Keywords: disease ecology, DNA metabarcoding, gastrointestinal parasite, ITS2, mixed infection, nemabiome
Abstract
Identifying factors that drive among-individual variation in mixed parasitic infections is fundamental to understanding the ecology and evolution of host–parasite interactions. However, a lack of non-invasive diagnostic tools to quantify mixed infections has restricted their investigation for host populations in the wild. This study applied DNA metabarcoding on parasite larvae cultured from faecal samples to characterize mixed strongyle infections of 320 feral horses on Sable Island, Nova Scotia, Canada, in 2014 to test for the influence of host (age, sex and reproductive/social status) and environmental (location, local density and social group membership) factors on variation. Twenty-five strongyle species were identified, with individual infections ranging from 3 to 18 species with a mean richness (±1 s.d.) of 10.8 ± 3.1. Strongyle eggs shed in faeces were dominated by small strongyle (cyathostomins) species in young individuals, transitioning to large strongyles (Strongylus spp.) in adults. Egg counts were highest in young individuals and in the west or centre of the island for most species. Individuals in the same social group had similar parasite communities, supporting the hypothesis that shared environment may drive parasite assemblages. Other factors such as local horse density, sex, date and reproductive/social status had minimal impacts on infection patterns. This study demonstrates that mixed infections can be dynamic across host ontogeny and space and emphasizes the need to consider species-specific infection patterns when investigating mixed infections.
Introduction
Understanding the ecology and evolution of host–parasite interactions requires knowledge of the intrinsic and environmental factors that drive among-individual variation in infection (Watson, 2013). This includes, for example, assessing the relative contributions of host age (Dobson and Pacala, 1988), genetics (Bishop, 2012), local density (Patterson and Ruckstuhl, 2013) and environmental heterogeneity (Pullan et al., 2012) on variation in infection status. However, despite concurrent infections by multiple parasite species (herein called ‘mixed infections’) being the norm (Bordes and Morand, 2009), most research in this field has so far focussed on single-species infection or indiscriminate aggregate measures of infection (e.g. fecal egg counts of major parasite clades). A greater consideration of mixed infections is important since co-occurring parasite species can have unique consequences not apparent in single infections (Hoarau et al., 2020). For example, variation in the composition of mixed infections can alter infection pathology (Ezenwa et al., 2021), and may result in unexpected epidemiological patterns such as increased transmission (Lass et al., 2013). Identifying factors driving variation in mixed infections is therefore essential to accurately assess their ecological and evolutionary consequences.
While still limited, literature on the role of intrinsic and extrinsic factors in shaping variation in mixed infections is beginning to emerge. Host age is hypothesized to play a key role, with younger individuals often having higher prevalence and abundance of certain parasite species (e.g. Parascaris spp. in horses (Fabiani et al., 2016); Trichostrongylus spp. in Soay sheep (Sinclair et al., 2016)) if juveniles have yet to develop acquired resistance (Fabiani et al., 2016; Sinclair et al., 2016). Similarly, variation between sexes has been documented in multiple host species (Bucknell et al., 1995; Santoro et al., 2014; Sweeny et al., 2022), possibly due to physiological differences, such as testosterone depressing immune responses in males (Ezenwa et al., 2012), or higher reproductive investment in females (Houdijk, 2008; Sweeny et al., 2022). Mixed infections can also vary across space in response to mechanisms such as dispersal limitation (Moss et al., 2020), propensity for certain climatic regions (Abbas et al., 2023), or sympatry among host species (Avramenko et al., 2018). Furthermore, mixed infection can vary across temporal scales, with seasonal patterns of parasite egg shedding driven by species-specific variation in life histories (Sargison et al., 2022; Sweeny et al., 2022), or as a consequence of priority effects that alter interactions among parasite species (Karvonen et al., 2019).
Despite increasing interest, testing hypotheses on the drivers of mixed infections remains challenging outside of clinical settings (Hoarau et al., 2020). Invasive sampling techniques to quantify parasite infection, such as kill-and-count or deworming treatment, are often difficult to implement for wild populations, especially in the case of longitudinal individual-based population studies, when hosts are elusive, or for populations of conservation concern (Aivelo and Medlar, 2018). Furthermore, invasive techniques are often resource intensive, which often limit conducting comprehensive surveys of parasite infections under field conditions. In contrast, non-invasive methods, such as fecal egg counts (FEC), are easier to employ, require fewer resources and limit negative impacts on study populations. However, traditional techniques relying on morphological features of eggs or larvae require significant training for accurate identification (Bradbury et al., 2022), or lack resolution due to limited differences in features among taxa (Aivelo and Medlar, 2018). Fortunately, advancements in DNA metabarcoding techniques now permit characterization of mixed parasitic infections using non-invasive samples (Aivelo and Medlar, 2018). In particular, amplification and sequencing of the ITS2 gene region using parasite DNA isolated from feces (commonly referred to as ‘nemabiome’ sequencing) has been utilized to characterize mixed parasite infections in multiple domestic (e.g. cattle; Avramenko et al., 2018, sheep; Redman et al., 2019, horses; Poissant et al., 2021) and wildlife (primates; Pafčo et al., 2018, reindeer; Davey et al., 2021, moose; Davey et al., 2023, roe deer; Beaumelle et al., 2022) species.
The need for parasite species distinction is particularly important for strongyle nematode infections of equids. There are at least 64 strongyle species (Family: Strongylidae) that infect the gastrointestinal tract of equines upon ingestion of parasite larvae from contaminated environments (Lichtenfels et al., 2008). Strongyle species are the most common parasite of horses and can vary in life history and pathology (Khan et al., 2015). Species in the Strongylus genus migrate through host tissue during larval development, with S. vulgaris notable for causing severe inflammation and thrombosis in the mesenteric artery that can be fatal (McCraw and Slocombe, 1976). Cyathostomins (Subfamily: Cyathostominae) do not migrate but encyst directly in the large intestine, and may cause larval cyathostominosis, a highly fatal syndrome associated with mass emergence of encysted larvae (Corning, 2009). Necropsy and deworming studies in horses have shown that the composition of mixed strongyle infections can vary across ontogeny (Steuer et al., 2022; Boisseau et al., 2023a), between sexes (Sallé et al., 2018) and environments (Saeed et al., 2019). Recent studies applying nemabiome sequencing in domestic horses further indicate that mixed strongyle infections may be influenced by reproductive status and climatic zones (Abbas et al., 2023) and vary across months (Poissant et al., 2021; Sargison et al., 2022). Furthermore, the composition of strongyle infections were found to be similar among 2 sympatric equine species (plains zebra and Grevy's zebra), emphasizing the influence of shared environment for generalist parasites such as strongyles (Tombak et al., 2021). However, the nemabiome approach has yet to be applied in a population of wild/feral equines to investigate drivers of among-individual variation in infections in the wild, limiting an accurate assessment of how this variation can influence the health of free-living equine populations.
The feral population of horses on Sable Island, Nova Scotia, Canada, provides a unique model for studying the ecology and evolution of host-parasite infections in the wild. The population has been subject to a long-term individual-based study since 2007 (Debeffe et al., 2016; Gold et al., 2019). Strict limits on human intervention mean that this population has never been given anthelmintics or veterinary care, providing a unique opportunity to study mixed infections under natural conditions. Previous studies have shown that Sable Island horses have higher strongyle fecal egg counts (FECs) relative to domestic horse populations (Debeffe et al., 2016). Furthermore, a general decline in FEC is observed across horse age (Debeffe et al., 2016), though older individuals shed higher proportions of Strongylus spp. eggs based on fecal cultures (Jenkins et al., 2020). Mean FEC was higher in female horses (1287 EPG) than males (1043 EPG). Individuals with higher energy requirements, such as lactating females (1437 EPG) had higher mean FEC than non-lactating females (672 EPG), though, there were no clear differences in mean FEC between bachelor males (916 EPG) and band stallions (898 EPG) (Debeffe et al., 2016). Finally, strongyle FEC was shown to vary along the length of the island, with FEC declining from west to east, possibly associated with denser vegetation and greater availability of permanent freshwater ponds in the west and center of the island (Debeffe et al., 2016). However, our current understanding of the ecology of strongyle infections in this population lacks species-specific resolution, particularly for the non-Strongylus species that constitute most of the species diversity (Poissant et al., 2021).
In this study, extensive host and environmental data was combined with nemabiome sequencing to identify factors associated with mixed infection composition and species-specific prevalence and FEC in the Sable Island horse population. Since age is associated with significant physiological change and strongyle FEC (Debeffe et al., 2016), we predicted that mixed infections would vary across ontogeny. Specifically, we expected younger individuals that have yet to develop acquired resistance to have higher strongyle diversity and FEC. Similarly, energy-limited individuals were predicted to have more diverse and abundant infections due to physiological differences in sex or reproductive status. Finally, given that strongyle species are environmentally transmitted, we expected individuals sharing the same space to have more similar infections.
Materials and methods
Study site and host population
Sable Island National Park Reserve (43°55′N; −60°00′W) is a crescent-shaped sandbar 49 km long and 1.2 km wide at its widest point, located approximately 175 km off the east coast of Nova Scotia, Canada (Fig. 1). The island is home to a population of feral horses introduced in the 18th century which has been protected from human interference since 1961, including any handling, veterinary care, or supplemental feeding (Frasier et al., 2016). Since 2007, annual surveys have been conducted during the late breeding season (July–September) as part of a long-term individual-based study (Gold et al., 2019; Regan et al., 2020). Each day (weather permitting), 1 of 7 sections of the island was surveyed, resulting in complete coverage of the island approximately once a week, and multiple times over a field season. Horses were approached on foot and their appearance (coat color, markings, etc.), age class (e.g. foal, yearling, adult), sex, social band membership, time of observation and location within 5 m using a GPS recorded. Photographs of each horse were also taken to facilitate subsequent identification through comparison with a comprehensive photographic database.
Figure 1.
Map of Sable Island National Park Reserve, Nova Scotia, Canada (from Gold et al., 2019).
As the study started in 2007, individuals born since 2006 could be aged accurately since foals and yearlings are easily distinguishable. Individuals born prior to 2006 were pooled in a single cohort assumed to be born in 2005. Given that most foals are born prior to the start of the field season, the exact birth date of individuals is unknown. Thus, we defined age of individuals in years, with foals defined as young-of-the-year (0 years old), increasing by 1 year every subsequent field season the individual was observed (e.g. yearlings have an age of 1 year). We expect most foals to be born in spring, thus, most foals are likely 0–4 months old at the start of the field season. The center of an individual's home range was inferred using the median longitude of all sightings for that individual, and local horse density associated with an individual was calculated as the total number of horses, excluding foals, whose median location was within an 8000-m radius of the individual's median location; this corresponds to the area used by horses for foraging (Marjamäki et al., 2013; Debeffe et al., 2016). Since strongyle parasites are transmitted through contaminated vegetation, local horse density was calculated as a function of total vegetated area (Marjamäki et al., 2013).
The social system of the horse is characterized by female-defense polygyny. Breeding groups (bands) are typically comprised of an adult male (stallion), breeding females (mares) and juveniles/subadults, although on some occasions multi-male bands do occur (i.e. a subordinate adult male may be present) (Contasti et al., 2012). Bands are generally stable throughout the duration of the field season, with most social dispersal occurring overwinter (Marjamäki et al., 2013). Unattached ‘bachelor’ males often associate in groups that are more ephemeral but still relatively stable (McDonnell and Murray, 1995). Hence, social groups for bachelors were defined as the unique grouping of individuals that were most often observed together over the field season. If a bachelor was observed in multiple social groupings an equal number of times, it was arbitrarily assigned to the largest social group. Female reproductive status was inferred by the presence or absence of a nursing foal (young-of-the-year), while male social status was defined as either band stallion (tending a harem), subordinate (in a band but not the dominant stallion), or bachelor.
Parasite sample collection
Freshly dropped feces linked to specific individuals were collected either opportunistically during surveys or by targeted observation using disposable nitrile gloves, with one subsample typically stored on icepacks and another at ambient temperature. Special attention was given to avoid environmental contamination (i.e. sand or vegetation) and multiple areas of a fecal mass was sampled, whenever possible. Samples for this study were collected between July 23 and September 5, 2014.
Strongyle abundance for each fecal sample was measured as fecal egg counts (FEC), which were conducted on the day of collection using 4 ± 0.1 g of feces and 26 mL Sheather's sugar solution (specific gravity of 1.27) following a previously reported modified McMaster protocol (Debeffe et al., 2016). Counts were multiplied by 25 to calculate strongyle abundance in units of eggs per gram of feces (EPG). FECs were typically, but not always, performed using feces kept cool on icepacks, although earlier research indicated that FECs are not impacted by storage temperature in the field (Debeffe et al., 2016).
For each fecal sample, a strongyle L3 coproculture was also conducted following methods described in Poissant et al. (2021). Briefly, 30 ± 1 g of feces kept at ambient temperature was mixed with 3 heaped teaspoons of vermiculite and moistened with tap water in a glass tumbler. Coprocultures were covered with a petri dish and placed in a 26°C incubator for 6 days, misted with tap water and randomly shuffled in the incubator every other day. On the evening of the sixth day, a modified Baermann ‘glass-over-petri-dish’ technique (Roberts and O'Sullivan, 1950) was performed, with L3 larvae harvested the following morning. While species may respond differently to culture conditions, experimental evidence indicates that most eggs incubated at 26°C will reach the infective L3 stage by 3–4 days (Mfitilodze and Hutchinson, 1987) and that eggs of different species should have relatively similar hatching rates (Rupasinghe and Ogbourne, 1978). Harvested L3 larvae were fixed in 1–2 mL of 70% ethanol, stored at −20°C in the field and then at −80°C upon return to the mainland.
DNA preparation and sequencing
A total of 731 larvae samples were collected from 504 individuals in 2014 (population size was 552). For this study, we randomly selected one sample from 320 different individuals. Samples were processed using protocols described in Poissant et al. (2021). The total number of L3 larvae per sample was estimated from the average worm count of two 5 μL aliquots, and approximately 2500 larvae were aliquoted. Aliquots were centrifuged at 14 000 RPM for 2 min and most of the ethanol removed, followed by the addition of 900 μL of lysis buffer (recipe in Avramenko et al., 2015). This process was repeated 2 more times to remove as much ethanol as possible and L3 were finally resuspended in 300 μL of lysis buffer. Larvae were then incubated at 95°C for 15 min and frozen at −80°C for a minimum of 12 h to encourage tissue lysis. Nine microlitre of proteinase K (20 mg mL−1) were then added and samples incubated at 50°C on a shaking incubator (300 rpm) until L3 were no longer visible (12–24 h). Proteinase K was then deactivated by incubating samples at 95°C for 15 min and a 1:10 dilution made using molecular grade water and stored at −80°C until further processing.
When larval samples had less than 2500 L3 larvae, the entire sample was processed (n = 14/320, range: 750–2400 L3). Excluding these samples from analyses did not quantitatively or qualitatively impact results, thus, these samples were retained for subsequent analyses.
Sequencing library preparation followed protocols described in Avramenko et al. (2015). Briefly, the ITS2 region was amplified in a first polymerase chain reaction (PCR) using the NC1 and NC2 primers (Gasser et al., 1993) with up to 3 random bases preceding the priming sequence and an adapter sequence to allow incorporation of barcodes in a subsequent PCR. Thermocycling consisted of 95°C for 3 min, followed by 25 cycles of 98°C for 20 s, 62°C for 15 s, 72°C for 15 s and a final extension at 72°C for 2 min. PCR products were purified using AMPure XP magnetic beads (Beckman Coulter, USA) at a ratio of 1:1 followed by gel electrophoresis to confirm successful amplification. A second PCR was performed using the initial PCR product to add unique combinations of indexes using the following thermocycler conditions: 98°C for 45 s, followed by 7 cycles of 98°C for 20 s, 63°C for 20 s and a final extension at 72°C for 2 min. Amplification was followed by a second round of magnetic bead purification. DNA concentration was quantified using a microvolume spectrophotometer and samples were pooled into libraries with approximately 100 ng of DNA per sample. The total concentration of DNA in the libraries was quantified with real-time PCR using the Universal KAPA Library Quantification Kits (Kapa Biosystems, USA), followed by sequencing on an Illumina MiSeq sequencer using 12 pM libraries with 20% PhiX sequencing control (Illumina, USA). The 320 samples considered in this study were sequenced across 3 separate runs; 2 used a v2 500-cycle Reagent Kit (n = 24, 15) and 1 used a v3 600-cycle Reagent Kit (n = 281) (Illumina, USA). Pooled libraries for the 2 v2 500-cycle kits included additional samples from Sable Island horses (repeat samples from the same individuals not considered herein), other horse populations, as well as cattle, bison and domestic sheep. The library for the v3 600-cycle kit contained only Sable Island horse samples.
Bioinformatics pipeline
Each sequencing run was independently analyzed through an in-house bioinformatics pipeline (Poissant et al., 2021; pipeline version 2 available at https://data.mendeley.com/datasets/vhyysw8xt2/2) with minor modifications. First, as different sequencing kits result in different read lengths, reads obtained from the v3 600-cycle kit were trimmed to a maximum length of 250 bp prior to analyses using the DADA2 function filterAndTrim with the parameter truncLen set to 250 to parallel read lengths generated from v2 500-cycle kits. Primers and adapters were then removed using CutAdapt v4.1 (Martin, 2011), followed by processing through DADA2 v1.26 (Callahan et al., 2016). The variable lengths of the ITS2 gene region for equine strongyles results in species-specific variation in the length of overlap when merging paired reads (Poissant et al., 2021). To avoid a filtering bias against species with a shorter ITS2 gene region which have a longer overlapping region and a higher likelihood of random errors detected during merging, the maximum number of mismatches permitted during merging was based on percent mismatch (relative to the length of the overlapping region) rather than an absolute number of mismatches. For each sequencing run, a mismatch threshold of 2.5% was empirically identified as suitable to retain relatively abundant amplicon sequence variants (ASV) while limiting rare sequences containing an excessive number of errors. Following processing through DADA2, ITSx v1.1.3 (Bengtsson-palme et al., 2013) was used to remove the conserved gene regions flanking the ITS2, and taxonomy assigned to resulting ASVs using a curated ITS2 reference database (details below) and the DADA2 assignTaxonomy function with assignment confidence set to ≥80%. To account for possible index hopping, any species that represented less than 1/2500 of the proportion of reads in a sample was removed (i.e. ~1 larvae out of the 2500). Non-equine strongyle sequences that were present in mixed sequencing runs, but not in the run containing only Sable Island horse samples, were removed assuming these species were likely due to index hopping from other host species. Finally, any sample with less than 2500 total reads were not considered for analyses, to ensure sequencing depth was sufficient to represent the parasite communities.
ITS2 reference database
Version 1.4.0 of the Nematode ITS2 Sequence Database (Workentine et al., 2020) was obtained from www.nemabiome.ca/its2-database.html. ITS2 sequences of strongyle (family Strongylidae) nematode parasites of equines (family Equidae) were further curated replicating the steps taken in Poissant et al. (2021). First, individual species with various synonymous names were renamed to a common name following Lichtenfels et al. (2008). Of the 323 equine strongyle ITS2 sequences present in the database, 40 incomplete sequences were identified following alignment using MAFFT v7.490 (Katoh et al., 2002) and visual inspection using UGENE v42 (Okonechnikov et al., 2012) and removed. To identify potential errors in species identification for GenBank entries, IQ-TREE v.1.6.12 (Nguyen et al., 2015) was used to construct a phylogenetic tree using maximum likelihood approaches using the remaining 283 sequences. Similar to Poissant et al. (2021), one sequence did not cluster with other sequences of the same species and was removed (accession number: Y08619.1; Cyathostomum catinatum). Ultimately, 282 sequences remained, representing 43 of the 64 equine strongyle species recognized in Lichtenfels et al. (2008), with 1 to 44 sequences available per species. Version 1.4.0 contains 18 new sequences compared to version 1.3.0 used in Nielsen et al. (2022), but no new species. Only ITS2 sequences of strongyle species described in Lichtenfels et al. (2008) were curated following the described steps. Any other ITS2 sequences from the nematode database were left unchanged, even if they are known to infect horses.
Estimating correction factors
Nemabiome sequencing may result in biased species relative proportions due to species-specific variation in the number of cells, copy numbers of the ITS2 gene region and PCR efficiency (Avramenko et al., 2015). To account for this, Avramenko et al. (2015) sequenced mock communities of morphologically identified L3 to estimate correction factors for cattle strongyles. Due to the inability to create mock communities for equine strongyles, Poissant et al. (2021) compared the relative proportions of L3 species identified morphologically (S. vulgaris, S. equinus and S. edentatus, non-Strongylus species) in field samples with the proportion of reads assigned to those species using nemabiome sequencing. However, species-specific biases (estimated as the regression between molecular and morphological relative proportion) reported in Poissant et al. (2021) do not take into consideration that the observed proportion of reads assigned to a given species is a function of both the morphological:molecular bias for that species as well as those of other species present. For example, if bias results in one species being over-represented in a sample, the relative proportion of other species will decrease as a result.
To obtain more accurate estimates of correction factors, subsamples of L3 larvae fixed in 10% formalin were morphologically identified replicating methods in Poissant et al. (2021). Across 57 paired samples, a total of 70 489 larvae were identified, ranging from 102 to 6370 larvae per sample (median = 954). Due to limited distinguishing features among strongyle L3 larvae, morphological identification (according to Russell, 1948) was only possible for certain species including Strongylus vulgaris and S. equinus. Larvae containing 16 or more distinct intestinal cells, exclusive of those identified as S. vulgaris and S. equinus, were considered as S.edentatus, as molecular results indicated low prevalence and abundance of other candidate Strongylin species. Although a few additional species and genera could be identified, (e.g. Gyalocephalus capitatus and Poteriostomum spp.), low prevalence and abundance (both morphological and molecular) precluded accurate estimation of their correction factors. Ultimately, L3 were morphologically classified as either 1 of the 3 Strongylus species or as non-Strongylus.
Molecular species classifications were grouped to parallel morphological classifications. Based on a maximum-likelihood phylogenetic tree, unclassified ASVs (which represented ASVs with less than 80% confidence in species assignment) clustered with non-Strongylus species and were grouped together. Correction factors were estimated for the 3 Strongylus species and the non-Strongylus group simultaneously using the nls function in R using the following formulation:
where molij and morphij are row vectors of molecular and morphological proportions for each taxon i in each sample j, respectively, X is a design matrix (of 0s and 1s) linking each element of morphij to the appropriate element of C, a column vector containing taxon-specific correction factors for each taxa, and ci and morphi are taxon-specific correction factors and row vectors of morphological proportions in each sample for all n taxon, respectively. To allow for estimating multiple correction factors simultaneously, the correction factor for non-Strongylus was set to 1. As such, resulting correction factors for Strongylus species reflect how many reads these species generate per L3 larvae relative to non-Strongylus species. The correction factors for S. edentatus, S. equinus and S. vulgaris relative to non-Strongylus species were estimated to be 1.523, 1.809 and 4.658, respectively, indicating that all Strongylus species yielded more reads per L3 larvae than non-Strongylus species. To correct molecular proportions for Strongylus species, the number of reads assigned to each species were divided by the correction factors prior to calculating relative proportions. Sample FEC were then multiplied with the relative proportion of each parasite species to estimate species-specific FEC. One sample had zero EPG, but larvae were successfully harvested from the coproculture, thus a minimum of one egg was assumed for the FEC (25 EPG).
Statistical analyses
Species diversity and species-specific analyses
Species diversity of an individual's nemabiome was measured across various univariate indices: species richness, Inverse Simpson diversity index and Shannon's diversity index (normalized, ranging from 0 to 1). Diversity indices were calculated using the diversity function in the R package ‘vegan’ 2.6–4 (Oksanen et al., 2022). The Inverse Simpson index places greater weight on species evenness (dominance of certain species), while Shannon's index considers both species richness and evenness.
To test which host and environmental factors were associated with species diversity indices, species-specific prevalence and species-specific FEC, generalized linear models (GLMs) were fitted using glmmTMB in the R package ‘glmmTMB’ v1.1.6 (Brooks et al., 2023). A global model was constructed which included: age (3rd order orthogonal polynomial), sex (2 level factor), median longitude (standardized to a mean of 0 and s.d. = 1, 2nd order orthogonal polynomial), an interaction between linear age and linear longitude, ordinal date (continuous; standardized to a mean of 0 and s.d. = 1) and local horse density (continuous). The model included age2 and age3 to account for possible curvilinear relationships between age and parasite infection, for example, if resistance peaks or dips during certain life-stages (e.g. immunosenescence Froy et al., 2019). Longitude and the interaction between age and longitude, found to explain strongyle FEC in earlier research (Debeffe et al., 2016), were also included in addition to longitude2 to test for possible non-linear spatial patterns. The species richness models used a Poisson probability distribution (family = poisson), while the Inverse Simpson and the Shannon's diversity index models used a Gaussian probability distribution (family = gaussian). Prevalence was modeled using binomial models (family = binomial), while species-specific FECs were cube-root transformed and modeled using zero-inflated GLMs using the linear parameterization of the tweedie probability distribution (ziformula = ~1, family = tweedie) to account for overdispersion common in parasite abundance measures (Wilson et al., 1996). Species-specific models were limited to parasite species with at least 10 positive samples, resulting in a total of 19 species for which prevalence and FEC were modelled.
A multi-model inference approach was used for model selection (Burnham and Anderson, 2002). Starting with the global model described above, all possible combinations of fixed effects were constructed and ranked by AICc, and conditional model averaging with models with ΔAICc <2 was implemented using the R package ‘MuMIn’ v1.47.5 (Bartoń, 2023) to calculate parameter estimates. Prevalence and FEC for certain species could not be model-averaged (i.e. global models could not converge if prevalence is too high/low, thus AIC weights could not be calculated). For those cases, the model with the lowest AICc was chosen for interpretation. Finally, a Spearman correlation test was used to test if species-specific prevalence and FEC were associated.
Community analyses
Jaccard and Bray-Curtis dissimilarity matrices were calculated using species-specific FEC to quantify differences in mixed infection composition between samples using the vegdist function from the vegan package (Oksanen et al., 2022) and visualized using a principal coordinate analysis (PCoA). Jaccard dissimilarity only considers species presence–absence, while Bray–Curtis dissimilarity incorporates species presence–absence and their abundance. To test which host and environmental factors were associated with infection species composition, a permutational analysis of variance (PERMANOVA) was conducted on the Jaccard and Bray–Curtis distances using the adonis2 function in the vegan package with 9999 permutations (Oksanen et al., 2022). Parameters in the PERMANOVA included: age (3rd order polynomial), sex (2-level factor), median longitude (2nd order polynomial, standardized to a mean of 0 and s.d. = 1), ordinal date (continuous; standardized to a mean of 0 and s.d. = 1) and local horse density (continuous). Foals (age 0) were identified to have significantly higher variation in species composition relative to other age groups (i.e. greater beta dispersion). As PERMANOVA is unable to distinguish if significant associations are due to variation within-groups or between-groups, a second PERMANOVA was conducted excluding foals to meet the assumption of multivariate homoscedasticity. Given that the no-foal PERMANOVA had near identical results to the full model, the interpretations and visualizations are based on the full model. To simplify visualization of the PCoA, individuals were grouped into age classes: juveniles (age 0–1), subadults (age 2–3), adults (age >3). To confirm that visualizations and the PERMANOVA had consistent interpretations regardless of how age was classified, 2 additional PERMANOVAs were constructed in which age (as a continuous variable) was replaced with (1) age class (3 levels) or (2) age as a categorical variable (in years, 10 levels). Given that all models were quantitatively and qualitatively similar, only the PERMANOVA with age as a continuous variable is presented.
To test for the effects of reproductive and social status on strongyle community composition, PERMANOVAs were conducted on an adult-only (age >3) subsets of the data, with social status (males: bachelor or band stallion) or reproductive status (females: with foal or without a foal) replacing sex. Subsequently, sex-specific adult-only PERMANOVAs were conducted to further investigate differences (Supplementary Materials). Finally, a univariate PERMANOVA was conducted to estimate how much variation in parasite communities was attributed to social group.
Results
Sequencing outputs, quality filtering and taxonomic assignment
Sequencing resulted in 5838–84 704 read pairs per sample (35 990 ± 9742 [ ± 1 s.d.]). Following filtering and processing, between 3264 and 33 673 amplicons remained per sample (15 657 ± 4376 [
± 1 s.d.]). Following filtering and processing, between 3264 and 33 673 amplicons remained per sample (15 657 ± 4376 [ ± 1 s.d.]). The proportions of reads with taxonomy assigned were high, with most samples having less than 2% unclassified reads and only 6 samples having more than 10% (maximum: 15.4%). The majority of unclassified ASVs were assigned to Cylicostephanus calicatus and Cylicocyclus ashworthi at a confidence level below the 80% threshold.
± 1 s.d.]). The proportions of reads with taxonomy assigned were high, with most samples having less than 2% unclassified reads and only 6 samples having more than 10% (maximum: 15.4%). The majority of unclassified ASVs were assigned to Cylicostephanus calicatus and Cylicocyclus ashworthi at a confidence level below the 80% threshold.
Alpha diversity
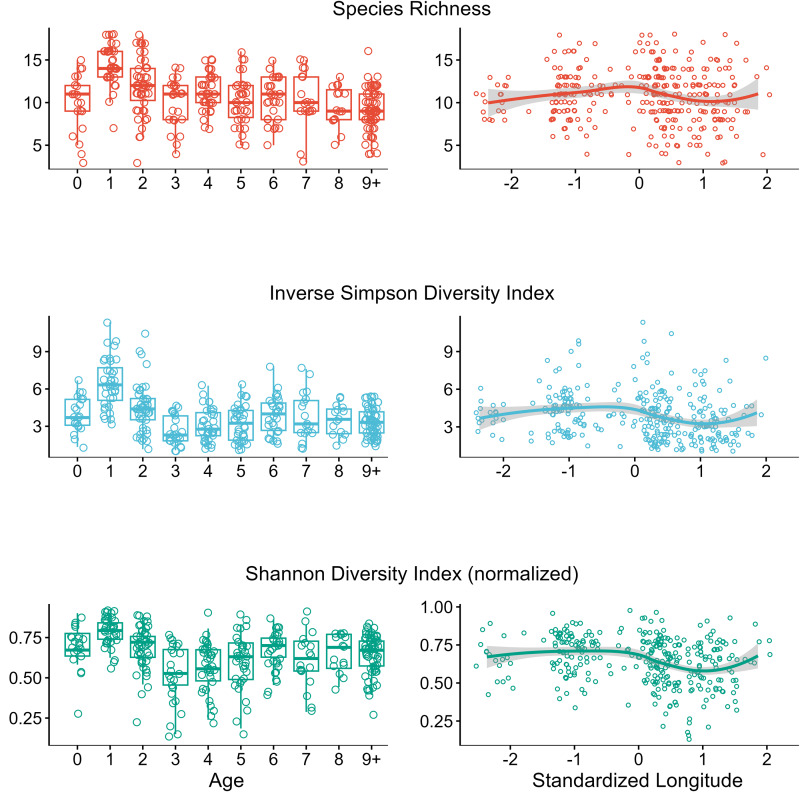
A total of 25 strongyle species were identified, with individual samples containing between 3 and 18 species with a mean richness (± 1 s.d.) of 10.8 ± 3.1. Species richness was highest in yearlings (14.7 ± 2.6), and significantly decreased with age (P < 0.01, Table 1) with the oldest individuals (age 9+) having the lowest richness (9.31 ± 2.5). Similarly, the Inverse Simpson and Shannon indexes decreased with horse age (P < 0.01, Table 1, Fig. 2). However, positive curvilinear age associations (age2, P < 0.01, Table 1) for both indexes suggest that the evenness of strongyle communities are higher in younger (i.e. 1–3 years old) and older adults (i.e. 8–9 years old) (Fig. 2). Furthermore, both Inverse Simpson and Shannon indexes decreased west to east, suggesting that horses in the east have lower diversity and evenness of strongyle parasite communities (P < 0.01, Table 1).
Table 1.
Model averaging results (conditional) for alpha diversity measures of strongyle parasites communities of Sable Island horses (n = 320) in response to host and environmental factors
| Species richness | Inverse simpson diversity index | Shannon diversity index | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | Adjusted SE | z | P | Estimate | Adjusted SE | z | P | Estimate | Adjusted SE | z | P | |
| Intercept | 2.37 | 0.018 | 132.46 | 2 × 10−16 | 4.21 | 0.68 | 6.21 | 2 × 10−16 | 0.72 | 0.066 | 10.92 | 2 × 10−16 |
| Age | −1.89 | 0.31 | 6.04 | 2 × 10−16 | −10.95 | 1.69 | 6.47 | 2 × 10−16 | −0.51 | 0.13 | 3.81 | 0.00014 |
| Age2 | −0.12 | 0.31 | 0.40 | 0.69 | 5.92 | 1.68 | 3.53 | 0.0042 | 0.65 | 0.13 | 4.81 | 1.5 × 10−6 |
| Age3 | 0.45 | 0.31 | 1.56 | 0.12 | – | – | – | – | 0.15 | 0.13 | 1.15 | 0.25 |
| Longitude | −0.50 | 0.31 | 1.61 | 0.11 | −6.90 | 1.77 | 3.89 | 0.00010 | −0.77 | 0.14 | 5.37 | 1.0 × 10−7 |
| Longitude2 | −0.58 | 0.32 | 1.84 | 0.066 | −3.21 | 1.73 | 1.86 | 0.063 | −0.15 | 0.15 | 1.75 | 0.33 |
| Age:longitude | −4.27 | 5.47 | 0.78 | 0.44 | −8.94 | 29.30 | 0.31 | 0.76 | 1.23 | 2.32 | 0.53 | 0.60 |
| Sex | 0.020 | 0.035 | 0.59 | 0.55 | 0.21 | 0.19 | 1.13 | 0.26 | 0.010 | 0.015 | 0.68 | 0.50 |
| Local horse density | – | – | – | – | −0.035 | 0.026 | 1.33 | 0.18 | −0.0034 | 0.0019 | 1.75 | 0.080 |
| Ordinal date | −0.020 | 0.17 | 1.14 | 0.26 | −0.12 | 0.095 | 1.26 | 0.20 | – | – | – | – |
Models were considered for averaging if they had an ΔAICc <2. Dashes indicate fixed effects that were not present in any top model. Bolded rows indicate significant (P < 0.05) factors.
Figure 2.
Strongyle parasite species richness, Inverse Simpson diversity index and Shannon diversity index for 320 horses on Sable Island, Nova Scotia, Canada, across host age and standardized longitude. Each point indicates an individual sample. Boxplots on age plots show mean values for each age (in years). Lines in longitude plots describing the conditional means with a 95% confidence interval.
Species-specific prevalence
The prevalence of the 25 species of strongyles detected in Sable Island horses varied greatly (Table 2) with declines across horse age being the most common patterns followed by declines from west to east (Figs 3 & 4). At the population level, the 3 Strongylus species had the highest prevalence with S. edentatus found in 96.6% of the population, followed by S. equinus (95.0%) and S. vulgaris (89.1%). Non-migratory large strongyles had lower prevalence comparatively, with Triopdontophorus brevicauda, T. serratus and Craterostomum acuticaudatum found in 19.7%, 14.4% and 1.4% of samples, respectively. Seven small strongyle species had population-wide prevalence greater than 50%, with Cyathostomum catinatum (88.1%), Cylicostephanus longibursatus (87.8%) and Cylicocyclus nassatus (78.4%) being the most common. Other species were less common, such as Cylicocyclus insigne (37.5%) and Cylicocyclus elongatus (15.9%). Five small strongyle species had a population prevalence of less than 3% (n < 9/320) (e.g. Poteriostomum imparidentatum, Cyathostomum pateratum), with Cylicostephanus bidentatus only found in a single sample.
Table 2.
Population-wide prevalence and mean fecal egg counts (FEC) for 25 strongyle species identified using DNA metabarcoding in 320 horses sampled on Sable Island, Nova Scotia, Canada, in 2014
| Species | Population prevalence (%) | Mean FEC (EPG ± 1 s.d.) | Prevalence rank in domestic horsesa (Bellaw and Nielsen, 2020) |
|---|---|---|---|
| Strongylus edentatus | 96.56 | 311.75 ± 290.71 | – |
| Strongylus equinus | 95.00 | 545.77 ± 725.82 | – |
| Strongylus vulgaris | 89.06 | 41.11 ± 62.65 | – |
| Cyathostomum catinatum | 88.13 | 91.33 ± 151.89 | 1 |
| Cylicostephanus longibursatus | 87.81 | 120.11 ± 166.86 | 3 |
| Cylicocyclus nassatus | 78.44 | 117.81 ± 226.22 | 2 |
| Coronocyclus coronatus | 77.19 | 55.91 ± 94.75 | 5 |
| Cylicostephanus calicatus | 71.25 | 11.94 ± 16.98 | 6 |
| Cylicostephanus minutus | 68.13 | 70.23 ± 123.24 | 8 |
| Cylicocyclus leptostomum | 67.81 | 45.44 ± 82.62 | 7 |
| Cylicocyclus ashworthi | 49.38 | 12.82 ± 28.79 | 10 |
| Cylicocyclus insigne | 37.50 | 16.89 ± 41.87 | 9 |
| Cylicostephanus goldi | 33.75 | 4.88 ± 12.42 | 4 |
| Coronocyclus labratus | 31.56 | 8.18 ± 25.79 | 12 |
| Coronocyclus labiatus | 28.75 | 4.51 ± 12.98 | 13 |
| Petrovinema poculatum | 21.88 | 1.69 ± 5.54 | 15 |
| Triodontophorus brevicauda | 19.69 | 16.43 ± 56.15 | – |
| Cylicocyclus elongatus | 15.94 | 12.49 ± 50.67 | 17 |
| Triodontophorus serratus | 14.38 | 3.45 ± 11.70 | 16 |
| Poteriostomum imparidentatum | 2.50 | 0.10 ± 0.91 | 14 |
| Craterostomum acuticaudatum | 2.19 | 0.09 ± 0.71 | – |
| Cyathostomum pateratum | 1.56 | 0.05 ± 0.48 | 11 |
| Cylicodontophorus bicoronatus | 1.56 | 0.04 ± 0.36 | 18 |
| Gyalocephalus capitatus | <1.94 | 0.01 ± 0.15 | – |
| Cylicostephanus bidentatus | <1.31 | 0.01 ± 0.13 | – |
Figure 3.
Prevalence of 25 strongyle species identified by DNA metabarcoding across host age for 320 Sable Island horses in 2014. Lines show the conditional means with a 95% confidence interval. Each point indicates the mean prevalence for each age with error bars estimated as 95% bootstrap confidence intervals.
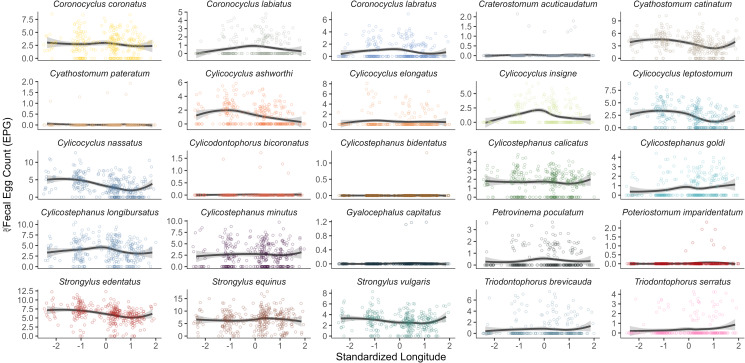
Figure 4.
Prevalence of 25 strongyle species identified by DNA metabarcoding across a west to east gradient on Sable Island (standardized to a mean of 0, s.d. of 1) for 320 Sable Island horses in 2014. Each point indicates an individual sample, with lines describing the conditional means with a 95% confidence interval.
The relationship between prevalence and host age differed among strongyle species (Fig. 3). The prevalence of the Strongylus species was lowest in foals and stayed relatively stable following an increase from age 0 to 3 (Fig. 3), with S. equinus and S. vulgaris being the only species that significantly increased in prevalence across ontogeny (P < 0.05, Table 3). An increase in prevalence across age was also noted for Cylicostephanus goldi. Otherwise, the prevalence of most species (12/19 tested) declined as horse age increased (P < 0.05, Table 3; Fig. 3). Prevalence appeared to peak at certain ages for some species (negative coefficient for significant age2 associations), such as Cylicocyclus insigne and Petrovinema poculatum prevalences peaking at age 4 (61.7% and 46.8%, respectively; Fig. 2). In contrast, Triodontophotus brevicauida, T. serratus and Cylicocyclus elongatus had the opposite curvilinear trend with significant dips in prevalence in young adults following by an increase in older individuals. Furthermore, significant age3 associations were observed for 9 species, suggesting stabilization of prevalence in adults following a peak (e.g. Cylicocyclus nassatus) or dip (e.g. Coronocyclus coronatus) (P < 0.05, Table 3; Fig. 3).
Table 3.
Model averaging results (conditional) for specie-specific prevalence of 19 strongyle parasite species found in Sable Island horses (n = 320) in response to host and environmental factors
| Species | Intercept | Age | Age2 | Age3 | Longitude | Longitude2 | Age:Longitude | Sex (M) | Local horse density | Ordinal date | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Est. | P | Est. | P | Est. | P | Est. | P | Est. | P | Est. | P | Est. | P | Est. | P | Est. | P | Est. | P | |
| Strongylus edentatusa | 5.89 | <0.01 | 21.20 | 0.11 | −33.42 | <0.01 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Strongylus equinusa | 35.31 | 0.03 | 610.36 | 0.04 | 172.33 | 0.06 | – | – | 8.20 | 0.12 | – | – | – | – | – | – | – | – | – | – |
| Cyathostomum catinatum | 1.54 | 0.23 | 10.83 | <0.01 | −1.79 | 0.64 | 9.16 | <0.01 | – | – | 3.50 | 0.39 | 0.69 | 0.08 | – | – | 0.07 | 0.25 | −0.13 | 0.53 |
| Strongylus vulgaris | 2.33 | <0.01 | 6.71 | 0.08 | 3.88 | 0.29 | −7.67 | 0.04 | −12.54 | <0.01 | – | – | −0.24 | 0.51 | −76.07 | 0.34 | – | – | 0.39 | 0.035 |
| Cylicostephanus longibursatus | 2.33 | <0.01 | −12.23 | <0.01 | −1.73 | 0.60 | 5.06 | 0.12 | −4.92 | 0.15 | 1.04 | 0.75 | 0.15 | 0.68 | 35.13 | 0.59 | −0.03 | 0.43 | 0.096 | 0.59 |
| Cylicocyclus nassatus | 1.99 | 0.04 | – | – | 4.20 | 0.15 | −8.63 | <0.01 | 21.26 | <0.01 | 6.62 | 0.16 | −0.12 | 0.68 | – | – | – | – | – | – |
| Coronocyclus coronatus | 2.75 | 0.08 | −11.41 | <0.01 | −4.95 | 0.04 | 7.85 | <0.01 | −6.67 | <0.05 | 6.42 | 0.13 | 0.13 | 0.67 | −53.31 | 0.26 | −0.07 | 0.12 | −0.22 | 0.14 |
| Cylicostephanus calicatus | 3.82 | <0.01 | −8.20 | <0.01 | −2.52 | 0.28 | 2.45 | 0.29 | −1.43 | 0.62 | 8.56 | 0.02 | 0.20 | 0.45 | −101.58 | 0.02 | −0.10 | 0.02 | −0.20 | 0.14 |
| Cylicocyclus leptostomum | 0.87 | 0.01 | −9.47 | <0.01 | – | – | −0.85 | 0.72 | −9.34 | <0.01 | −4.24 | 0.07 | 0.10 | 0.71 | 63.45 | 0.17 | −0.01 | 0.78 | −0.13 | 0.31 |
| Cylicostephanus minutus | 1.01 | 0.03 | −13.74 | <0.01 | 1.45 | 0.55 | – | – | 1.78 | 0.44 | −2.66 | 0.24 | −0.28 | 0.29 | – | – | −0.03 | 0.33 | −0.14 | 0.28 |
| Cylicocyclus ashworthi | 0.06 | 0.92 | −4.52 | 0.05 | – | – | −2.12 | 0.33 | −12.02 | <0.01 | −5.16 | 0.04 | −0.48 | 0.05 | 70.33 | 0.09 | 0.03 | 0.34 | −0.17 | 0.18 |
| Cylicocyclus insigne | −1.05 | 0.21 | −2.17 | 0.36 | −8.19 | <0.01 | −1.64 | 0.46 | −0.88 | 0.73 | −11.24 | <0.01 | 0.37 | 0.13 | – | – | 0.04 | 0.25 | – | – |
| Cylicostephanus goldi | −0.75 | <0.01 | 4.81 | 0.04 | 2.16 | 0.32 | −4.54 | 0.04 | 7.73 | <0.01 | – | – | – | – | 35.93 | 0.38 | – | – | 0.43 | <0.01 |
| Coronocyclus labratus | −2.15 | 0.12 | −22.11 | <0.01 | 2.98 | 0.28 | 8.69 | <0.01 | −5.62 | 0.08 | −8.46 | 0.01 | 0.13 | 0.65 | −76.09 | 0.17 | 0.07 | 0.11 | −0.089 | 0.54 |
| Coronocyclus labiatus | −1.21 | 0.01 | −12.70 | <0.01 | −4.69 | 0.07 | −2.58 | 0.28 | 5.09 | 0.11 | −9.69 | <0.01 | 0.20 | 0.46 | −79.50 | 0.17 | 0.02 | 0.66 | −0.53 | <0.01 |
| Petrovinema poculatum | 0.65 | 0.63 | −7.13 | 0.02 | −5.79 | 0.03 | 4.25 | 0.11 | 2.05 | 4.62 | −1.51 | 0.62 | 0.21 | 0.47 | – | – | −0.08 | 0.07 | −0.082 | 0.56 |
| Triodontophorus brevicauda | −2.26 | <0.01 | −27.66 | <0.01 | 9.87 | 0.03 | 9.86 | 0.01 | 3.55 | 0.25 | – | – | 0.41 | 0.22 | – | – | −0.02 | 0.69 | −0.067 | 0.69 |
| Cylicocyclus elongatus | −2.97 | <0.01 | −34.72 | <0.01 | 13.41 | 0.03 | 11.96 | <0.01 | 2.56 | 0.45 | −2.28 | 0.49 | 0.45 | 0.23 | – | – | −0.02 | 0.67 | −0.37 | 0.052 |
| Triodontophorus serratus | −6.44 | <0.01 | −35.81 | <0.01 | 51.13 | <0.01 | 46.33 | <0.01 | 8.65 | 0.048 | −5.78 | 0.28 | 0.64 | 0.11 | −50.36 | 0.47 | 0.09 | 0.16 | −0.065 | 0.76 |
Models were considered for averaging if they had an ΔAICc <2. Significant fixed effects are bolded (P < 0.05). Dashes indicate fixed effects that were not present in any top model.
Indicates species which could not be model averaged due to difficulty in fitting a global model, for which the top models (ΔAICc = 0) was used for parameter estimates. Dashes indicate fixed effects not present in the top model for these species.
Prevalence was found to vary along the length of the island for certain species (Fig. 4). Five species decreased in prevalence from west to east, such as Cylicocyclus nassatus, which decreased from 90% in the west to 60% in the east (P < 0.01, Table 3, Fig. 4). In contrast, the prevalence of Cylicostephanus goldi and Triodontophorus serratus increased towards the east side of the Island (P < 0.01, Table 3). A few species exhibited curvilinear trends along the length of the island (i.e. significant longitude2 associations), with Cylicocyclus ashworthi, Cylicocyclus insigne, Coronocyclus labratus and Coronocyclus labiatus having significantly higher prevalence near the center (P < 0.05, Table 3). Only Cylicostephanus calicatus was less common in the center of the island (P = 0.02)
Ordinal date was significantly associated with 3 species, with Cyathostomum catinatum and Cylicostephanus goldi becoming more prevalent as the field season progressed, while Coronocyclus labiatus became less prevalent (P = 0.019, Table 1). Cylicostephanus calicatus was the only species to vary (negatively) with density (P < 0.05, Table 3) and age:longitude interactions (P = 0.02). Horse sex was not associated with the prevalence of any species.
Species-specific fecal egg counts
Similar to prevalence patterns, species-specific FEC varied greatly among parasite species (Table 2). Strongylus equinus and S. edentatus had noticeably higher mean FEC (± 1 s.d.) relative to other species with 545.8 ± 725.8 and 311.8 ± 290.7 EPG, respectively. In contrast to the other Strongylus species, S. vulgaris, had a mean of 41.1 ± 62.7 EPG. Among the small strongyles, Cylicostephanus longibursatus, Cylicocyclus nassatus and Cyathostomum catinatum had the highest mean FEC (120.1 ± 166.9, 117.8 ± 226.2 and 91.3 ± 151.9 EPG, respectively). Most species had much lower mean FEC, typically <20 (Table 2). Patterns of species-specific FEC closely paralleled prevalence, with higher FEC associated with higher prevalence (Spearman's rank correlation coefficient = 0.93, P < 0.0001), though, the highly prevalent Cylicostephanus calicatus (prevalence = 71.3%) had relatively low mean FEC (11.9 ± 17.0 EPG).
There was clear variation across age for Strongylus FEC, with foals and adults having the lowest FEC relative to yearlings and subadults (Fig. 5). Species-specific FEC of non-migratory large strongyles exhibited patterns similar to those seen with prevalence (Figs 5 & 6), with yearlings having the highest FEC for the Triodontophorus brevicauda (145.6 ± 143.6 EPG) and T. serratus (26.7 ± 24.5 EPG, age = 1). For 9 species, FEC significantly decreased with age (Table 4), whereas only Strongylus equinus and Cyathostomum catinatum showed an increase. Negative age2 associations were identified for 6 species indicating an increase in FEC, typically peaking between 1 and 3 years old, followed by a decline in individuals >3 years-old (Fig. 5). Conversely, 4 species had significant positive associations with age2, suggesting that younger and older adults had higher FEC of these species (Fig. 5). Finally, most species tested (11/19) had significant positive associations with age3 suggesting that for most species, FEC peaks in younger individuals, followed by stabilization into adulthood (Fig. 5).
Figure 5.
Fecal egg counts (FEC) of 25 strongyle species across host age for 320 Sable Island horses in 2014. Species-specific FEC was calculated by multiplying aggregate strongyle FEC by species-specific relative abundance estimated from DNA metabarcoding. Each point indicates an individual sample, with lines describing the conditional means with a 95% confidence interval. Note that independent y-axis were used per species to better visualize trends.
Figure 6.
Fecal egg counts (FEC) of 25 strongyle species identified by DNA metabarcoding across a west to east gradient on Sable Island (standardized to a mean of 0, s.d. of 1) for 320 Sable Island horses in 2014. Species-specific FEC was calculated by multiplying aggregate strongyle FEC by species-specific relative abundance estimated from DNA metabarcoding. Each point indicates an individual sample, with lines describing the conditional means with a 95% confidence interval. Note that independent y-axis were used per species to better visualize trends.
Table 4.
Model averaging results (conditional) for specie-specific fecal egg counts of 19 strongyle parasite species found in Sable Island horses (n = 320) in response to host and environmental factors. Models were considered for averaging if they had an ΔAICc <2
| Species | Zero-Inflation | Intercept | Age | Age2 | Age3 | Longitude | Longitude2 | Age:Longitude | Sex (M) | Local horse density | Ordinal date | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Est. | P | Est. | P | Est. | P | Est. | P | Est. | P | Est. | P | Est. | P | Est. | P | Est. | P | Est. | P | Est. | P | |
| Strongylus edentatus | −3.37 | <0.01 | 1.83 | <0.01 | – | – | −1.71 | <0.01 | 1.99 | <0.01 | −2.13 | <0.01 | −0.31 | 0.31 | – | – | −0.05 | 0.15 | <0.01 | 0.50 | −0.020 | 0.27 |
| Strongylus equinus | −5.02 | <0.01 | 1.82 | <0.01 | 4.62 | <0.01 | −6.80 | <0.01 | 4.24 | <0.01 | – | – | −0.22 | 0.63 | – | – | −0.08 | 0.13 | – | – | – | – |
| Cyathostomum catinatum | −2.11 | <0.01 | 1.12 | <0.01 | −1.29 | <0.01 | 1.29 | 0.01 | 1.15 | 0.02 | −1.46 | <0.01 | 0.27 | 0.52 | 3.74 | 0.61 | −0.05 | 0.28 | <0.01 | 0.51 | – | – |
| Strongylus vulgaris | −2.09 | <0.01 | 1.50 | <0.01 | 1.05 | 0.04 | −0.32 | 0.52 | −0.16 | 0.74 | −2.41 | <0.01 | −0.27 | 0.57 | 4.38 | 0.60 | −0.16 | < 0.05 | −0.01 | 0.08 | – | – |
| Cylicostephanus longibursatus | −3.27 | <0.01 | 1.57 | <0.01 | −3.06 | <0.01 | −2.39 | <0.01 | 0.48 | 0.49 | −1.81 | 0.02 | −1.29 | 0.09 | −17.17 | 0.18 | −0.10 | 0.19 | −0.01 | 0.24 | −0.055 | 0.15 |
| Cylicocyclus nassatusa | −1.96 | <0.01 | 1.39 | <0.01 | −0.44 | 0.57 | – | – | – | – | −4.47 | <0.01 | – | – | 18.75 | 0.11 | – | – | – | – | – | – |
| Coronocyclus coronatus | −1.38 | <0.01 | 1.21 | <0.01 | −3.51 | <0.01 | −0.68 | 0.32 | 2.59 | <0.01 | −1.05 | 0.09 | −1.01 | 0.08 | – | – | −0.03 | 0.68 | −0.01 | 0.61 | – | – |
| Cylicostephanus calicatus | −0.91 | <0.01 | 0.84 | <0.01 | 0.11 | 0.80 | 0.29 | 0.49 | 0.32 | 0.45 | −0.76 | 0.06 | −0.88 | 0.03 | −11.81 | 0.11 | −0.05 | 0.29 | – | – | – | – |
| Cylicocyclus leptostomum | −0.81 | <0.01 | 1.12 | <0.01 | −2.52 | <0.01 | −2.74 | <0.01 | 0.85 | 0.14 | −4.18 | <0.01 | - | - | −14.42 | 0.23 | −0.10 | 0.11 | <0.01 | 0.88 | −0.033 | 0.33 |
| Cylicostephanus minutus | −0.79 | <0.01 | 1.23 | <0.01 | −5.03 | <0.01 | – | – | 2.12 | <0.01 | −1.17 | 0.03 | – | – | – | – | – | – | – | – | – | – |
| Cylicocyclus ashworthi | – | – | 0.25 | 0.02 | −2.07 | 0.15 | −2.14 | 0.11 | – | – | −7.68 | <0.01 | −3.98 | 0.01 | 24.49 | 0.32 | −0.38 | 0.011 | – | – | −0.14 | 0.051 |
| Cylicocyclus insigne | 0.49 | <0.01 | −0.01 | 0.99 | −1.15 | 0.19 | −1.65 | 0.03 | 1.74 | 0.01 | 1.66 | 0.16 | −5.29 | <0.01 | – | – | −0.05 | 0.51 | 0.03 | 0.01 | −0.050 | 0.19 |
| Cylicostephanus goldia | – | – | 0.77 | <0.01 | −0.55 | 0.37 | −1.22 | 0.04 | 2.25 | <0.01 | – | – | – | – | – | – | – | – | – | – | – | – |
| Coronocyclus labratusa | 0.77 | <0.01 | 1.69 | <0.01 | −1.98 | 0.03 | - | - | 13.64 | 0.02 | −1.92 | 0.02 | – | – | – | – | – | – | −0.03 | <0.01 | −0.11 | <0.01 |
| Coronocyclus labiatusa | −19.39 | 1.00 | −0.79 | <0.01 | −9.67 | <0.01 | −4.69 | 0.02 | – | – | 3.11 | 0.19 | −7.58 | <0.01 | – | – | – | – | – | – | −0.40 | <0.01 |
| Petrovinema poculatuma | −18.62 | 1.00 | −1.10 | <0.01 | −5.24 | 0.06 | −6.84 | 0.01 | 4.30 | 0.09 | – | – | – | – | – | – | – | – | – | – | – | – |
| Triodontophorus brevicauda | −19.28 | 1.00 | −1.36 | <0.01 | −25.18 | <0.01 | 8.10 | 0.03 | 7.97 | 0.01 | – | – | −1.31 | 0.55 | – | – | 0.20 | 0.42 | – | – | −0.16 | 0.19 |
| Cylicocyclus elongatusa | −19.74 | 1.00 | −1.74 | <0.01 | −26.59 | <0.01 | 10.02 | 0.04 | 10.00 | <0.01 | – | – | – | – | – | – | – | – | – | – | – | |
| Triodontophorus serratus | −20.24 | 1.00 | −2.56 | <0.01 | −23.52 | <0.01 | 21.49 | <0.01 | 18.81 | <0.01 | 4.52 | 0.11 | −0.95 | 0.73 | – | – | 0.33 | 0.27 | 0.06 | 0.50 | −0.11 | 0.46 |
Significant fixed effects are bolded (P < 0.05). Dashes indicate fixed effects that were not present in any top model.
Indicates species which could not be model averaged due to difficulty in fitting a global model, for which the top models (ΔAICc = 0) was used for parameter estimates. Dashes indicate fixed effects not present in the top model for these species.
Fecal egg counts decreased from west to east for 9 species (P < 0.05, Table 4, Fig. 6). In contrast, no species had higher FEC in the east (Table 4). Cylicostephanus calicatus, Cylicocyclus ashworthi, Cylicocyclus insigne and Coronocyclus labiatus were more common in the center of the island (longitude2; P < 0.05, Table 4).
Compared to horse age and location, other host and environmental factors were not as commonly associated with FEC across parasite species. Sex differences were only observed for Cyathostomum catinatum and Cylicocyclus ashowrthi with FEC being higher in males than in females (P < 0.05, Table 4). FEC of only 2 strongyle species had significant positive associations with local horse density (Cylicocyclus insigne and Coronocyclus labratus), and only Coronocyclus labratus and Coronocyclus labiatus decreased in FEC as the field season progressed (Table 4). No significant age:longitude interactions were identified.
Community analyses
For the whole population, age explained the greatest proportion of variation in strongyle species composition for both Jaccard (R2 = 0.11, P = 0.001), and Bray–Curtis (R2 = 0.16, P = 0.001) dissimilarities followed by longitude (Jaccard R2 = 0.03, P < 0.01; Bray–Curtis R2 = 0.041, P < 0.01), and sex (Jaccard R2 = 0.0170, P = 0.005; Bray–Curtis R2 = 0.0191, P = 0.002). In contrast, ordinal date and horse density did not explain significant amounts of variation (Table 5). Juveniles (foals and yearlings) and subadults clustered together (age 2–3) appeared to cluster together with age class (Fig. 7), however, adults (age > 3) did not appear to cluster closely (Supplementary Fig. S1).
Table 5.
Results of permutational analysis of variance (PERMANOVA) testing the influence of host and environmental factors across the whole population and adults (age >3) describing the variation in community composition using Jaccard and Bray-Curtis dissimilarity indexes
| Jaccard dissimilarity index | Bray-curtis dissimilarity index | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| d.f. | SS | R2 | F | P | d.f. | SS | R2 | F | P | |
| Population (n = 320) | ||||||||||
| poly(Age, 3) | 3 | 10.08 | 0.11 | 13.00 | 0.001 | 3 | 10.97 | 0.16 | 20.84 | 0.001 |
| poly(Longitude, 2) | 2 | 2.88 | 0.030 | 5.57 | 0.001 | 2 | 2.84 | 0.041 | 8.08 | 0.001 |
| Sex | 1 | 0.67 | 0.007 | 2.58 | 0.0037 | 1 | 0.63 | 0.0091 | 3.60 | 0.0016 |
| Ordinal date | 1 | 0.26 | 0.0028 | 1.02 | 0.40 | 1 | 0.21 | 0.0030 | 1.18 | 0.30 |
| Local horse density | 1 | 0.21 | 0.0022 | 0.82 | 0.65 | 1 | 0.10 | 0.0015 | 0.59 | 0.79 |
| Residual | 311 | 80.39 | 0.85 | 311 | 54.58 | 0.78 | ||||
| Adults (n = 193) | ||||||||||
| poly(Age, 3) | 3 | 2.57 | 0.051 | 3.60 | 0.001 | 3 | 2.31 | 0.068 | 5.00 | 0.001 |
| poly(Longitude, 2) | 2 | 2.03 | 0.040 | 4.27 | 0.001 | 2 | 1.83 | 0.054 | 5.95 | 0.001 |
| Social/Reproductive status | 3 | 1.72 | 0.034 | 2.41 | 0.003 | 3 | 1.46 | 0.043 | 3.15 | 0.004 |
| Ordinal date | 1 | 0.14 | 0.0029 | 0.60 | 0.86 | 1 | 0.084 | 0.0025 | 0.55 | 0.77 |
| Local horse density | 1 | 0.24 | 0.0047 | 1.0012 | 0.40 | 1 | 0.14 | 0.0042 | 0.92 | 0.45 |
| Residual | 182 | 43.28 | 0.86 | 182 | 28.00 | 0.82 | ||||
Significant fixed effects are bolded (P < 0.05).
Figure 7.
Principal coordinate analysis of the (A) Jaccard and (B) Bray-Curtis dissimilarity matrix of parasitic strongyle community composition among 320 Sable Island horses in 2014. Each point represents a strongyle parasite community, with colour indicating horse age class (Juvenile, Subadult, Adult) with ellipses for the 95% confidence interval for each age class. Discrimination between samples of different ages (in years) are available in the Supplementary Materials.
The importance of age and longitude were consistent considering only adults (Table 5), along with social or reproductive status explaining significant, but minimal variation in strongyle community composition (Jaccard R2 = 0.034, P = 0.002; Bray–Curtis R2 = 0.043, P = 0.002). This variation appeared to be driven by females with foals clustering separately from other adults (Supplementary Fig. 2). For adult females models (Supplementary Table S1), age explained the greatest amount of variation in community composition (Jaccard R2 = 0.074, P = 0.001; Bray–Curtis R2 = 0.093, P = 0.0008), followed by longitude (Jaccard R2 = 0.055, P = 0.0016; Bray–Curtis R2 = 0.068, P = 0.0014), and reproductive status (Jaccard R2 = 0.017, P = 0.01; Bray–Curtis R2 = 0.031, P = 0.014). For adult males models (Supplementary Table S11), age explained the most variation (Jaccard R2 = 0.063, P = 0.002; Bray–Curtis R2 = 0.078, P = 0.004), followed by longitude (Jaccard R2 = 0.049, P = 0.001; Bray–Curtis R2 = 0.063, P = 0.001), and local horse density (Jaccard R2 = 0.018 P = 0.020; Bray-Curtis R2 = 0.022, P = 0.023).
Finally, the univariate PERMANOVA only including social band indicated that variation in the composition of strongyle communities were similar within social bands (Jaccard R2 = 0.37, d.f. = 111, dfresidual = 208, P = 0.004; Bray-Curtis R2 = 0.38, d.f. = 111, d.f.residual = 208, P = 0.014).
Discussion
This study applied a non-invasive method to conduct a comprehensive population-wide study of the drivers of variation in strongyle-parasite communities for a free-living, feral horse population with no individual subjected to anthelmintic treatment. Decomposing aggregate strongyle infections to the species level revealed that host and environmental factors can impact infection patterns differentially across parasite species. Consistent with our prediction, host age was broadly negatively associated with the prevalence and FEC of multiple strongyles. In addition, non-linear patterns of prevalence and FEC were common, highlighting that mixed infections vary across host ontogeny. In contrast, horse sex and life history differences (e.g. social status) appeared to have minimal impact on species-specific infection patterns, nor did they greatly influence the species composition of mixed infections. Horse median location on the island was the only environmental factor that was consistently associated with mixed infections, paralleling a previously reported decrease in aggregate FEC along the west to east gradient of the island (Debeffe et al., 2016). Finally, horses in the same social band had similar communities of parasites, emphasizing the possible role of local habitat and space utilization in structuring parasite communities. Taken together, our results reveal that mixed infections are driven by complex associations with host and environmental factors acting uniquely across parasite species.
Our analyses revealed the presence of at least 25 strongyle species infecting Sable Island horses. This is 5 more compared to a previous study that only examined 4 horses (Poissant et al., 2021): 4 cyathostomins (Poteriostomum imparidentatum, Cyathostomum pateratum, Cylicodontophorus bicoronatus, Cylicostephanus bidentatus) and one strongylin (Craterostomum acuticaudatum). As these 5 species have a prevalence of less than 3%, the difference between studies is likely driven by sample coverage. Given that Cylicocyclus bidentatus was only found in a single individual, further studies with greater sample coverage across temporal scales may be warranted to verify presence of rare species in this population. Patterns of cyathostomin species prevalence on Sable Island are similar to those of domestic horses (Bellaw and Nielsen, 2020), with high prevalence of core species like Cyathostomum catinatum, Cylicocyclus nassatus, Cylicostephanus longibursatus, though Cylicostephanus goldi is comparatively less common on Sable Island. As noted in Jenkins et al. (2020), the high prevalence of Strongylus spp. on Sable Island is in contrast to infection patterns of domestic or managed horse populations where these species tend to be rare (Mfitilodze and Hutchinson, 1990; Kuzmina et al., 2016; Sargison et al., 2022; Abbas et al., 2023). One proposed mechanism for the low prevalence of Strongylus spp. in managed populations is a dispersal-fecundity trade-off in which high fecundity species (e.g. Strongylus spp.; Kuzmina et al., 2012) have lower dispersal capacity compared to low-fecundity species (e.g. Cyathostomins) (Sallé et al., 2018). Specifically, high fecundity species may be unable to colonize all hosts due to environmental constraints that limit successful dispersal (Sallé et al., 2018). However, the high prevalence of Strongylus spp. in our study, and other wild horse populations (Harvey et al., 2019; Poissant et al., 2021), suggest that a dispersal-fecundity trade-off may not be applicable for equine strongyles in the wild. This inference is further supported by the high correlation between mean species-specific FEC and prevalence across strongyle species. Instead, the differences in Strongylus spp. prevalence between wild and managed horse populations may be due to anthelmintic use (Saeed et al., 2019; Bellaw and Nielsen, 2020). It may be that variation in parasite life history such as prepatent period, migratory behaviour, or encystment patterns may result in differences in susceptibility to anthelmintics. In contrast, other species like Triodontophorus spp. were predominantly found in yearlings (consistent with clinical observations; Reinemeyer and Nielsen, 2018), suggesting that certain species-specific infection patterns may be governed by the host (i.e. acquired resistance), as opposed to parasite-specific traits.
Horse age was consistently associated with strongyle infection, with 1–3-year-old horses having the highest parasite prevalence and FEC. Linear and non-linear patterns across horse age were varied across parasite species, contributing to an age-structured pattern of beta diversity. The linear decline in prevalence and FEC across ontogeny indicates increasing strongyle resistance as individuals age, consistent with strongyle infection patterns in domestic (Boisseau et al., 2023a) and Sable Island (Debeffe et al., 2016) horses. Furthermore, significant non-linear age patterns of FEC across multiple species may reflect biological processes that vary across host ontogeny. For example, foals may exhibit lower infection burdens because they have limited parasite exposure (not weaned, thus not consuming as much contaminated herbage), or it may be that certain parasite species have yet to mature due to variation in pre-patent periods among strongyle species (Round, 1969). Once exposed, younger horses may be more susceptible to parasite infections if they have not developed resistance or may have energetic trade-offs between growth and resistance (van der Most et al., 2011), resulting in a peak in FEC. As individuals transition to adulthood (age >3), individuals may have had sufficient time to develop resistance, resulting in the decrease in species-specific FEC. Subsequently, adults appear to maintain relatively stable and chronic infections (significant age3 associations) for certain species, which may reflect tolerance or the high cost of maintaining resistance (Colditz, 2008).
The cumulation of the various age trends in species-specific FEC resulted in predictable age-structured shifts of parasite community composition, with 1–2-year-olds having the highest species richness, followed by a decrease in older individuals. This is consistent with classic succession theory in which communities progress to a climax state followed by a decrease in complexity to a stable plateau (Miller and TerHorst, 2012). Succession patterns have been observed in microparasite communities of other long-lived mammals (African buffalo; Syncerus caffer) (Glidden et al., 2023) and could be a consequence of temporally mediated niche differentiation (Dini-Andreote et al., 2014). Similar changes in the composition of parasite communities across host age were also documented in other wild systems such as helminth parasites of Soay Sheep (Craig et al., 2006) and helminth and coccidian parasites of wild zebra and springbok (Turner and Getz, 2010). Such shifts highlight the dynamic nature of mixed infections which can be obscured when using aggregate measures of infections. For instance, Strongylus spp. is likely to disproportionately influence trends in aggregate strongyle FEC in Sable Island horses, masking species-specific patterns of less abundant species. Aggregate FEC would not be able to detect that Cyathostomum catinatum FEC is stable across ontogeny, or that Triodontophorus spp. FEC peak in yearlings. Species-specific patterns across host age may provide critical context to assess the health of animals, for example, while the presence of Triodontophorus spp. is typical in 1–2 year olds, its presence in adults could indicate weakened immunity.
Prevalence and FEC for most parasite species were higher on the western side of the island, broadly consistent with previously reported patterns observed for strongyle FEC in this population (Debeffe et al., 2016; Gold et al., 2019). Proposed explanations for this west to east decline include greater water availability and vegetation density on the western side of the island (Contasti et al., 2012; Rozen-Rechels et al., 2015) which may enhance the viability of parasite larvae in the environment (Debeffe et al., 2016). These patterns may also be explained by the heterogeneous distribution of horses along the island. In particular, overlapping home ranges are known to influence parasite species sharing among wild ungulate species (Stephens et al., 2019). Thus, non-linear prevalence patterns may be driven by horses in the center of the island having more overlapping home ranges with horses from both the east and the west of the island. Host space utilization may also explain certain opposing trends in parasite abundance across the island. There were a handful of species that increased in prevalence towards the east, opposite of the patterns observed in overall strongyle burden (Debeffe et al., 2016), which may be a consequence of variation in host population demography. For example, Triodontophorus serratus is predominantly found in yearlings, and there is a greater density of yearlings in the eastern half (Contasti et al., 2013).
Of all variables considered, social band explained the greatest proportion of among-individual variation in the composition of strongyle communities. For environmentally transmitted parasites such as strongyles, social groups may be exposed to the same parasites since they share the same habitat. Such patterns have been observed in the gastrointestinal parasite communities of meerkats (Suricata suricatta; Leclaire and Faulkner, 2014), and are broadly consistent with expectations that shared habitat utilization (i.e. sympatry) result in similar parasite community between host species (Archie and Ezenwa, 2011; Tombak et al., 2021; Beaumelle et al., 2022). A similar influence of social group was observed for gastrointestinal microbiome communities of Sable Island horses (Stothart et al., 2021). Though bacteria can be directly transmitted between individuals (e.g. via social grooming), this is not the case for strongylid nematodes, and it is likely that the similar strongyle parasite communities is due to grazing in shared habitats that are contaminated by strongyle larvae. Given that the gastrointestinal microbiome of horses can modulate the host response to strongyle infection (Boisseau et al., 2023b), it may also be the case that social bands effects are being modulated by microbiome diversity. Finally, since FEC is heritable in Sable Island horses (Gold et al., 2019), social band effects could also be partly due to the fact that bands often contain relatives (parent-offspring pairs and siblings).
Species-specific differences in seasonal trends have been documented for equine strongyles (Sargison et al., 2022; Abbas et al., 2023), but only 4 species in our study exhibited significant variation across the field season. This, in turn, translated to no significant shifts in the composition of parasite communities across ordinal date for either Jaccard and Bray–Curtis dissimilarities. Our contrasting result likely reflects the small sampling window of this study (July 23–September 5). Additionally, it should be noted that our results are based on samples from a single year. Longitudinal variation in infection can differ by parasite species (e.g. strongyles vs coccidia in Soay Sheep; Hayward et al., 2022) and parasite community composition can change across years (e.g. parasite communities of minnows; Hirtle et al., 2023). Though long-term changes in non-strongylid parasite species of domestic horses are recognized (Tolliver et al., 1987; Sallé et al., 2020), there is limited species-specific information for most strongyle species. Though aggregate strongyle FEC does not appear to vary much over consecutive years for Sable Island horses (Gold et al., 2019), inter-annual variation in species-specific FEC have yet to be tested. Longer-term studies will be necessary to test if the observed stability of strongyle FEC in Sable Island horses conceal long-term species-specific patterns.
Our results indicated limited systematic differences in species-specific prevalence and FEC between the sexes, as in domestic equines (Sallé et al., 2018). Although sex differences in parasite infection may be expected due to physiological differences (e.g. testosterone acting as an immune suppressor (Ezenwa et al., 2012)), differences between sexes in Sable Island horses appears to be limited to variation in life history, with females with foals having different parasite communities from all other adults (Supplementary Fig. S2). Such patterns are consistent with aggregate strongyle FECs of Sable Island horses (Debeffe et al., 2016), and suggest that sex-specific variation in life history may drive parasite community composition. However, the amount of variation explained by reproductive or social status were minimal relative to other factors such as age and location. Similar results were seen for local host density, with significant, but minimal, variation in species composition attributed to density for adult males, paralleling patterns of aggregate FEC seen in Sable Island horses (Debeffe et al., 2016).
While DNA metabarcoding has clear benefits for the study of mixed infections, it also has limitations. For example, index hopping can lead to false positives (Wright and Vetsigian, 2016), and the sequencing of a limited number of larvae may prevent the detection of low abundance species. Index hopping could be mitigated in future studies by using dual index primers (Wright and Vetsigian, 2016). Furthermore, detection of individual species could possibly be improved using eDNA approaches using feces, as metabarcoding using DNA extracted from feces is possibly sensitive enough to identify the presence of parasite species even in the absence of eggs or larvae (Davey et al., 2023).
Another common limitation of DNA metabarcoding studies is the inability to account for species-level variation in gene copy numbers, PCR efficiency and number of cells on abundance estimates (Avramenko et al., 2015; Poissant et al., 2021). To account for this, a novel approach was implemented where correction factors of multiple species were estimated concurrently in a single regression model, as opposed to multiple species-specific regressions. This approach suggested that the relative proportion of Strongylus vulgaris was heavily overestimated when using molecular characterization, with a correction factor of 4.66. The other Strongylus spp. were also overestimated with molecular characterization, but to a lesser extent. The correction factors calculated in this study are higher in magnitude compared to a previous study with less samples (Poissant et al., 2021), but are consistent in relative biases between species. As noted previously (Poissant et al., 2021), the overestimation of Strongylus spp. using molecular methods are likely a reflection of variation in cell number among species since Strongylus spp. larvae have a greater number of cells, especially S. vulgaris which has 1.5–4 times more cells than other species. The inability to identify cyathostomins larvae to species using morphology prevented us from calculating species-specific correction factors for this group in the current study. However, a possible solution to this would be to identify individual larvae using DNA barcoding, and subsequently assessing their relative ITS-2 amplification efficiency using qPCR (Courtot et al., 2023) or by generating mock communities (Avramenko et al., 2015).
This study highlights that the prevalence and egg counts of parasite species respond uniquely across host and environmental factors in feral horses. Variation in mixed strongyle infections was shown to vary across host age, with shifts occurring in younger individuals followed by relative stability in adulthood. Prevalence and FEC of some species also varied across the length of Sable Island, emphasizing the role of host habitat use on parasite infections. Furthermore, species-specific data, as reported here, suggest that aggregate measures of parasite infection can be dominated by species with high fecundity and prevalence, which masks species-specific infection patterns of other species. As different parasite species can result in different pathologies and alter epidemiological patterns, knowledge of variation in species-specific infection among individuals will provide valuable context for quantifying the consequences of parasites in host populations.
Supporting information
Ahn et al. supplementary material
Acknowledgements
The authors would like to recognize and thank the many students, research assistants and volunteers who have contributed to data collection and sample processing for the Sable Island Horse Project. The authors are grateful for the in-kind and logistic support provided by Parks Canada Agency, Fisheries and Oceans Canada (DFO), Canada Coast Guard, the Bedford Institute of Oceanography (DFO Science), Environment and Climate Change Canada, Sable Aviation and Sable Island Station (Meteorological Service of Canada). The authors also thank Dr Emily Jenkins (University of Saskatchewan) for assistance in aquiring funding and laboratory equipment.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182024001185.
Data availability statement
Nemabiome DNA metabarcoding data have been deposited in the NCBI SRA under the BioProject accession code PRJNA1190982. Detailed sample metadata and code will be available from the corresponding authors upon reasonable request.
Author contributions
JP, PDM and JSG secured research funding. JP, PDM, JSG and SA conceived and designed the study. JP and PDM led sample collection. JP, EMR, JB, SG and SA conducted laboratory and bioinformatics analyses. SA led data analyses and writing of the manuscript. All authors contributed to the writing and editing of the final manuscript.
Financial support
Funding for this project was provided by the Natural Science and Engineering Research Council of Canada (PDM, Discovery Grant No. 2022-04584), (JP, Discovery Grant No. 2019-04388), (JSG, Discovery Grant No. 2015-03976); the Canada Foundation for Innovation (PDM, Leaders Opportunity Grant No. 25046); the University of Calgary NSERC-CREATE Host-Parasite Interactions Training Program; and the L. David Dubé and Heather Ryan Veterinary Health and Research Fund. SA was supported by a University of Calgary Eyes High Doctoral Recruitment and NSERC Canada Graduate Doctoral Scholarships.
Competing interests
The authors declare none.
Ethical standards
Sample collection and processing was performed under Parks Canada Agency Research and Collections Permit SINP-2021-38998, University of Saskatchewan Animal Care Protocol 20090032, University of Calgary Animal Care Protocol AC18-0078, and University of Kentucky (USA) Animal Care Protocol 2012-1046.
References
- Abbas G, Ghafar A, Bauquier J, Beasley A, Ling E, Gauci CG, El-Hage C, Wilkes EJA, McConnell E, Carrigan P, Cudmore L, Hurley J, Beveridge I, Nielsen MK, Stevenson MA, Jacobson C, Hughes KJ and Jabbar A (2023) Prevalence and diversity of ascarid and strongylid nematodes in Australian thoroughbred horses using next-generation sequencing and bioinformatic tools. Veterinary Parasitology 323, 110048. doi: 10.1016/j.vetpar.2023.110048 [DOI] [PubMed] [Google Scholar]
- Aivelo T and Medlar A (2018) Opportunities and challenges in metabarcoding approaches for helminth community identification in wild mammals. Parasitology 145, 608–621. doi: 10.1017/S0031182017000610 [DOI] [PubMed] [Google Scholar]
- Archie EA and Ezenwa VO (2011) Population genetic structure and history of a generalist parasite infecting multiple sympatric host species. International Journal for Parasitology 41, 89–98. doi: 10.1016/j.ijpara.2010.07.014 [DOI] [PubMed] [Google Scholar]
- Avramenko RW, Redman EM, Lewis R, Yazwinski TA, Wasmuth JD and Gilleard JS (2015) Exploring the gastrointestinal “nemabiome”: deep amplicon sequencing to quantify the species composition of parasitic nematode communities. PLoS ONE 10, e0143559. doi: 10.1371/journal.pone.0143559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avramenko RW, Bras A, Redman EM, Woodbury MR, Wagner B, Shury T, Liccioli S, Windeyer MC and Gilleard JS (2018) High species diversity of trichostrongyle parasite communities within and between Western Canadian commercial and conservation bison herds revealed by nemabiome metabarcoding. Parasites & Vectors 11, 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoń K (2023) MuMIn: Multi-Model Inference. Available at https://cran.r-project.org/web/packages/MuMIn/inde
- Beaumelle C, Redman E, Verheyden H, Jacquiet P, Bégoc N, Veyssière F, Benabed S, Cargnelutti B, Lourtet B, Poirel MT, de Rijke J, Yannic G, Gilleard JS and Bourgoin G (2022) Generalist nematodes dominate the nemabiome of roe deer in sympatry with sheep at a regional level. International Journal for Parasitology 52, 751–761. doi: 10.1016/j.ijpara.2022.07.005 [DOI] [PubMed] [Google Scholar]
- Bellaw JL and Nielsen MK (2020) Meta-analysis of cyathostomin species-specific prevalence and relative abundance in domestic horses from 1975–2020: emphasis on geopraphical region and specimen collection method. Parasites & Vectors 13, 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson-palme J, Ryberg M, Hartmann M, Branco S, Wang Z, Godhe A, De Wit P, Marisol S, Ebersberger I, De Sousa F, Amend AS, Jumpponen A, Unterseher M, Kristiansson E, Abarenkov K, Bertrand YJK, Sanli K, Eriksson KM, Vik U, Veldre V and Nilsson RH (2013) Improved software detection and extraction of ITS1 and ITS2 from ribosomal ITS sequences of fungi and other eukaryotes for analysis of environmental sequencing data. Methods in Ecology and Evolution 4, 914–919. doi: 10.1111/2041-210X.12073 [DOI] [Google Scholar]
- Bishop SC (2012) A consideration of resistance and tolerance for ruminant nematode infections. Frontiers in Genetics 3, 1–7. doi: 10.3389/fgene.2012.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisseau M, Mach N, Basiaga M, Kuzmina T, Laugier C and Sallé G (2023a) Patterns of variation in equine strongyle community structure across age groups and gut compartments. Parasites and Vectors 16, 1–12. doi: 10.1186/s13071-022-05645-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisseau M, Dhorne-Pollet S, Bars-Cortina D, Courtot É, Serreau D, Annonay G, Lluch J, Gesbert A, Reigner F, Sallé G and Mach N (2023b) Species interactions, stability, and resilience of the gut microbiota – Helminth assemblage in horses. iScience 26, 106044. doi: 10.1016/j.isci.2023.106044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordes F and Morand S (2009) Parasite diversity: an overlooked metric of parasite pressures? Oikos 118, 801–806. doi: 10.1111/j.1600-0706.2008.17169.x [DOI] [Google Scholar]
- Bradbury RS, Sapp SGH, Potters I, Mathison BA, Frean J, Mewara A, Sheorey H, Tamarozzi F, Couturier MR, Chiodini P and Pritt B (2022) Where have all the diagnostic morphological parasitologists gone? Journal of Clinical Microbiology 60, 1–12. doi: 10.1128/jcm.00986-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks M, Bolker B, Kristensen K, Maechler M, Magnusson A, McGillycuddy M, Skaug H, Nielsen A, Berg C, van Bentham K, Sadat N, Lüdecke D, Lenth R, O'Brien J, Geyer CJ, Jagan M, Wiernik B and Stouffer DB (2023) glmmTMB: Generalized Linear Mixed Models using Template Model Builder. Available at https://cloud.r-project.org/web/packages/glmmTMB
- Bucknell DG, Gasser RB and Beveridge I (1995) The prevalence and epidemiology of gastrointestinal parasites of horses in Victoria, Australia. International Journal for Parasitology 25, 711–724. doi: 10.1016/0020-7519(94)00214-9 [DOI] [PubMed] [Google Scholar]
- Burnham KP and Anderson DR (2002) Formal inference from more than one model: Multimodel Inference (MMI). In Model Selection Multimodel Inference. New York, NY: Springer, pp. 149–205. doi: 10.1016/j.ecolmodel.2003.11.004. [DOI] [Google Scholar]
- Callahan BJ, Mcmurdie PJ, Rosen MJ, Han AW, Johnson AJA and Holmes SP (2016) DADA2: high-resolution sample inference from Illumina amplicon data. Nature Methods 13, 581–587. doi: 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colditz IG (2008) Six costs of immunity to gastrointestinal nematode infections. Parasite Immunology 30, 63–70. doi: 10.1111/j.1365-3024.2007.00964.x [DOI] [PubMed] [Google Scholar]
- Contasti AL, Tissier EJ, Johnstone JF and McLoughlin PD (2012) Explaining spatial heterogeneity in population dynamics and genetics from spatial variation in resources for a large herbivore. PLoS ONE 7, e47858. doi: 10.1371/journal.pone.0047858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contasti AL, Van Beest FM, Vander Wal E and McLoughlin PD (2013) Identifying hidden sinks in growing populations from individual fates and movements: the feral horses of Sable Island. Journal of Wildlife Management 77, 1545–1552. doi: 10.1002/jwmg.625 [DOI] [Google Scholar]
- Corning S (2009) Equine cyathostomins: a review of biology, clinical significance and therapy. Parasites and Vectors 2, 1–6. doi: 10.1186/1756-3305-2-S2-S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtot É, Boisseau M, Dhorne-Pollet S, Serreau D, Gesbert A, Reigner F, Basiaga M, Kuzmina T, Lluch J, Annonay G, Kuchly C, Diekmann I, Krücken J, von Samson-Himmelstjerna G, Mach N and Sallé G (2023) Comparison of two molecular barcodes for the study of equine strongylid communities with amplicon sequencing. PeerJ 11, 1–29. doi: 10.7717/peerj.15124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig BH, Pilkington JG and Pemberton JM (2006) Gastrointestinal nematode species burdens and host mortality in a feral sheep population. Parasitology 133, 485–496. doi: 10.1017/S0031182006000618 [DOI] [PubMed] [Google Scholar]
- Davey ML, Utaaker KS and Fossøy F (2021) Characterizing parasitic nematode faunas in faeces and soil using DNA metabarcoding. Parasites and Vectors 14, 1–13. doi: 10.1186/s13071-021-04935-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey ML, Kamenova S, Fossøy F, Solberg EJ, Davidson R, Mysterud A and Rolandsen CM (2023) Faecal metabarcoding provides improved detection and taxonomic resolution for non-invasive monitoring of gastrointestinal nematode parasites in wild moose populations. Parasites and Vectors 16, 1–15. doi: 10.1186/s13071-022-05644-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeffe L, McLoughlin PD, Medill SA, Stewart K, Andres D, Shury T, Wagner B, Jenkins E, Gilleard JS and Poissant J (2016) Negative covariance between parasite load and body condition in a population of feral horses. Parasitology 143, 983–997. doi: 10.1017/S0031182016000408 [DOI] [PubMed] [Google Scholar]
- Dini-Andreote F, De Cássia Pereira E, Silva M, Triadó-Margarit X, Casamayor EO, Van Elsas JD and Salles JF (2014) Dynamics of bacterial community succession in a salt marsh chronosequence: evidences for temporal niche partitioning. ISME Journal 8, 1989–2001. doi: 10.1038/ismej.2014.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson AP and Pacala SW (1988) The relation between the number of parasites/host and host age: population dynamic causes and maximum likelihood estimation. Parasitology 96, 197–210. doi: 10.1017/S0031182000081762 [DOI] [PubMed] [Google Scholar]
- Ezenwa VO, Stefan Ekernas L and Creel S (2012) Unravelling complex associations between testosterone and parasite infection in the wild. Functional Ecology 26, 123–133. doi: 10.1111/j.1365-2435.2011.01919.x [DOI] [Google Scholar]
- Ezenwa VO, Budischak SA, Buss PE, Seguel M, Luikart G, Jolles AE and Sakamoto K (2021) Natural resistance to worms exacerbates bovine tuberculosis severity independently of worm coinfection. Proceedings of the National Academy of Sciences of the United States of America 118, 1–9. doi: 10.1073/pnas.2015080118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiani JV, Lyons ET and Nielsen MK (2016) Dynamics of Parascaris and Strongylus spp. parasites in untreated juvenile horses. Veterinary Parasitology 230, 62–66. doi: 10.1016/j.vetpar.2016.11.002 [DOI] [PubMed] [Google Scholar]
- Frasier TR, Lucas Z, McLeod BA and McLoughlin PD (2016) The horses of Sable Island (Ch. 16). In Freedman B (ed.) Sable Island: Explorations in Ecology & Biodiversity. Markham, ON: Fitzhenry & Whiteside, pp. 271–299. [Google Scholar]
- Froy H, Sparks AM, Watt K, Sinclair R, Bach F, Pilkington JG, Pemberton JM, McNeilly TN and Nussey DH (2019) Senescence in immunity against helminth parasites predicts adult mortality in a wild mammal. Science (New York, N.Y.) 365, 1296–1298. doi: 10.1126/science.aaw5822 [DOI] [PubMed] [Google Scholar]
- Gasser RB, Chilton NB, Hoste H and Beveridge I (1993) Rapid sequencing of rDNA from single worms and eggs of parasitic helminths. Nucleic Acids Research 21, 2525–2526. doi: 10.1093/nar/21.10.2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glidden CK, Karakoç C, Duan C, Jiang Y, Beechler B, Jabbar A and Jolles AE (2023) Distinct life history strategies underpin clear patterns of succession in microparasite communities infecting a wild mammalian host. Molecular Ecology 32, 3733–3746. doi: 10.1111/mec.16949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold S, Regan CE, McLoughlin PD, Gilleard JS, Wilson AJ and Poissant J (2019) Quantitative genetics of gastrointestinal strongyle burden and associated body condition in feral horses. International Journal for Parasitology: Parasites and Wildlife 9, 104–111. doi: 10.1016/j.ijppaw.2019.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AM, Meggiolaro MN, Hall E, Watts ET, Ramp D and Šlapeta J (2019) Wild horse populations in south-east Australia have a high prevalence of Strongylus vulgaris and may act as a reservoir of infection for domestic horses. International Journal for Parasitology: Parasites and Wildlife 8, 156–163. doi: 10.1016/j.ijppaw.2019.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward AD, Behnke JM, Childs DZ, Corripio-Miyar Y, Fenton A, Fraser MD, Kenyon F, McNeilly TN, Pakeman RJ, Pedersen AB, Pemberton JM, Sweeny AR, Wilson K and Pilkington JG (2022) Long-term temporal trends in gastrointestinal parasite infection in wild Soay sheep. Parasitology 149, 1749–1759. doi: 10.1017/S0031182022001263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirtle SV, Ahn S and Goater CP (2023) A congeneric and non-randomly associated pair of larval trematodes dominates the assemblage of co-infecting parasites in fathead minnows (Pimephales promelas). Parasitology 150, 1006–1014. doi: 10.1017/S0031182023000859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoarau AOG, Mavingui P and Lebarbenchon C (2020) Coinfections in wildlife: focus on a neglected aspect of infectious disease epidemiology. PLoS Pathogens 16, 1–5. doi: 10.1371/journal.ppat.1008790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdijk JGM (2008) Influence of periparturient nutritional demand on resistance to parasites in livestock. Parasite Immunology 30, 113–121. doi: 10.1111/j.1365-3024.2008.00992.x [DOI] [PubMed] [Google Scholar]
- Jenkins E, Backwell AL, Bellaw JL, Colpitts J, Liboiron A, McRuer D, Medill S, Parker S, Shury T, Smith M, Tschritter C, Wagner B, Poissant J and McLoughlin P (2020) Not playing by the rules: unusual patterns in the epidemiology of parasites in a natural population of feral horses (Equus caballus) on Sable Island, Canada. International Journal for Parasitology: Parasites and Wildlife 11, 183–190. doi: 10.1016/j.ijppaw.2020.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karvonen A, Jokela J and Laine AL (2019) Importance of sequence and timing in parasite coinfections. Trends in Parasitology 35, 109–118. doi: 10.1016/j.pt.2018.11.007 [DOI] [PubMed] [Google Scholar]
- Katoh K, Misawa K, Kuma KI and Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30, 3059–3066. doi: 10.1093/nar/gkf436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MA, Roohi N and Rana MA (2015) Strongylosis in equines: a review. Journal of Animal and Plant Sciences 25, 1–9. [Google Scholar]
- Kuzmina TA, Lyons ET, Tolliver SC, Dzeverin II and Kharchenko VA (2012) Fecundity of various species of strongylids (Nematoda: Strongylidae)-parasites of domestic horses. Parasitology Research 111, 2265–2271. doi: 10.1007/s00436-012-3077-5 [DOI] [PubMed] [Google Scholar]
- Kuzmina TA, Dzeverin I and Kharchenko VA (2016) Strongylids in domestic horses: influence of horse age, breed and deworming programs on the strongyle parasite community. Veterinary Parasitology 227, 56–63. doi: 10.1016/j.vetpar.2016.07.024 [DOI] [PubMed] [Google Scholar]
- Lass S, Hudson PJ, Thakar J, Saric J, Harvill E, Albert R and Perkins SE (2013) Generating super-shedders: co-infection increases bacterial load and egg production of a gastrointestinal helminth. Journal of the Royal Society Interface 10, 20120588. doi: 10.1098/rsif.2012.0588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclaire S and Faulkner CT (2014) Gastrointestinal parasites in relation to host traits and group factors in wild meerkats Suricata suricatta. Parasitology 141, 925–933. doi: 10.1017/S0031182013002333 [DOI] [PubMed] [Google Scholar]
- Lichtenfels JR, Kharchenko VA and Dvojnos GM (2008) Illustrated identification keys to strongylid parasites (strongylidae: Nematoda) of horses, zebras and asses (Equidae). Veterinary Parasitology 156, 4–161. doi: 10.1016/j.vetpar.2008.04.026 [DOI] [PubMed] [Google Scholar]
- Marjamäki PH, Contasti AL, Coulson TN and Mcloughlin PD (2013) Local density and group size interacts with age and sex to determine direction and rate of social dispersal in a polygynous mammal. Ecology and Evolution 3, 3073–3082. doi: 10.1002/ece3.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet Journal 17, 10–12. [Google Scholar]
- McCraw BM and Slocombe JOD (1976) Strongylus vulgaris in the horse: a review. Canadian Veterinary Journal 17, 150–157. [PMC free article] [PubMed] [Google Scholar]
- McDonnell SM and Murray SC (1995) Bachelor and harem stallion behavior and endocrinology. Biology of Reproduction 52, 577–590. doi: 10.1093/biolreprod/52.monograph_series1.577 [DOI] [Google Scholar]
- Mfitilodze MW and Hutchinson GW (1987) Development and survival of free-living stages of equine strongyles under laboratory conditions. Veterinary Parasitology 23, 121–133. doi: 10.1016/0304-4017(87)90030-6 [DOI] [PubMed] [Google Scholar]
- Mfitilodze MW and Hutchinson GW (1990) Prevalence and abundance of equine strongyles (Nematoda : Strongyloidea) in tropical Australia. Journal of Parasitology 76, 487–494. [PubMed] [Google Scholar]
- Miller TE and TerHorst CP (2012) Testing successional hypotheses of stability, heterogeneity, and diversity in pitcher-plant inquiline communities. Oecologiac 170, 243–251. doi: 10.1007/s00442-012-2292-1 [DOI] [PubMed] [Google Scholar]
- Moss WE, McDevitt-Galles T, Calhoun DM and Johnson PTJ (2020) Tracking the assembly of nested parasite communities: using β-diversity to understand variation in parasite richness and composition over time and scale. Journal of Animal Ecology 89, 1532–1542. doi: 10.1111/1365-2656.13204 [DOI] [PubMed] [Google Scholar]
- Nguyen LT, Schmidt HA, Von Haeseler A and Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution 32, 268–274. doi: 10.1093/molbev/msu300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MK, Steuer AE, Anderson HP, Gavriliuc S, Carpenter AB, Redman EM, Gilleard JS, Reinemeyer CR and Poissant J (2022) Shortened egg reappearance periods of equine cyathostomins following ivermectin or moxidectin treatment: morphological and molecular investigation of efficacy and species composition. International Journal for Parasitology 52, 787–798. doi: 10.1016/j.ijpara.2022.09.003 [DOI] [PubMed] [Google Scholar]
- Okonechnikov K, Golosova O, Fursov M, Varlamov A, Vaskin Y, Efremov I, German Grehov OG, Kandrov D, Rasputin K, Syabro M and Tleukenov T (2012) Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics (Oxford, England) 28, 1166–1167. doi: 10.1093/bioinformatics/bts091 [DOI] [PubMed] [Google Scholar]
- Oksanen J, Simpson GL, Blanchet GF, Kindt R, Legendre P, Minchin PR, O'Hara RB, Solymos P, Stevens MHH, Szoecs E, Wagner H, Barbour M, Bolker B, Borcard D, Carvalho G, Chirico M, De Caceres M, Durand S, Evangelista HBA, FitzJohn R, Friendly M, Furneaux B, Hannigan G, Hill MO, Lahti L, McGlinn D, Ouellette M-H, Cunha ER, Smith T, Stier A, Ter Braak CJF and Weedon J (2022). vegan: an R package for community ecologists. Available at https://cran.r-project.org/web/packages/vegan/
- Pafčo B, Čížková D, Kreisinger J, Hasegawa H, Vallo P, Shutt K, Todd A, Petrželková KJ and Modrý D (2018) Metabarcoding analysis of strongylid nematode diversity in two sympatric primate species. Scientific Reports 8, 5933. doi: 10.1038/s41598-018-24126-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JEH and Ruckstuhl KE (2013) Parasite infection and host group size: a meta-analytical review. Parasitology 140, 803–813. doi: 10.1017/S0031182012002259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poissant J, Gavriliuc S, Bellaw JL, Redman EM, Avramenko RW, Robinson D, Workentine ML, Shury TK, Jenkins EJ, Mcloughlin PD, Nielsen MK and Gilleard JS (2021) A repeatable and quantitative DNA metabarcoding assay to characterize mixed strongyle infections in horses. International Journal for Parasitology 51, 183–192. [DOI] [PubMed] [Google Scholar]
- Pullan RL, Sturrock HJW, Soares Magalhães RJ, Clements ACA and Brooker SJ (2012) Spatial parasite ecology and epidemiology: a review of methods and applications. Parasitology 139, 1870–1887. doi: 10.1017/S0031182012000698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman E, Queiroz C, Bartley DJ, Levy M, Avramenko RW and Stuart J (2019) Veterinary Parasitology Validation of ITS-2 rDNA nemabiome sequencing for ovine gastrointestinal nematodes and its application to a large scale survey of UK sheep farms. Veterinary Parasitology 275, 108933. doi: 10.1016/j.vetpar.2019.108933 [DOI] [PubMed] [Google Scholar]
- Regan CE, Medill SA, Poissant J and McLoughlin PD (2020) Causes and consequences of an unusually male-biased adult sex ratio in an unmanaged feral horse population. Journal of Animal Ecology 89, 2909–2921. doi: 10.1111/1365-2656.13349 [DOI] [PubMed] [Google Scholar]
- Reinemeyer CR and Nielsen MK (2018) Pathology of parasitism and impact on performance. In Handbook of Equine Parasite Control. Hoboken, NJ: John Wiley & Sons, Inc, pp. 25–44. [Google Scholar]
- Roberts F and O'Sullivan P (1950) Methods for egg counts and larval cultures for strongyles infesting the gastro-intestinal tract of cattle. Australian Journal of Agricultural Research 1, 99–103. doi: 10.1071/ar9500099 [DOI] [Google Scholar]
- Round MC (1969) The prepatent period of some horse nematodes determined by experimental infection. Journal of Helminthology 43, 185–192. doi: 10.1017/S0022149X00004016 [DOI] [PubMed] [Google Scholar]
- Rozen-Rechels D, van Beest FM, Richard E, Uzal A, Medill SA and Mcloughlin PD (2015) Density-dependent, central-place foraging in a grazing herbivore: competition and tradeoffs in time allocation near water. Oikos 124, 1142–1150. doi: 10.1111/oik.02207 [DOI] [Google Scholar]
- Rupasinghe D and Ogbourne CP (1978) Laboratory studies on the effect of temperature on the development of the free-living stages of some strongylid nematodes of the horse. Zeitschrift für Parasitenkunde 55, 249–253. doi: 10.1007/BF00390377 [DOI] [Google Scholar]
- Russell AF (1948) The development of helminthiasis in thoroughbred foals. The Journal of Comparative Pathology and Therapeutics 58, 107–127. doi: 10.1016/s0368-1742(48)80009-3 [DOI] [PubMed] [Google Scholar]
- Saeed MA, Beveridge I, Abbas G, Beasley A, Bauquier J, Wilkes E, Jacobson C, Hughes KJ, El-Hage C, O'Handley R, Hurley J, Cudmore L, Carrigan P, Walter L, Tennent-Brown B, Nielsen MK and Jabbar A (2019) Systematic review of gastrointestinal nematodes of horses from Australia. Parasites and Vectors 12, 188. doi: 10.1186/s13071-019-3445-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallé G, Kornaś S and Basiaga M (2018) Equine strongyle communities are constrained by horse sex and species dipersal-fecundity trade-off. Parasites and Vectors 11, 1–11. doi: 10.1186/s13071-018-2858-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallé G, Guillot J, Tapprest J, Foucher N, Sevin C and Laugier C (2020) Compilation of 29 years of postmortem examinations identifies major shifts in equine parasite prevalence from 2000 onwards. International Journal for Parasitology 50, 125–132. doi: 10.1016/j.ijpara.2019.11.004 [DOI] [PubMed] [Google Scholar]
- Santoro M, Mattiucci S, Cipriani P, Bellisario B, Romanelli F, Cimmaruta R and Nascetti G (2014) Parasite communities of icefish (Chionodraco hamatus) in the Ross Sea (Antarctica): influence of the host sex on the helminth infracommunity structure. PLoS ONE 9, 1–7. doi: 10.1371/journal.pone.0088876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargison N, Chambers A, Chaudhry U, Costa Júnior L, Doyle SR, Ehimiyein A, Evans M, Jennings A, Kelly R, Sargison F, Sinclair M and Zahid O (2022) Faecal egg counts and nemabiome metabarcoding highlight the genomic complexity of equine cyathostomin communities and provide insight into their dynamics in a Scottish native pony herd. International Journal for Parasitology 52, 763–774. doi: 10.1016/j.ijpara.2022.08.002 [DOI] [PubMed] [Google Scholar]
- Sinclair R, Melville L, Sargison F, Kenyon F, Nussey D, Watt K and Sargison N (2016) Gastrointestinal nematode species diversity in Soay sheep kept in a natural environment without active parasite control. Veterinary Parasitology 227, 1–7. doi: 10.1016/j.vetpar.2016.07.020 [DOI] [PubMed] [Google Scholar]
- Stephens PR, Altizer S, Ezenwa VO, Gittleman JL, Moan E, Han B, Huang S and Pappalardo P (2019) Parasite sharing in wild ungulates and their predators: effects of phylogeny, range overlap, and trophic links. Journal of Animal Ecology 88, 1017–1028. doi: 10.1111/1365-2656.12987 [DOI] [PubMed] [Google Scholar]
- Steuer AE, Anderson HP, Shepherd T, Clark M, Scare JA, Gravatte HS and Nielsen MK (2022) Parasite dynamics in untreated horses through one calendar year. Parasites and Vectors 15, 1–12. doi: 10.1186/s13071-022-05168-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stothart MR, Greuel RJ, Gavriliuc S, Henry A, Wilson AJ, McLoughlin PD and Poissant J (2021) Bacterial dispersal and drift drive microbiome diversity patterns within a population of feral hindgut fermenters. Molecular Ecology 30, 555–571. doi: 10.1111/mec.15747 [DOI] [PubMed] [Google Scholar]
- Sweeny AR, Corripio-Miyar Y, Bal X, Hayward AD, Pilkington JG, McNeilly TN, Nussey DH and Kenyon F (2022) Longitudinal dynamics of co-infecting gastrointestinal parasites in a wild sheep population. Parasitology 149, 593–604. doi: 10.1017/S0031182021001980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolliver SC, Lyons ET and Drudge JH (1987) Prevalence of internal parasites in horses in critical tests of activity of parasiticides over a 28-year period (1956-1983) in Kentucky. Veterinary Parasitology 23, 273–284. doi: 10.1016/0304-4017(87)90013-6 [DOI] [PubMed] [Google Scholar]
- Tombak KJ, Hansen CB, Kinsella JM, Pansu J, Pringle RM and Rubenstein DI (2021) The gastrointestinal nematodes of plains and Grevy's zebras: phylogenetic relationships and host specificity. International Journal for Parasitology: Parasites and Wildlife 16, 228–235. doi: 10.1016/j.ijppaw.2021.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner WC and Getz WM (2010) Seasonal and demographic factors influencing gastrointestinal parasitism in ungulates of etosha national park. Journal of Wildlife Diseases 46, 1108–1119. doi: 10.7589/0090-3558-46.4.1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Most PJ, De Jong B, Parmentier HK and Verhulst S (2011) Trade-off between growth and immune function: a meta-analysis of selection experiments. Functional Ecology 25, 74–80. doi: 10.1111/j.1365-2435.2010.01800.x [DOI] [Google Scholar]
- Watson MJ (2013) What drives population-level effects of parasites? Meta-analysis meets life-history. International Journal for Parasitology: Parasites and Wildlife 2, 190–196. doi: 10.1016/j.ijppaw.2013.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K, Grenfell BT and Shaw DJ (1996) Analysis of aggregated parasite distributions: a comparison of methods. Functional Ecology 10, 592–601. doi: 10.2307/2390169 [DOI] [Google Scholar]
- Workentine ML, Chen R, Zhu S, Gavriliuc S, Shaw N, de Rijke J, Redman EM, Avramenko RW, Wit J, Poissant J and Gilleard JS (2020) A database for ITS2 sequences from nematodes. BMC Genetics 21, 10–13. doi: 10.1186/s12863-020-00880-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright ES and Vetsigian KH (2016) Quality filtering of Illumina index reads mitigates sample cross-talk. BMC Genomics 17, 1–7. doi: 10.1186/s12864-016-3217-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ahn et al. supplementary material
Data Availability Statement
Nemabiome DNA metabarcoding data have been deposited in the NCBI SRA under the BioProject accession code PRJNA1190982. Detailed sample metadata and code will be available from the corresponding authors upon reasonable request.