Abstract
Liver fibrosis, characterized by scar tissue accumulation due to liver injury, poses significant barriers to liver-targeted gene therapy. Current clinical trials exclude patients with fibrosis, as intact liver architecture is considered essential for efficient and safe adeno-associated viral vector (AAV)-mediated gene delivery. Here, we show that liver fibrosis reduces the efficiency of hepatocyte transduction by AAV8 vectors across three mouse models with diverse fibrotic patterns. This inefficiency stems primarily from decreased vector uptake by the liver rather than loss of vector genomes due to hepatocyte turnover. Additionally, fibrosis alters blood vector clearance and redistributes AAV particles to extra-hepatic organs, such as spleen, lung, and kidney. At the cellular level, fibrosis decreases AAV genome content in hepatocytes while increasing it in non-parenchymal liver cells and splenic immune cells. Importantly, the capsid variant AAV-KP1 retains transduction efficiency in fibrotic livers, highlighting its potential for expanding gene therapy applications to fibrotic diseases.
Subject terms: Liver fibrosis, Gene therapy
Ferriero et al. show that liver fibrosis reduces the efficiency of AAV-based gene therapy by limiting liver uptake and altering vector distribution to other organs. The impact of fibrosis varies depending on the AAV variant, highlighting the need for tailored approaches in fibrotic diseases.
Introduction
Liver fibrosis is the deposition of scar tissue in the liver as a result of liver injury. Liver fibrosis can progress to cirrhosis, a condition characterized by altered organ architecture, aberrant vasculature, and multiple regenerative nodules resulting in portal hypertension, hepatocellular carcinoma, and organ failure. The estimated prevalence of liver fibrosis in the general population is up to 25% with an estimated prevalence of up to 2% for advanced liver fibrosis or cirrhosis1. Several inherited liver diseases are characterized by progressive and severe liver fibrosis, including progressive familial cholestasis, Wilson disease, and α-1 antitrypsin deficiency2. Moreover, improved therapies and medical care are increasing the lifespan of patients potentially unmasking in the near future liver fibrosis as a more relevant issue in other inherited diseases with hepatic injury, which is not currently a well-established manifestation but has been reported only anecdotally3,4.
Liver-directed gene therapy by AAV vectors achieved remarkable success in the clinic. Encouraging results from a successful hemophilia B trial5 paved the way for a number of interventional clinical trials for hemophilias and several inherited disorders6, with three AAV-based products for the treatment of hemophilia A and B have recently been given market authorization7–9. Clinically relevant liver fibrosis is currently an exclusion criterion in most liver-directed gene therapy clinical trials for reasons of caution based on a putative reduced therapeutic efficacy. A variety of biological and physical changes associated with the development of liver fibrosis could indeed affect the efficacy and safety of AAV-mediated gene therapy: (i) the loss of sinusoid fenestrations and deposition of extracellular matrix in the perisinusoidal space (also known as capillarization) reduce vascular permeability10 and may hamper the infection of hepatocytes by blood-borne AAV vectors; (ii) the infiltration and proliferation of immune cells may enhance vector scavenging and immune reaction against the vector11,12; (iii) the inflammation associated with fibrosis may contribute to priming of immune cells13; (iv) the regenerative responses induced by liver damage may result in hepatocyte proliferation, episomal vector dilution and short term expression of the therapeutic gene14. However, very few studies have investigated AAV vector delivery in animal models with fibrotic livers and there is no data in human patients correlating liver fibrosis to efficacy or safety outcomes. To fill this gap, in the present study, we performed a thorough investigation of AAV-mediated gene transfer to the liver of several disease mouse models with liver fibrosis and provide relevant insights on the impact of fibrosis on vector biodistribution and transduction.
Results
Liver fibrosis impairs hepatocyte transduction by AAV8
We investigated AAV-mediated liver gene transfer in three mouse models of liver fibrosis resembling different patterns of fibrosis development and progression: (i) thioacetamide (TAA)-treatment, a well-established model of peri-central to pan-lobular fibrosis15; (ii) the Atp7b−/− mouse, which recapitulates Wilson disease with hepatic copper accumulation resulting in necroinflammation and extensive perisinusoidal fibrosis16; (iii) the Abcb4−/− mouse, a widely used model of biliary fibrosis resembling clinical features of progressive familial intrahepatic cholestasis type 3 (PFIC3) and primary sclerosing cholangitis17.
Male fibrotic and control mice received intravenous (i. v.) injections of an AAV2/8 that expresses green fluorescent protein (GFP) under the control of a hepatocyte-specific promoter (AAV2/8-TBG-eGFP) at a dose of 1 × 1013 (TAA) or 5 × 1012 (Atp7b−/−and Abcb4−/−) genome copies (gc)/kg and were sacrificed after 14 days (Atp7b−/−and Abcb4−/−), or after 42 days (TAA) post-injection. Sirius Red staining of harvested livers showed that TAA-treated, Atp7b−/−, and Abcb4−/− mice exhibited increased collagen deposition (Fig. 1A and Fig. S1A) and reduced number of GFP-positive cells and GFP protein levels compared to control mice (Fig. 1B, C and Fig. S1B, C). Consistent with reduced transduction, hepatic vector genome content was significantly reduced in fibrotic mice (Fig. 1D). Because sex significantly affects AAV vector-mediated transduction of murine liver18, female mice were also investigated and they showed reduced hepatic transduction and vector genome copies compared with controls, consistent with the data in male mice (Fig. S2). To assess whether the impaired transduction could be overcome with higher vector doses, mice were injected with 5 × 1013 gc/kg of AAV2/8-TBG-eGFP. Similarly to what was observed for the lower dose, fibrotic livers showed significantly reduced transduction and vector genome content compared to non-fibrotic livers (Fig. S3) To further explore the inverse relationship between AAV transduction and fibrosis, we administered the AAV2/8-TBG-eGFP to younger (16 weeks of age) Atp7b−/−, displaying reduced liver fibrosis (Fig. S4A, B). This milder fibrosis did not significantly affect transgene expression or vector genome levels compared to vehicle-treated controls (Fig. S4C–E). Correlation analysis showed a strong to very strong inverse correlation between liver fibrosis and AAV transduction efficiency in TAA-induced and Abcb4−/− mice and their respective control mice (Fig. S5). In contrast, no significant correlation was found for Atp7b−/− and Atp7b+/− mice, although this may be related to overestimation of GFP-positive area in Atp7b−/− mice, which is likely caused by an increase in hepatocyte size related to copper storage16 (Fig. S5).
Fig. 1. Reduced AAV8 transduction in liver fibrosis mouse models.
The AAV2/8-TBG-GFP vector was injected into male mice treated with thioacetamide (TAA, n = 8) or vehicle (PBS, n = 6) at a dose of 1 × 1013 gc/kg, and into 18-week-old male Atp7b−/− (n = 6) and Atp7b+/− (control, n = 5) mice, and 14-week-old male Abcb4−/− (n = 5) and Abcb4+/− (control, n = 7) mice at a dose of 5 × 1012 gc/kg. Mice were sacrificed 42 days (TAA) or 14 days (Atp7b−/− and Abcb4−/−) post-injection. A Representative images of Sirius Red staining of liver sections. Scale bar: 100 µm. B Representative images of immunofluorescence performed on liver sections using an anti-GFP antibody. Nuclei are counterstained with DAPI. Scale bar: 100 µm. C Representative image from western blot analysis of whole liver lysates using an anti-GFP antibody. β-actin (ACTB) and p115 were used as loading controls. D Vector genome copy (GC) analysis performed by qPCR. (two-tailed t-test) Data are reported as mean ± standard error.
Liver fibrosis impairs hepatic AAV8 uptake, delays vector blood clearance, and changes vector biodistribution
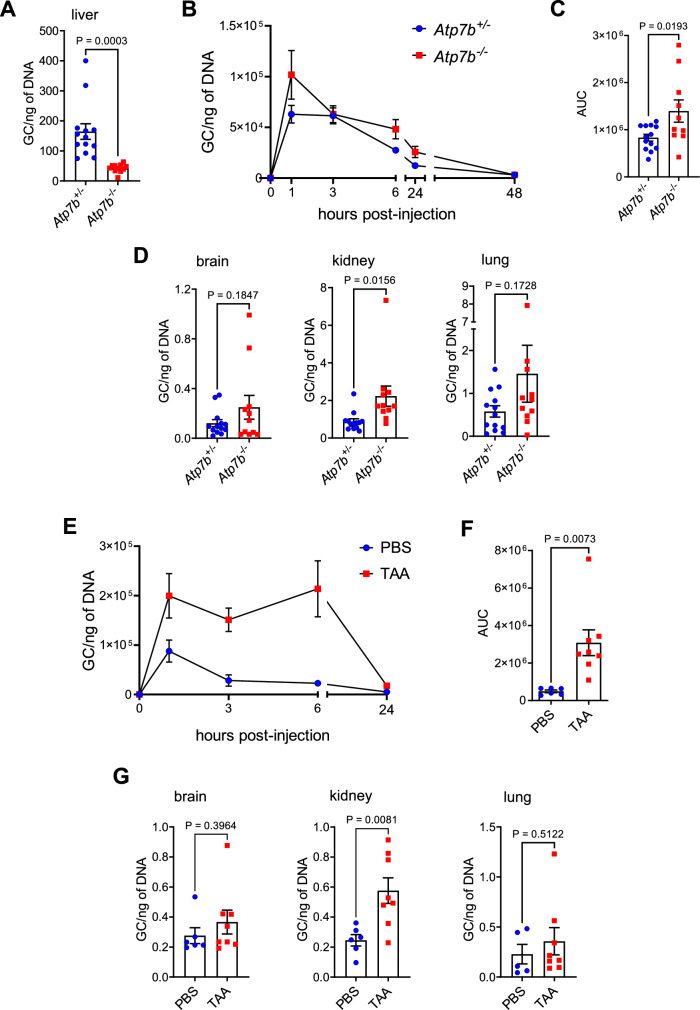
Chronic liver damage is usually associated with a regenerative response and hepatocyte proliferation has been described in all mouse models investigated in this study16,19. Therefore, we asked whether the reduced transduction efficiency observed at 14 days post-injection was due to reduced vector uptake by the liver cells or to episomal vector dilution because of hepatocyte proliferation. To address this question, Atp7b−/− and Abcb4−/− mice were sacrificed 2 days after vector administration, before the onset of vector dilution. There was a significant reduction of vector genome content in fibrotic Atp7b−/− livers compared to non-fibrotic Atp7b+/− mice (Fig. 2A), suggesting impaired vector uptake in livers with fibrosis. Analysis of vector blood clearance by qPCR analysis showed delayed vector blood clearance in Atp7b−/− mice compared to control Atp7b+/− mice (Fig. 2B, C). Moreover, compared to controls, Atp7b−/− mice showed an increasing trend in the lung and brain (Fig. 2D) and a significant increase in the kidney of vector genome content (Fig. 2D). The increase in AAV vector genomes in the kidney was associated with increased mesangial cellularity and matrix deposition in 3 out 10 Atp7b−/− mice (Fig. S6A). Other extra-hepatic organs showed similar vector genome copies in Atp7b−/−and Atp7b+/− mice (Fig. S6B). Similarly, TAA-treated mice showed delayed vector blood clearance (Fig. 2E, F) and changes in vector biodistribution with a significant increase in vector genome content in the kidney and a non-significant increase in the lung and brain (assayed at 42 days post-vector administration) (Fig. 2G).
Fig. 2. Delayed clearance and altered biodistribution of AAV8 in TAA-treated and Atp7b−/− mice.
A–D The AAV2/8-TBG.GFP vector was administered to 18-week-old male Atp7b−/− (n = 11) and Atp7b+/− (control, n = 13) mice. Mice were sacrificed 2 days post-injection. A Vector genome copy (GC) analysis in the liver. B Vector GC analysis in the plasma and C quantification of the area under the curve (AUC). D Vector GC analysis in extra-hepatic organs. E–G The AAV2/8-TBG-GFP vector was injected in male wild-type mice treated with thioacetamide (TAA, n = 8) or vehicle (PBS, n = 6) as control. Mice were sacrificed 42 days post-vector administration. E Vector GC analysis in the plasma and F quantification of the area under the curve (AUC). G Vector GC analysis in extra-hepatic organs. Data are reported as mean ± standard error. (two-tailed t-test).
Vector genome content in the liver was also reduced as early as 2 days post-vector administration in Abcb4−/− mice (Fig. 3A), further supporting the concept that impaired transduction is primarily due to reduced vector uptake by the liver rather than vector dilution in replicating hepatocytes. According to what we observed in Atp7b−/− and TAA-induced mouse models, circulating vector levels showed a higher peak at early time points post-injection in Abcb4−/− mice (Fig. 3B), while overall blood vector clearance was similar to controls (Fig. 3C). Analysis of vector biodistribution in extra-hepatic organs showed a significant increase of vector genome content in the lung and spleen and a non-significant increase in the heart of male Abcb4−/− mice (Figs. 3D and S7A). Increased viral uptake by the spleen was associated with capsule and trabeculae thickening, follicular hyperplasia, and ectasia of blood vessels in Abcb4−/− mice (Fig. S7B, C).
Fig. 3. Delayed clearance and altered biodistribution of AAV8 in Abcb4−/− mice.
14-week-old male Abcb4−/− (n = 7) and Abcb4+/− (control, n = 6) mice were administered with 5 × 1012 gc/kg of AAV2/8-TBG-GFP vector and sacrificed 2 days post-injection. A Vector genome copy (GC) analysis in livers (P = 0.00002). B Vector GC analysis in the plasma and C quantification of the area under the curve (AUC) in Abcb4−/− (n = 14) and Abcb4+/− (control, n = 11) mice administered with AAV2/8-TBG-GFP. D Vector GC analysis in extra-hepatic organs from male Abcb4−/− (n = 8) and Abcb4+/− (control, n = 6) mice (two-tailed t-test or, B only, repeated measure two-way ANOVA plus Sidak post-hoc). Data are reported as mean ± standard error.
Liver fibrosis increases vector uptake by hepatic non-parenchymal cells and splenocytes of myeloid origin
Fibrosis significantly modifies liver cellularity and alters the biological and morphological characteristics of liver cells. To investigate whether these changes affect the distribution of the AAV among liver cell types, we analyzed vector genome content in FACS-isolated liver cell fractions at 4 h post-injection. This early time point was chosen to avoid the loss of vector genomes by intracellular degradation, particularly in non-parenchymal liver cells (NPCs). Analysis of vector genome content in liver cell fractions from Atp7b−/− mice revealed a decrease in hepatocytes (0.3-fold) and an increase in dendritic cells (DCs, 7.2-fold), endothelial cells (ECs, 2.3-fold), hepatic stellate cells (HSCs, 25.1-fold), and Kupffer cells (KCs, 5.3-fold) compared to cell fractions from control mice (Fig. 4A). Interestingly, increased uptake by ECs was associated with a significant reduction of sinusoidal fenestrations in Atp7b−/− mice (Fig. 4B, C). Co-staining analysis for GFP and NPC-specific markers of livers at 14 days post-vector administration suggested no transgene expression within NPCs (Fig. S8). Similarly, in TAA-treated mice, reduced vector genome content in hepatocytes was accompanied by increased uptake in ECs and a non-significant increase in HSCs and KCs. (Fig. S9A) The hepatocytes fractions from Abcb4−/− mice showed a reduction of AAV genomes compared to controls (Fig. 4D). However, NPC fractions showed similar vector genome content in Abcb4−/− and control Abcb4+/− mice, except for DCs which showed a significant 1.5-fold increase in Abcb4−/− mice (Figs. 4D and S9B). No changes in the number of sinusoidal fenestrations were found in these mice (Fig. S9C, D). Gene expression analysis of AAV8 receptors, namely AAVR20 and LamR21 revealed significant changes in Atp7b−/− livers with higher (3.8-fold) AAVR and LamR expression in hepatocytes and greater upregulation in HSC and DC (487.0- and 16.5-fold for LamR, and 81.4- and 16.1-fold for AAVR, respectively) compared to controls (Fig. 4E, F). However, no significant differences in AAVR or LamR levels were observed in EC and KC fractions between Atp7b−/− and Atp7b+/− livers (Fig. S10A, B). In contrast, liver cell fractions from Abcb4−/− and Abcb4+/− mice showed no significant changes in AAVR or LamR expression, except for a 3.0-fold increase in LamR expression in DC fractions from Abcb4−/− livers (Figs. 4G and S10C, D).
Fig. 4. Increased AAV8 distribution to liver non-parenchymal cells in Atp7b−/− mice and to myeloid splenocytes in Abcb4−/− mice.
A–C AAV2/8-TBG-GFP vector was administered to 18-week-old male Atp7b−/− (n = 5) and Atp7b+/− (control, n = 5) mice. Mice were sacrificed 4 h post-injection. A Vector genome copy (GC) analysis by digital PCR in hepatocytes (Hep) and non-parenchymal liver cells: dendritic cell (DC), endothelial cell (EC), hepatic stellate cell (HSC), and Kupffer cell (KC) fractions. B Representative images from electron microscopy analysis on liver tissues. Arrows indicate endothelial fenestrations. Scale bar: 500 nm. C Quantification of fenestrations. The number of endothelial fenestrae per 1 µm of capillary wall length was counted in three animals per group. D The AAV2/8-TBG-GFP vector was administered to 10-week-old female Abcb4−/− (n = 11) and Abcb4+/− (n = 12) mice. Mice were sacrificed 4 h post-injection. Vector genome copy (GC) analysis by digital PCR in hepatocyte (Hep) and hepatic dendritic cell (DC) fractions. E–G Gene expression analysis by qPCR of AAV8 receptor E AAVR and F LamR in hepatocyte (Hep), dendritic cell (DC), and hepatic stellate cell (HSC) fractions from Atp7b−/− (n = 5) and Atp7b+/− (control, n = 7) mice and G LamR in dendritic cell (DC) fractions from Abcb4−/− (n = 5) and Abcb4+/− (control, n = 7) mice. H Vector genome copy (GC) analysis by digital PCR in splenic macrophages (MΦ) and dendritic cell (DC) fractions from Abcb4−/− (n = 5) and Abcb4+/− (n = 8) mice (two-tailed t-test). Data are reported as mean ± standard error.
Given the increased amount of vector in the spleen, we also analyzed genome content in splenocyte fractions from Abcb4−/− mice and found a significant increase in splenic macrophages (2.1-fold) and dendritic cells (3.1-fold) compared to controls (Fig. 4H), while no significant differences were found in T and B lymphocytes (Fig. S11). To interrogate the immunological consequences of vector redistribution to antigen-presenting cells (APCs), such as macrophages and dendritic cells, we investigated the cytokine profiles in plasma of Abcb4−/− mice injected with AAV, with Abcb4+/− mice serving as controls. Although there was a trend toward elevated concentrations of some cytokines such as IP10 (Fig. S12) in the AAV-injected Abcb4−/− mice compared to controls at the baseline or to vehicle-treated Abcb4−/− mice, overall no significant upregulation of cytokines was detected after AAV administration in Abcb4−/− and Abcb4+/− mice (Fig. S12). These findings suggest that despite its redistribution to cells with APC function, the AAV vector did not result in significant differences in systemic cytokine response in fibrotic livers, at least at the tested vector dose of 5 × 1012 gc/kg. Moreover, analysis of fibrosis in livers from AAV- versus vehicle-treated mice suggests that AAV does not accelerate fibrosis progression (Fig. S13).
Liver fibrosis's impact on liver transduction is capsid-dependent
We next investigated whether fibrosis impacts liver transduction by other AAV variants. Given its ability to traverse biological barriers22, we tested AAV9-mediated gene transfer in fibrotic livers. Consistent with the results of AAV8, AAV9 genome copies were significantly reduced in the liver and increased in the spleen compared to control mice at 2 and 14 days post-vector administration (Fig. S14A, B), and AAV9-mediated liver transduction was less efficient in Abcb4−/− versus Abcb4+/− mice (Fig. S14C, D). Similarly, AAV9 showed reduced hepatic vector genome content and transduction in Atp7b−/−compared to Atp7b+/− mice (Fig. S14E, F). To expand our screening for hepatocyte transduction in fibrotic livers to a larger number of AAV variants, we used an AAV testing kit23. This AAV kit contained an equimolar mix of n = 67 known AAV variants, including natural serotypes and isolates, as well as novel bioengineered variants. All the variants included in the kit express GFP under the control of a ubiquitous promoter and carry two unique 6-nucleotide barcode sequences for each capsid in the 3′UTR. Barcode analysis by next-generation sequencing (NGS) on DNA and RNA levels can thus provide information about the capacity of the AAV variant capacity to enter the cells (DNA, cell entry) and express the transgene (RNA, functional transduction). Atp7b−/− and Abcb4−/− mice, and age-matched control mice, were injected with a 1 × 1012 gc/Kg dose using the AAV testing Kit. Mice were sacrificed five days post-injection, and hepatocyte fractions were subjected to NGS. Overall genome content in hepatocytes from both Atp7b−/− and Abcb4−/− mice was reduced compared to controls, thus further confirming impaired vector uptake by fibrotic livers (Fig. S15A). Clustering analysis revealed that at the DNA level, fibrotic livers from Atp7b−/− and Abcb4−/− mice showed a higher degree of similarity between them rather than with their non-fibrotic controls, with Atp7b−/− livers having the most divergent profile from non-fibrotic ones (Fig. S15B). AAV-DJ and AAV-KP1 were the most represented in hepatocytes from Abcb4−/− mice and from control Atp7b+/− and Abcb4+/− mice (Fig. S15C, D). Surprisingly, AAV5 and AAV1 were the most abundant variants retrieved from Atp7b−/− hepatocytes (Fig. S15C). Conversely, fibrotic livers showed a less similar transduction profile at the RNA level (Fig. S15B). Among the most represented, several AAV variants were relatively more abundant in fibrotic hepatocytes including AAV-KP1, 2 and 3, AAV-DJ, AAV-NP22, AAV-NP-66, AAV5, and AAV1 in Atp7b−/− (Fig. 5A), and AAV-KP1, AAV-Rh10, AAV11, and AAV-Rh74 in Abcb4−/− (Fig. 5B). Because it was the most represented variant at the RNA level in both models (Fig. 5A, B), we investigated AAV-KP1 for hepatocyte transduction in fibrotic livers. An AAV2/KP1-TBG-eGFP vector was injected i.v. at a dose of 5 × 1012 gc/kg in male fibrotic Atp7b−/− and Abcb4−/− mice and in age- and sex-matched Atp7b+/− and Abcb4+/− controls (Fig. S16A, B). We found similar or even increased transgene expression and vector genome content in fibrotic versus non-fibrotic livers by AAV2/KP1-TBG-eGFP vector (Figs. 5C–E and S16C, D). Taken together, this data shows that impairment of AAV-mediated liver-directed gene transfer in fibrotic livers is capsid-dependent and AAV-KP1 transduction is unaffected or potentially higher in fibrotic livers.
Fig. 5. Liver fibrosis impacts gene transfer in a capsid-dependent fashion and does not affect AAV-KP1.
A, B The AAV testing kit was administered to 18-week-old male Atp7b−/− (n = 5), Atp7b+/− (control, n = 5) mice, and to 14-week-old male Abcb4−/− (n = 4) and Abcb4+/− (control, n = 6) mice at the dose of 1 × 1012 gc/kg. Mice were sacrificed at 5 days post-injection. Quantification of RNA expression for the twelve most represented AAV variants in A Atp7b−/− and Atp7b+/− control mice and B Abcb4−/− and Abcb4+/− control mice. The AAV2/KP1.TBG.GFP vector was administered to 18-week-old male Atp7b−/− (n = 7), Atp7b+/− (control, n = 7), and to 14-week-old male Abcb4−/− (n = 7) and Abcb4+/− (control, n = 2) mice at the dose of 5 × 1012 gc/kg. Mice were sacrificed at 14 days post-injection. C Representative images of immunofluorescence performed on liver sections using an anti-GFP antibody. Nuclei are counterstained with DAPI. Scale bar: 100 µm. D Representative image from western blot analysis of whole liver lysates using an anti-GFP antibody. p115 was used as a loading control. E Vector genome copy (GC) analysis performed by qPCR (two-tailed t-test). Data are reported as mean ± standard error.
Discussion
Liver-directed gene therapy has undergone tremendous developments over the last two decades24. AAVs are the vectors of choice for the treatment of inherited liver diseases, with some products achieving market authorization. Although previous studies suggested that fibrosis could be a barrier toward efficient gene transfer, very few data were available on AAV vectors in fibrotic livers. An early study inoculating AAV1 in rats with carbon tetrachloride (CCl4)- and thioacetamide (TAA)-induced cirrhosis showed no differences or even improvement of hepatocyte transduction compared to healthy animals25. Conversely, gene transfer using AAV8 in Abcb4−/− mice showed up to a 60-fold reduction in transgene expression when injected after fibrosis development19.
In this study, we investigated AAV-mediated cell entry and transduction in chemically- and genetically induced liver fibrosis models. Using three mouse models characterized by different types of liver fibrosis, we provided compelling evidence that fibrotic livers are transduced less efficiently by AAV8-based vectors. This was a common finding across all tested models, irrespective of the fibrosis patterns and etiologies, and it was consistent with previous evidence in Abcb4−/− mice19. The impact of fibrosis on AAV-mediated liver transduction was clearly stage-dependent, as illustrated by the inverse correlation between the levels of transgene expression and collagen deposition. This is further supported by the absence of detrimental effects observed in younger Atp7b−/− mice with mild to moderate peri-central fibrosis, while advanced peri-central fibrosis significantly impairs transduction. Although hepatocyte proliferation in response to damage was believed to play a major role in the reduction of transduction efficiency, we showed that decreased vector genome content in fibrotic livers compared to non-fibrotic livers was already observed at 2 days post-injection, a very early time point when the dilution effect is negligible. Therefore, liver fibrosis likely impairs vector uptake by liver cells. Although not resulting in productive cell entry and transgene expression, AAV8 associates with and accumulates on the surface of both KCs and ECs within minutes after i.v. delivery to healthy mice26. In contrast, AAV particles may fail to efficiently traverse vascular endothelium due to a scarcity of fenestrations and accumulate in ECs, as observed in fibrotic livers of Atp7b−/− and TAA-treated mice. Virion uptake by KCs is associated with cell death and activation of a local, self-limiting acute inflammatory response in non-fibrotic livers26,27. However, KCs are not a barrier to gene delivery to hepatocytes, because their depletion does not increase hepatocyte transduction26,28, while they induce a tolerogenic response toward the transgene product by Treg interaction29. Nevertheless, an inflamed and fibrotic hepatic milieu may impact KC response to AAV cell entry. In Wilson disease patients, the number and activation of KCs increase with disease progression30–32, and this may boost KC vector scavenging and immune reaction. Fibrosis may not only physically obstruct the vector path toward hepatocytes but may also modify AAV tropism for specific cell types by inducing changes in their plasma membrane receptor repertoire. In CCl4-induced liver fibrosis, AAV2 showed tropism for HSC, whose activation is associated with upregulation of fibroblast growth factor receptor-1α, an AAV2 co-receptor33. Similarly, AAV8-based vectors poorly transduce HSC in healthy livers, while we found a remarkable increase of AAV8 uptake by HSC in fibrotic Atp7b−/− mice, associated with upregulation of genes encoding for AAV8 receptors. While the TAA-induced fibrosis model also showed a generalized increase in NPC vector genome content, unexpectedly we did not find significant increases in the vector genome content and the expression of AAV8 receptors of NPCs in Abcb4−/− mice, except for DCs. However, HSC and KC are greatly expanded in this mouse model34. Therefore, while the viral uptake per cell was similar to control mice, the overall vector content in the HSC and KC compartments should be increased in fibrotic mice.
Persistent AAV circulation in the bloodstream increased the distribution of viral particles in extra-hepatic organs, especially in the kidney, brain, and lung of both Atp7b−/− and TAA-treated mice. Similarly, AAV circulating levels in Abcb4−/− mice showed a significantly higher peak at early time points post-vector administration. This was associated with increased spleen vector uptake and a generalized increase of vector genome content in extra-hepatic organs. The differences observed in AAV biodistribution among tested fibrotic models may reflect disease-specific alterations in the extra-hepatic tissues, affecting vector uptake. In Wilson disease, the kidney is susceptible to copper-induced injury35, and Atp7b−/− mice show renal copper storage and alterations of renal function36. Liver fibrosis induces portal hypertension causing splenomegaly that may enhance the number and phagocytic activity of splenic macrophages, known as hypersplenism37. Blood engorgement and increased scavenging of viral particles by resident macrophages may contribute to increased vector uptake by the spleen, and splenomegaly and hypersplenism are well-recognized features of PFIC338,39 and Abcb4−/− mice40. However, splenomegaly has also been described in Atp7b−/− mice41, which did not show increased vector genome content in the spleen compared to controls. This suggests that some specific alterations resulting from ABCB4 deficiency may be responsible for enhanced vector uptake. We found that increased viral uptake by the spleen in fibrotic Abcb4−/− mice is primarily driven by macrophages and DCs. Liver DCs also showed higher vector genome content compared to controls, which was associated with increased expression of AAV8 receptors. DCs and macrophages are crucial players in both innate and adaptive immune responses against AAV. Toll-like receptor engagement by AAV components in macrophages, DCs, and liver ECs leads to the generation of anti-AAV cytotoxic CD8+ T cells and antibodies42–46. Interestingly, hepatic KCs, DCs, and ECs showed higher vector genome content in Atp7b−/− mice compared to non-fibrotic controls. However, increased viral uptake by immunocompetent cells does not necessarily aggravate the immune response against AAV, and our preliminary study suggests that fibrotic liver does result in significant activation of the innate immunity against AAV8 in Abcb4−/− mice. Further investigations into the immune profile and safety of AAV in the context of fibrotic liver diseases are needed.
According to the current and previous studies, a higher vector dose should be needed to achieve a therapeutic benefit in patients with liver fibrosis. However, high vector doses have been associated with severe adverse events and death in the presence of pre-existing liver damage47 Vector administration before fibrosis development or increasing vector doses may represent a strategy to overcome the fibrosis. Nevertheless, fibrosis is already present at the time of diagnosis in several patients affected by inborn errors of metabolism48. Our data suggests that better vectors are needed to achieve effective and safer gene therapy for diseases with liver fibrosis, and vector engineering approaches aimed at de-targeting AAVs from non-hepatocytic cells may result in enhanced hepatocyte transduction. High-throughput screening of existing AAV variants revealed that, while different fibrosis patterns similarly impact AAV uptake by hepatocytes, the transduction profiles of tested AAV variants varied significantly across two liver fibrosis models. Of note, the two mouse models were maintained on different genetic backgrounds16,49 Although more in-depth investigations are needed, this finding points to intracellular AAV trafficking and processing as potential variables determining the outcomes of gene transfer to fibrotic livers. Interestingly, AAV1, previously shown to be unaffected by fibrosis in rat models25, was enriched in fibrotic hepatocytes, particularly in the Atp7b−/− model. Similarly, AAV5—on which two approved gene therapy products are based7,8—was recovered at relatively higher levels in fibrotic hepatocytes. Notably, approximately 20% of hemophilia patients who suffer from liver fibrosis or cirrhosis, are often associated with chronic hepatitis C virus infection or metabolic-associated steatotic liver disease50,51. At the RNA level, the AAV-KP1 capsid was the most enriched in both liver fibrosis models by AAV testing kit screening and it showed at least similar transduction efficiency in fibrotic and non-fibrotic mice when tested individually. AAV-KP1 is a shuffled bioengineered capsid holding 93% identity with AAV3B and efficiently transducing mouse hepatocytes52. Additionally, previous studies discovered that the deletion of amino acid T265 in the AAV-KP1 VP1 sequence enables this capsid to very efficiently transduce human hepatocytes in an in vivo xenograft mouse model53. As a strong heparan sulfate proteoglycan (HSPG) binder, AAV-KP1 showed a preferred tropism for periportal hepatocytes53. In contrast, AAV8 and AAV9 exhibit a predominantly perivenous transduction pattern in the murine liver54, and their transduction efficiency was significantly reduced in fibrotic conditions. Similarly, AAV-Rh10, another capsid with a preference for perivenous hepatocytes, showed relatively reduced viral entry and transgene expression in Atp7b−/− mice, although showing enrichment in Abcb4−/− hepatocytes compared to controls. These data support the intriguing hypothesis that liver fibrosis impairs AAV-mediated transduction in a zone-dependent manner, an area warranting further investigation.
In conclusion, we showed that liver fibrosis severely hampers liver gene transfer by AAV8 and AAV9 vectors and alters AAV biodistribution both at the organ and cell type level, depending on fibrosis etiology, pattern, and/or involvement of extra-hepatic organs. Moreover, increased viral uptake by immunocompetent and antigen-presenting cells in the fibrotic liver and the spleen may modify the AAV immune profile. Our findings also reveal that the impact of liver fibrosis on AAV-mediated gene transfer is capsid-dependent, with AAV-KP1 retaining effective transduction in fibrotic livers.
Overall, these data confirm that liver fibrosis has to be carefully evaluated prior to AAV administration in patients, and more extensive safety assessment is needed in patients with fibrotic livers. Moreover, this study also highlights the potential of specific AAV vectors as a platform for gene therapy in fibrotic diseases.
Methods
Ethics Statement
Research reported in this study complies with all relevant ethical regulations and was authorized by the Italian Ministry of Health (Authorization n° n. 843/2021-PR) and approved by ethical boards of TIGEM. All animal experiments were performed in line with the ARRIVE guidelines.
Animal procedures
Mouse procedures were carried out in accordance with the regulations of and authorized by the Italian Ministry of Health. Male and female C57BL/6 wild-type mice (Charles River Laboratories), Atp7b−/− and Atp7b+/− mice in hybrid C57BL/6 × 129S6/SvEv background49, and Abcb4−/− and Abcb4+/− mice in Balb/cJ background16 were housed under specific pathogen-free conditions at the TIGEM animal facility (Pozzuoli, Italy) with 12-h light/dark cycles and received food and water ab libitum. Animal welfare was constantly monitored, and euthanasia was performed according to ethical regulations. AAV2/8, AAV2/9, and AAV-KP1 vectors encoding GFP under the control of the TBG promoter were produced by triple transfection, as previously described55 (InnovaVector). Six-week-old male or female C57BL/6 wild-type mice were administered intraperitoneally with increasing doses of TAA or vehicle (PBS) starting at 50 mg for the first two weeks and gradually increased to 400 mg by the final 16 weeks15 and then intravenously (i.v.) injected with an AAV vector. Blood collection and animal sacrifice were performed at different time points post-vector administration, as indicated in the results section. At sacrifice, animals were perfused with PBS, and organs were harvested for further analysis. Plasma samples were analyzed with mouse cytokine & chemokine 36‐Plex ProcartaPlex 1A Panel (ThermoFisher) according to the manufacturer’s instructions. Investigators were blinded to group allocation during data collection and analysis.
Vector copy number analysis by qPCR
Genomic DNA was extracted from 30 to 50 mg of snap-frozen liver, spleen, kidney, lung, brain, bone marrow, and heart using standard phenol-chloroform and ethanol precipitation methods. Briefly, total DNA was isolated from liver tissues with Hirt solution (10 mM Tris, 10 mM EDTA pH 7.5, and 0.6% SDS) supplemented with 20 µg/µL Proteinase K (Euroclone) overnight at 55 °C with agitation. 100 µg/µL of RNAse A (Qiagen) were added to the mixture and incubated for one hour at 37 °C. DNA was recovered after two rounds of phenol-chloroform extraction and overnight precipitation in ethanol supplemented with 3 M sodium acetate. Pelleted DNA was centrifuged at 21.1 × g for 60 min at 4 °C, washed twice with 70% ethanol, dried at 55 °C, and resuspended in Milli-Q H2O. Plasma genomic DNA was purified using NucleoSpin Blood according to the manufacturer’s protocol (Macherey–Nagel). Vector genome copies from plasma and organs were investigated by real-time PCR targeting the TBG sequence (forward primer: AAACTGCCAATTCCACTGCTG; reverse primer: CCATAGGCAAAAGCACCAAGA), using the Sybr Green qPCR Master Mix (Roche) on a Light Cycler 480 II (Roche). The samples were run in duplicate. Data was analyzed with the Light Cycler 480 software Standard curves were generated using serial dilutions of linearized plasmid containing TBG-eGFP.
Histopathology
Livers, spleens, and kidneys from PBS-perfused mice were fixed in 4% paraformaldehyde for 12 h, stored in 70% ethanol, and embedded in paraffin blocks. Sirius Red staining was performed on 5 μm liver sections which were rehydrated and stained for 1 h in picrosirius red solution (0.1% Sirius Red in saturated aqueous solution of picric acid). After two changes of acidified water (0.5% acetic acid in water), sections were dehydrated, cleared in xylene, and mounted on a resinous medium. Images were captured using an Axio Scan.Z1 microscope (Zeiss) and analyzed by ImageJ for quantification of Sirius Red-positive areas. At least five images for each mouse were analyzed. For hematoxylin and eosin staining, 5 μm thick sections were rehydrated, stained with Mayer’s hematoxylin (Bio-Optica) and eosin Y (Sigma–Aldrich), dehydrated, and mounted in mounting medium (Leica Biosystems). Spleen fibrosis, capsule thickening, trabeculae thickening, follicle hyperplasia, and ectasia of blood vessels were scored on a scale of 0 (absent), 1 (mild), 2 (moderate), and 3 (severe). Pathology evaluation was performed by experienced pathologists (SC, OP, and EDN).
Immunofluorescence
For immunofluorescence, livers from PBS-perfused mice were fixed in 4% paraformaldehyde for 12 h, cryopreserved in 10%–30% (wt/vol) sucrose, frozen in optimal cutting temperature compound (Tissue-Tek O.C.T.; Kaltek) in OCT blocks. eGFP expression was evaluated on 5-μm frozen sections permeabilized with 0.2% Triton X-100 with 75 mM NH4Cl in PBS for 30′ at room temperature. Sections were washed twice in PBS and blocked for 60 min at room temperature with 5% donkey serum, 3% BSA, 20 mM MgCl2, 0.3% Triton X-100 in PBS and then incubated with chicken anti-GFP (1:200 - Abcam, Cat#13970) and anti-CD31 (1:200 - BD Bioscience, Cat#553373) anti-CD68 (1:200 - Bio-Rad, Cat#MCA1957), or anti-VCL (1:200 - Santa Cruz, Cat#SC-73614) antibodies overnight at 4 °C. The day after, sections were washed twice with 0.2% Triton X-100 in PBS and incubated for 60 min at room temperature with donkey anti-chicken Alexafluor 488 (Invitrogen, Cat# A78948) for GFP, donkey anti-rat (Invitrogen, Cat#A21209) for CD31 and CD68, donkey anti-mouse Alexafluor 594 (Invitrogen, Cat# A21203) for VCL, and DAPI (Invitrogen, Cat#1306). All secondary antibodies were used at a final concentration of 1:400. Sections were washed twice with 0.2% Triton X-100 in PBS and mounted with a Mowiol mounting medium. Images were captured using an Axio Scan.Z1 microscope (Zeiss) and analyzed by ImageJ for quantification of GFP-positive areas. At least five images for each mouse were analyzed.
Electron microscopy
For EM analyses, liver specimens were fixed using a 1% glutaraldehyde prepared in 0.2 M HEPES buffer (pH 7.3). Small blocks of liver tissue were then post‐fixed in 1% OsO4 and 0.5% uranyl acetate. After dehydration through a graded series of ethanol solutions and acetone, tissue samples were embedded in epoxy resin and polymerized at 60 °C for 72 h. From each sample, 60 nm thin sections were cut using a Leica EM UC7 ultramicrotome and images were acquired by FEI Tecnai-12 (FEI, Eindhoven, The Netherlands) electron microscope equipped with Veletta CCD camera. The number of endothelial fenestrae per 1 µm of capillary wall length was counted from randomly chosen fields.
Western blot analysis
Snap-frozen liver tissues were lysed in RIPA buffer supplemented with protease and phosphatase inhibitors (Sigma). Liver samples were disrupted using TissueLyzer LT (Qiagen) for 3 min at 30 Hrtz. Lysates were incubated for 30 min on ice and centrifuged for 20 min at 21.1 × g at 4 °C. The supernatant was collected, and protein content was determined by Bradford assay (Bio-Rad). Protein samples were separated by SDS-PAGE using 4%–15% polyacrylamide gels (Bio-Rad). Primary antibodies were rabbit anti-GFP (1:1000 - Santa Cruz; Cat#sc-8334), mouse anti-β-actin (1:1000 - Novusbio; Cat#NB600-501H), and rabbit anti-P115 (1:5000 - kindly provided by Maria Antonietta De Matteis’ laboratory, TIGEM), which were diluted in TBS-T (0.8% NaCl, 0.02% KCl, 0.3% Tris-base, 0.1% Tween 20)/5% milk (Bio-Rad). Proteins of interest were detected with horseradish peroxidase (HRP)-conjugated goat anti-mouse and goat anti-rabbit IgG antibodies (1:3000 - GE Healthcare). Peroxidase substrate was provided using the ECL Western Blotting Substrate kit (Pierce). Each western was run two times.
Isolation of liver and spleen cell fractions
Parenchymal and non-parenchymal cells were isolated from mouse livers using a modified protocol based on Pronase/collagenase digestion56. In brief, mouse livers were perfused through the inferior vena cava with an EGTA solution followed by enzymatic digestion with Pronase (Sigma–Aldrich) and then collagenase type D (Roche Applied Science). Next, livers were harvested, and liver cells were disassociated by digestion with Pronase/collagenase solution and filtered through a nylon filter (Corning) to remove undigested tissues and debris. The resulting cell suspension was centrifuged at 50 × g for 3 min at 4 °C. The cell pellet containing hepatocytes was collected and frozen in 90% FBS/10% DMSO for FACS analysis. The NPC-containing supernatant was centrifuged at 600 × g for 10 min at 4 °C. The pellet was washed with Ca2+- and Mg2+-free Hanks’ balanced salt solution (Gibco) and centrifuged at 70 × g for 3 min at 4 °C to minimize hepatocyte contamination. The final cell suspension was centrifuged at 600 × g for 10 min at 4 °C. The cell pellet was resuspended in 14% of Nicodenz (Proteogenix) and centrifuged at 1400 × g for 20 min at 4 °C. The interphase was centrifuged at 600 × g to obtain the NPC fraction that was collected and frozen in a freezing medium (90% FBS/10% DMSO) for FACS analysis.
FACS analysis
For isolation of liver cell fractions, sorting was performed on a BD FACSAria (Becton Dickinson) cell sorter. Flow cytometry analysis was performed by FACSDiva software (Becton Dickinson). The hepatocyte fraction was stained with anti‐CD31 and anti‐CD45 antibodies, and the double-negative population representing hepatocytes was collected separately from the CD31+ cells representing endothelial cells. Liver NPCs were stained separately with anti‐CD45, anti‐F4/80, and anti‐CD140b antibodies. For NPC sorting, following size gating using FSC-A vs. SSC-A (side scatter pulse height versus side scatter pulse area), a plot on CD45 was used to set gates for CD45+ or CD45− cells and generate a dot plot that discriminates immune cells from endothelial cells. For gating on CD45+ cells, we generated a dot plot of CD11c versus F4/80 to separate DC from KC, and the two cell populations were collected separately. A second gate on the CD45− population was used to generate a dot plot that identified HSC by sorting CD140b+ cells. All antibodies were monoclonal rat anti-mouse IgG and were used at a final concentration of 1:200 (Table S1). In order to reduce clogs during cell sorting, the cell suspension was filtered before the analysis. A dot plot of FSC-A vs. SSC-A was generated to identify single cells and exclude doublets, and an unstained sample was run for each experiment to minimize autofluorescence contamination in the collected stained fractions. Live/dead cells were discriminated by morphology. The gating strategy is depicted in Fig. S17.
Spleens were minced and digested in 5 ml of IMDM + 10% FCS (cIMDM) with 250 µg/ml of collagenase B (Roche) and 30 U/ml of DNase I (Sigma–Aldrich) for 30 min at 37 °C with gentleMACS (Miltenyi Biotec). After digestion, single-cell suspensions were passed through 70-µm strainers, and red blood cells were lysed with an ammonium chloride–potassium bicarbonate (ACK) lysis buffer. Cells were counted, resuspended at 100 × 106 cells per ml, and used per antibody staining reaction. Antibodies used are listed in Table S1. B cells, T cells, Dendritic Cells (cDCs), and Macrophages (MΦ) were sorted into complete IMDM by FACSAria as CD19+CD3– (B Cells), CD19–CD3+ (T cells), CD19–CD3–MHCII+CD11c+ (cDCs) and CD19–CD3–CD11b+F4/80+ (MΦ). The gating strategy is depicted in Fig. S18. Sort purity of >93% was confirmed by post-sort analysis before cells were used for further experiments.
Digital PCR (dPCR)
Genomic DNA and RNA were extracted from liver and spleen cell fractions using a DNA/RNA Mini Kit (Qiagen) as indicated by the manufacturer. For cDNA synthesis, 100 ng of RNA was retro-transcribed using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). QIAcuity dPCR reactions were conducted using the QIAcuity One System (Qiagen). Digital PCR reactions consisted of 40 μL reaction mixture per well containing 13.3 µL 3× EvaGreen PCR Master Mix (FAM channel) (Qiagen), 0.4 µM of each primer (Table S2), PCR-grade water and 10 μl of DNA or 3 μl of cDNA template. Reaction mixtures were prepared in standard 96-well PCR plates, mixed, and transferred into QIAcuity well Nanoplates (Qiagen) for partitioning using the Qiagen Priming Profile EVAGreen (RT-) PCR. Genomic DNA from cell populations or cDNA were amplified in duplicate under the following conditions: enzyme activation for 2 min at 95 °C and 40 cycles of 15 s at 95 °C, 15 s at 60 °C, 15 s at 62 °C and 5 min at 40 °C. Partitions were imaged with 200 ms exposure time, and the gain was set to 2 for the green channel. The QIAcuity Software Suite (Qiagen, version 2.5.0.1) was used with the manual global threshold approach that is based on the amplitude signal observed in negative control samples. For gene expression analysis, Rn18s was used as a reference gene.
AAV testing kit screening
The AAV Testing Kit was prepared as previously reported23 but in this study, the Kit contained n = 67 AAV variants. Each variant was individually produced twice with a unique barcode, so the AAV Testing Kit of 67 required 134 individual AAV preparations. AAV production was performed using the human embryonic kidney (HEK) 293T cell line (ATCC, Cat#CRL-3216) grown in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco) supplemented with 10% fetal bovine serum (FBS) (Sigma–Aldrich), 1×·penicillin-streptomycin (PS) (Gibco), and 25 mM HEPES (Gibco,). All individual AAV preparations were purified using standard iodixanol protocol57 and titered using droplet digital PCR (ddPCR) (Bio-Rad) using EvaGreen supermix (Bio-Rad) and eGFP oligos (EGFP-F: TCAAGATCCGCCACAACATC, and EFGP-R: TTCTCGTTGGGGTCTTTGCT). Following titration, all AAV preparations were diluted to the same concentration and re-titered. Final adjustments were made at the time of mixing so that the same number of vector genomes was mixed from each AAV preparation. The final mixed AAV Testing Kit was concentrated to 1 mL using Amicon Ultra-4 Centrifuge Filter Units with Ultracel−100 kDa membrane (EMD Millipore) and titered using ddPCR. A small aliquot of the AAV Testing Kit was used for NGS analysis to establish the exact ratio of each AAV in the mix. This NGS result was subsequently used to normalize NGS results obtained from AAV Testing Kit studies. Male Abcb4−/− and Abcb4+/− mice were injected with an AAV kit at 1 × 1012 vg/kg and sacrificed 5 days later. Hepatocyte cell fraction was isolated as previously described. DNA and RNA were isolated and cDNA was synthesized as previously reported23 without modifications. For amplification and recovery of the AAV barcode (BC) region for NGS analysis, the barcoded region of AAV-CMV-GFP-BC-WPRE cassette was amplified from 50 ng of total genomic and AAV Testing Kit vector DNA as well as 3 µL final cDNA product with BC_F forward (5′-GTTCAGCTGGAGTT CGTGACCGCCG) and BC_R reverse (5′-CAACATAGTTAAGAATACCAGTCAATCTT TCACAAATTTTGTAATCCAGAGG) primers. PCR was performed using the Q5 high-fidelity DNA polymerase (NEB, Cat# M0491L). and the resulting amplicons were purified and submitted to the Illumina amplicon-seq PE150 sequencing (Azenta, Suzhou, China). Raw NGS reads (fastq files) were converted to barcode abundance lists as previously reported58. The analysis was then finished using a custom R script to create the final data shown in this manuscript. NGS reads from the DNA and cDNA populations were normalized to the NGS results from the pre-transduction AAV Testing Kit. Analysis of vector genome copies was performed by digital droplet PCR (ddPCR) as previously described23, with minor modifications. Each sample was run in triplicate.
Statistics and reproducibility
Statistical analyses were performed using Prism 9 software (GraphPad). Two-tailed t-test, Mann–Whitney test, or repeated measure two-way ANOVA plus Sidak or one-way ANOVA plus Tukey post-hoc were used as statistical tests for mean comparisons. To compare variables reported with percentage values, we applied Spearman’s correlation between SR and GFP-positive areas computing the confidence interval at 95% and validating the values with the two-tailed t-test (approximated P value). The correlation scale considered for scoring the values is (absolute value): [0.80,1.00] very strong, [0.60, 0.79] strong, [0.40, 0.59] medium, [0.20, 0.39] weak, [0.00, 0.19] very weak. We used GraphPad Prism (ver. 10.3.1) for the calculation of the statistics. Statistical tests and experimental group sizes used for each experiment are reported in figure legends. No data was excluded from the analyses. Data are reported as mean ± standard error. All experiments (except AAV kit Screening) were repeated independently at least two times with similar results.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Source data
Acknowledgements
We are grateful to Alberto Auricchio and Graciana Diez-Roux for the helpful discussion and critical review of the manuscript. We are grateful to Antonella De Matteis for providing the anti-P115 antibody and to Mark Kay for providing the AAV-KP1 capsid plasmid. We thank Cathal Wilson and the TIGEM Scientific Office for English proofreading and Eugenio Del Prete and the TIGEM Bioinformatic core for statistical analysis. This work has been supported by the Telethon Foundation, the Horizon Europe EIC Pathfinder program under grant agreement No. 101071041 (AAVolution), the European Joint Programme on Rare Diseases (WilsonMed grant), and the PFIC Network. IB is supported by an AFM‐Telethon postdoctoral fellowship.
Author contributions
R.F. and G.B. designed and performed the experiments; A.P., S.P., and M.B. performed experiments; I.B. and L.F. performed FACS analysis of liver fractions; M.G. and G.M. performed FACS analysis of spleen fractions and cytokine profiling; S.C., E.D.N., and O.P. performed histopathology analysis; T.I., S.V., E.N., and C.P. provided technical support; A.W. and M.K. performed AAV testing kit analysis; E.P. and R.P. performed EM analysis; N.B. supervised FACS analysis of liver fractions; L.L. designed and supervised AAV testing kit screening; F.F. designed and supervised FACS analysis of spleen fractions and cytokine profiling; P.P. designed the experiments, supervised the study, and wrote the manuscript.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data generated in this study have been deposited in the Figshare database 10.6084/m9.figshare.28046117 Source data are provided with this paper.
Competing interests
R.F., N.B.P., and P.P. are inventors in patent n. WO2022184650 -Use of microRNAs in the treatment of fibrosis. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Rosa Ferriero, Gemma Bruno.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-57382-9.
References
- 1.Harris, R., Harman, D. J., Card, T. R., Aithal, G. P. & Guha, I. N. Prevalence of clinically significant liver disease within the general population, as defined by non-invasive markers of liver fibrosis: a systematic review. Lancet Gastroenterol. Hepatol.2, 288–297 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Oishi, K., Arnon, R., Wasserstein, M. P. & Diaz, G. A. Liver transplantation for pediatric inherited metabolic disorders: considerations for indications, complications, and perioperative management. Pediatr. Transpl.20, 756–769 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ranucci, G. et al. Chronic liver involvement in urea cycle disorders. J. Inherit. Metab. Dis.42, 1118–1127 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Burrow, T. A., Bove, K. E. & Grabowski, G. A. The liver in lysosomal storage diseases. In Liver Disease in Children 3 edn (eds Suchy, F. J., Sokol, R. J. & Balistreri, W. F.) (Cambridge Univ. Press, 2007).
- 5.Nathwani, A. C. et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med.365, 2357–2365 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piccolo, P., Rossi, A. & Brunetti-Pierri, N. Liver-directed gene-based therapies for inborn errors of metabolism. Expert Opin. Biol. Ther., 1–12 (2020). [DOI] [PubMed]
- 7.Blair, H. A. Valoctocogene roxaparvovec: first approval. Drugs82, 1505–1510 (2022). [DOI] [PubMed] [Google Scholar]
- 8.Heo, Y. A. Etranacogene dezaparvovec: first approval. Drugs83, 347–352 (2023). [DOI] [PubMed] [Google Scholar]
- 9.Dhillon, S. Fidanacogene elaparvovec: first approval. Drugs84, 479–486 (2024). [DOI] [PubMed] [Google Scholar]
- 10.Greuter, T. & Shah, V. H. Hepatic sinusoids in liver injury, inflammation, and fibrosis: new pathophysiological insights. J. Gastroenterol.51, 511–519 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Rogers, G. L. et al. Plasmacytoid and conventional dendritic cells cooperate in crosspriming AAV capsid-specific CD8(+) T cells. Blood129, 3184–3195 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Dijk, R. et al. Polyinosinic acid blocks adeno-associated virus macrophage endocytosis in vitro and enhances adeno-associated virus liver-directed gene therapy in vivo. Hum. Gene Ther.24, 807–813 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Xu, R., Zhang, Z. & Wang, F. S. Liver fibrosis: mechanisms of immune-mediated liver injury. Cell. Mol. Immunol.9, 296–301 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakai, H. et al. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J. Virol.75, 6969–6976 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim, Y. O., Popov, Y. & Schuppan, D. Optimized mouse models for liver fibrosis. Methods Mol. Biol.1559, 279–296 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Huster, D. et al. Consequences of copper accumulation in the livers of the Atp7b-/- (Wilson disease gene) knockout mice. Am. J. Pathol.168, 423–434 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hochrath, K. et al. The hepatic phosphatidylcholine transporter ABCB4 as modulator of glucose homeostasis. FASEB J.26, 5081–5091 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Davidoff, A. M., Ng, C. Y., Zhou, J., Spence, Y. & Nathwani, A. C. Sex significantly influences transduction of murine liver by recombinant adeno-associated viral vectors through an androgen-dependent pathway. Blood102, 480–488 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Siew, S. M. et al. Prevention of cholestatic liver disease and reduced tumorigenicity in a murine model of PFIC type 3 using hybrid AAV-piggyBac gene therapy. Hepatology70, 2047–2061 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Pillay, S. et al. An essential receptor for adeno-associated virus infection. Nature530, 108–112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akache, B. et al. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J. Virol.80, 9831–9836 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foust, K. D. et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol.27, 59–65 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westhaus, A. et al. High-throughput in vitro, ex vivo, and in vivo screen of adeno-associated virus vectors based on physical and functional transduction. Hum. Gene Ther.31, 575–589 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piccolo, P. & Brunetti-Pierri, N. Current and Emerging Issues in Adeno-Associated Virus Vector-Mediated Liver-Directed Gene Therapy. Hum. Gene Ther.36, 77–87 (2025). [DOI] [PubMed]
- 25.Sobrevals, L. et al. AAV vectors transduce hepatocytes in vivo as efficiently in cirrhotic as in healthy rat livers. Gene Ther.19, 411–417 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Carestia, A. et al. Modulation of the liver immune microenvironment by the adeno-associated virus serotype 8 gene therapy vector. Mol. Ther. Methods Clin. Dev.20, 95–108 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ronzitti, G., Gross, D. A. & Mingozzi, F. Human immune responses to adeno-associated virus (AAV) vectors. Front. Immunol.11, 670 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu, D. L., Chow, N. S. M., Bridle, B. W. & Wootton, S. K. Macrophage depletion via clodronate pretreatment reduces transgene expression from AAV vectors in vivo. Viruses13, 2002 (2021). [DOI] [PMC free article] [PubMed]
- 29.Breous, E., Somanathan, S., Vandenberghe, L. H. & Wilson, J. M. Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology50, 612–621 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johncilla, M. & Mitchell, K. A. Pathology of the liver in copper overload. Semin. Liver Dis.31, 239–244 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Björklund, J. et al. High hepatic macrophage activation and low liver function in stable Wilson patients - a Danish cross-sectional study. Orphanet J. Rare Dis.13, 169 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glavind, E. et al. The macrophage activation marker soluble CD163 is elevated and associated with liver disease phenotype in patients with Wilson’s disease. Orphanet J. Rare Dis.15, 173 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsui, T. Y. et al. rAAV-mediated stable expression of heme oxygenase-1 in stellate cells: a new approach to attenuate liver fibrosis in rats. Hepatology42, 335–342 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Wei, G. et al. Synthetic human ABCB4 mRNA therapy rescues severe liver disease phenotype in a BALB/c.Abcb4(-/-) mouse model of PFIC3. J. Hepatol.74, 1416–1428 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schilsky, M. L. & Mistry, P. K. Chapter 42 - Wilson disease and the kidney. In Genetic Diseases of the Kidney (eds Lifton, R. P., Somlo, S., Giebisch, G. H. & Seldin D. W.) (Academic Press, 2009).
- 36.Gray, L. W. et al. Urinary copper elevation in a mouse model of Wilson’s disease is a regulated process to specifically decrease the hepatic copper load. PLoS ONE7, e38327 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li, L. et al. The spleen in liver cirrhosis: revisiting an old enemy with novel targets. J. Transl. Med.15, 111 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzales, E. et al. Outcomes of 38 patients with PFIC3: impact of genotype and of response to ursodeoxycholic acid therapy. JHEP Rep.5, 100844 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schatz, S. B. et al. Phenotypic spectrum and diagnostic pitfalls of ABCB4 deficiency depending on age of onset. Hepatol. Commun.2, 504–514 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikenaga, N. et al. A new Mdr2(-/-) mouse model of sclerosing cholangitis with rapid fibrosis progression, early-onset portal hypertension, and liver cancer. Am. J. Pathol.185, 325–334 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Ho, W. I. et al. Liposome-encapsulated curcumin attenuates HMGB1-mediated hepatic inflammation and fibrosis in a murine model of Wilson’s disease. Biomed. Pharmacother.152, 113197 (2022). [DOI] [PubMed] [Google Scholar]
- 42.Zhu, J., Huang, X. & Yang, Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J. Clin. Investig.119, 2388–2398 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martino, A. T. et al. The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9–dependent innate immune responses in the liver. Blood117, 6459–6468 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hösel, M. et al. Toll-like receptor 2–mediated innate immune response in human nonparenchymal liver cells toward adeno-associated viral vectors. Hepatology55, 287–297 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Rogers, G. L. et al. Plasmacytoid and conventional dendritic cells cooperate in crosspriming AAV capsid-specific CD8+ T cells. Blood129, 3184–3195 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuranda, K. et al. Exposure to wild-type AAV drives distinct capsid immunity profiles in humans. J. Clin. Investig.128, 5267–5279 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duan, D. Lethal immunotoxicity in high-dose systemic AAV therapy. Mol. Ther.31, 3123–3126 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scorza, M. et al. Genetic diseases that predispose to early liver cirrhosis. Int. J. Hepatol.2014, 713754 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buiakova, O. I. et al. Null mutation of the murine ATP7B (Wilson disease) gene results in intracellular copper accumulation and late-onset hepatic nodular transformation. Hum. Mol. Genet.8, 1665–1671 (1999). [DOI] [PubMed] [Google Scholar]
- 50.Qvigstad, C. et al. The elevated prevalence of risk factors for chronic liver disease among ageing people with hemophilia and implications for treatment. Medicine97, e12551 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen, M. C. et al. Prevalence of non-alcoholic fatty liver disease and associated factors in patients with moderate or severe hemophilia: a multicenter-based study. Clin. Appl. Thromb. Hemost.28, 10760296221128294 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pekrun, K. et al. Using a barcoded AAV capsid library to select for clinically relevant gene therapy vectors. JCI Insight4, e131610 (2019). [DOI] [PMC free article] [PubMed]
- 53.Cabanes-Creus, M. et al. Single amino acid insertion allows functional transduction of murine hepatocytes with human liver tropic AAV capsids. Mol. Ther. Methods Clin. Dev.21, 607–620 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dane, A. P., Wowro, S. J., Cunningham, S. C. & Alexander, I. E. Comparison of gene transfer to the murine liver following intraperitoneal and intraportal delivery of hepatotropic AAV pseudo-serotypes. Gene Ther.20, 460–464 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Maddalena, A. et al. High-throughput screening identifies kinase inhibitors that increase dual adeno-associated viral vector transduction in vitro and in mouse retina. Hum. Gene Ther.29, 886–901 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mederacke, I., Dapito, D. H., Affò, S., Uchinami, H. & Schwabe, R. F. High-yield and high-purity isolation of hepatic stellate cells from normal and fibrotic mouse livers. Nat. Protoc.10, 305–315 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khan, I. F., Hirata, R. K. & Russell, D. W. AAV-mediated gene targeting methods for human cells. Nat. Protoc.6, 482–501 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cabanes-Creus, M. et al. Novel human liver-tropic AAV variants define transferable domains that markedly enhance the human tropism of AAV7 and AAV8. Mol. Ther. Methods Clin. Dev.24, 88–101 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study have been deposited in the Figshare database 10.6084/m9.figshare.28046117 Source data are provided with this paper.