3467–3473 (2018). [DOI] [PubMed] [Google Scholar]
- 80.Zhang, P.-C., Han, J. & Zhang, J. Pd/PC-Phos-catalyzed enantioselective intermolecular denitrogenative cyclization of benzotriazoles with allenes and N-allenamides. Angew. Chem. Int. Ed.58, 11444–11448 (2019). [DOI] [PubMed] [Google Scholar]
- 81.Chu, H., Cheng, J., Yang, J., Guo, Y.-L. & Zhang, J. Asymmetric dearomatization of indole by palladium/PC-Phos-catalyzed dynamic kinetic transformation. Angew. Chem. Int. Ed.59, 21991–21996 (2020). [DOI] [PubMed] [Google Scholar]
- 82.Ogoshi, T., Akutsu, T., Yamafuji, D., Aoki, T. & Yamagashi, T. Solvent- and achiral-guest-triggered chiral inversion in a planar chiral pseudo[1]catenane. Angew. Chem. Int. Ed.52, 8111–8115 (2013). [DOI] [PubMed] [Google Scholar]
- 83.Brandt, J. R. et al. Redox-triggered chirality switching and guest-capture/release with a pillar[6]arene-based molecular universal joint. Angew. Chem. Int. Ed.59, 8094–8098 (2020). [DOI] [PubMed] [Google Scholar]
- 84.Yao, J. et al. Overtemperature-protection intelligent molecular chiroptical photoswitches. Nat. Commun.12, 2600 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brandt, J. R., Salerno, F. & Fuchter, M. J. The added value of small-molecule chirality in technological applications. Nat. Rev. Chem.1, 0045 (2017). [Google Scholar]
- 86.Lee, D.-M., Song, J.-W., Lee, Y.-J., Yu, C.-J. & Kim, J.-H. Control of circularly polarized electroluminescence in induced twist structure of conjugate polymer. Adv. Mater.29, 1700907 (2017). [DOI] [PubMed] [Google Scholar]
- 87.Ma, C., Sun, Y., Yang, J., Guo, H. & Zhang, J. Catalytic asymmetric synthesis of Tröger’s base analogues with nitrogen stereocenter. ACS Cent. Sci.9, 64–71 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun, C., Potter, B. & Morken, J. P. A catalytic enantiotopic-group-selective Suzuki reaction for the construction of chiral organoboronates. J. Am. Chem. Soc.136, 6534–6537 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Patel, N. D. et al. Computationally assisted mechanistic investigation and development of Pd-catalyzed asymmetric Suzuki−Miyaura and Negishi cross-coupling reactions for tetra-ortho-substituted biaryl synthesis. ACS. Catal.8, 10190–10209 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pearce-Higgins, R. et al. An enantioselective Suzuki−Miyaura coupling to form axially chiral biphenols. J. Am. Chem. Soc.144, 15026–15032 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The data generated in this study are provided in the Supplementary Information file. The experimental procedures, data of NMR, and HRMS have been deposited in the Supplementary Information file. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre under deposition numbers CCDC 2373070 (3a) and 2375474 (4l) (Supplementary Data 1). Copies of the data can be obtained free of charge via www.ccdc.cam.ac.uk/getstructures. All other data supporting the findings of the study, including experimental procedures and compound characterization, are available within the paper and its Supplementary Information, or from the corresponding author upon request.