Abstract
NCIC-CTG MA.5 and DBCG 89D are symmetrically designed randomized trials comparing adjuvant cyclophosphamide, epirubicin, and fluorouracil with cyclophosphamide, methotrexate, and fluorouracil in high-risk breast cancer patients. In a joint analysis we evaluate the predictive value in terms of anthracycline benefit of molecular subtyping by PAM50. A statistically significant interaction (P = 0.008) between continuous Risk of Recurrence (ROR) score and treatment regimen is evident, translating into a clear distinct treatment effect according to ROR score category with HR 0.51 for ROR score ≥ 72 and HR 1.10 for ROR score < 52 (Pinteraction = 0.004). The analysis provides evidence of the benefit from anthracycline in HER2-enriched subtype; for patients with discordance of HER2 subtype and clinical HER2 status, HER2-enriched subtype was predictive of anthracycline benefit whereas clinical HER2 positive status was not. Anthracycline-based adjuvant chemotherapy may safely be withheld for patients with a low ROR score while the benefit increases with increasing ROR score.
Subject terms: Tumour biomarkers, Predictive markers
Introduction
While the meta-analyses conducted by the Early Breast Cancer Trialists Collaborative Group (EBCTCG)1–3 demonstrated an overall benefit from adjuvant anthracycline chemotherapy, its use has persistently been restricted to breast cancer patients with a sufficiently high clinical risk or specific molecular characteristics4,5. The reluctance to recommend anthracyclines to all patients where chemotherapy is indicated is in part due to their adverse events including risks of heart failure and secondary leukemia. Furthermore, the heterogeneous results of individual trials have to some degree been associated with different patient selection criteria, motivating a search for predictive markers of benefit from anthracyclines in the National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG) MA.5 and the Danish Breast Cancer Group (DBCG) 89D trials6,7. These independent trials had symmetrical designs comparing cyclophosphamide, methotrexate, and fluorouracil (CMF) chemotherapy to cyclophosphamide, epirubicin, and fluorouracil (CEF) in patients with early breast cancer. Trastuzumab was not available when these trials were performed, but TOP2A alterations were retrospectively linked to anthracycline sensitivity8–10. Other attempts to develop biomarkers or combinations have included HER2, CEP17 and TIMP1 but have not led to the adoption of markers in the clinical setting11,12. An individual-patient meta-analysis verified a moderate predictive value of HER2 positivity and TOP2A alterations but also highlighted the importance of subtypes, which are further investigated in the current study13.
The predictive significance of the PAM50-based Prosigna assay for adjuvant chemotherapy with epirubicin and the discrepancies between PAM50 gene expression-based subtyping and immunohistochemical- or ISH-determined HER2 status were investigated in the NCIC-CTG MA.5 and DBCG 89D trials14,15. The results indicated that the effect is primarily driven by the PAM50 subtype, with the discordant groups following the intrinsic subtype, i.e. patients with HER2-negative tumors and a HER2-enriched subtype appeared to benefit from epirubicin, whereas patients with HER2-positive tumors but a non-HER2-enriched subtype received no benefit. Further, the benefit of epirubicin correlated with the Risk Of Recurrence (ROR) score, but without convincing statistical significance. However, these observations in each separate trial were underpowered due to limited numbers and a combined analysis of the trials might provide the largest possible power to give robust estimates.
Results
Patient and tumor characteristics
Individual patient data on PAM50 was retrieved from 1140 (67%) of the eligible patients randomized and Table 1 describes patient and tumor characteristics reflecting that both trials selected high-risk patients. A majority of the patients was premenopausal, with a tumor size above 20 mm, high-grade malignancy and node-positive disease (Table 1). Both trials were performed before trastuzumab was introduced and HER2 status was established retrospectively. The MA.5 trial included a higher proportion of patients with luminal subtypes (ER-positive, HER2-negative), in accordance with the differential inclusion criteria. The treatment effect in this joint analysis was similar to the effect observed in the original study populations of 1696 patients in total; a HR favoring CEF for DRFS (adjusted HR 0.73; 95% CI 0.60 to 0.88) and OS (adjusted HR 0.89; 95% CI 0.74 to 1.06)6,7,14,15.
Table 1.
Patient and tumor characteristics for the study population
| Treatment arm | Total | ||||||
|---|---|---|---|---|---|---|---|
| CMF | CEF | ||||||
| N | Col% | N | Col% | N | Col% | ||
| 595 | 545 | 1140 | |||||
| Age | < 50 | 428 | 72 | 391 | 72 | 819 | 72 |
| ≥ 50 | 167 | 28 | 154 | 28 | 321 | 28 | |
| Menopausal status | Premenopausal | 486 | 82 | 444 | 81 | 929 | 81 |
| Postmenopausal | 109 | 18 | 101 | 19 | 211 | 19 | |
| Tumor size | ≤ 2 cm | 240 | 40 | 199 | 37 | 439 | 39 |
| > 2, ≤ 5 cm | 301 | 51 | 289 | 53 | 590 | 52 | |
| > 5 cm | 42 | 7 | 37 | 7 | 79 | 7 | |
| Unknown | 12 | 2 | 20 | 4 | 32 | 3 | |
| Nodal status | Negative | 121 | 20 | 123 | 23 | 244 | 21 |
| 1-3 positive LN | 262 | 44 | 234 | 43 | 496 | 44 | |
| ≥ 4 positive LN | 212 | 36 | 188 | 35 | 400 | 35 | |
| Malignancy Grade | I | 47 | 8 | 34 | 6 | 81 | 7 |
| II | 244 | 41 | 227 | 42 | 471 | 41 | |
| III | 274 | 46 | 260 | 48 | 534 | 47 | |
| Not graded | 30 | 5 | 24 | 4 | 54 | 5 | |
| ER status | Positive | 221 | 37 | 202 | 37 | 423 | 37 |
| Negative | 247 | 42 | 221 | 41 | 468 | 41 | |
| Unknown | 127 | 21 | 122 | 22 | 249 | 22 | |
| HER2 status | Positive | 162 | 27 | 152 | 28 | 312 | 27 |
| Negative | 433 | 73 | 393 | 72 | 826 | 73 | |
| Trial | MA.5 | 233 | 39 | 221 | 41 | 454 | 40 |
| DBCG 89D | 362 | 61 | 324 | 59 | 686 | 60 | |
ROR score
The association of the continuous ROR score with 10-year DR rate was not proportional across regimens over time and therefore analyzed separately (Fig. 1) for the early (0 to ≤ 2.5 years) and late periods ( > 2.5 to 10 years). Treatment with CEF reduced the risk of recurrence as compared to CMF and the risk reduction increased with an increasing continuous ROR score. Thus, a statistically significant interaction (Pinteraction = 0.001) was observed between increase in ROR score and treatment group (Table 2). This translates into 10 year rates for distant recurrence of 49.0% and 57.6% for patients in the CMF arm having ROR score of 50 and 60, respectively, and a more moderate raise from 44.1% to 46.7% for patients in the CEF arm. The effect of increasing ROR score on the risk of distant recurrence is most pronounced in the first 2.5 years (Table 2; Fig. 1A, B). Further subdividing in patients with HER2-positive and patients with HER2-negative disease revealed a statistically significant interaction between ROR score and treatment regimen (Pinteraction = 0.009, including both early and late periods) and between ROR score and HER2 status (Pinteraction = 0.01 in unadjusted analysis) whereas the interaction between ROR score and treatment regimen is not heterogenous across HER2 status (Pinteraction = 0.46 and Pinteraction = 0.80 for early and late periods, respectively). The analysis of time to recurrence and overall survival as well as adjusted analyses provided similar results (Supplemental Table 1, Supplemental Table 2 and Table 2).
Fig. 1. Distant recurrence rate by continuous ROR score for patients in the CMF regimen and patients in the CEF regimen and according to HER2 status.
HER2N HER2-negative, HER2P HER2-positive. Hazard ratios and corresponding 95% CI for a 10-point difference in continuous ROR score are shown according to follow-up time; A Early (0–≤2.5 years) and B Late periods (>2.5–10 years).
Table 2.
Unadjusted and adjusted* results for ROR score continuous (10-point) on DR according to treatment group, divided according to follow-up time and HER2 status
| All | HER2-negative | HER2-positive | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | (95% CI) | Pinteraction | HR | (95% CI) | HR | (95% CI) | Pinteraction | |
| Unadjusted analysis | ||||||||
| 0.001 | ||||||||
| CMF | 1.27 | 1.19;1.36 | ||||||
| CEF | 1.09 | 1.01;.1.17 | ||||||
| ≤ 2.5 yrs | 0.008 | 0.009 | ||||||
| CMF | 1.43 | 1.31;1.57 | 1.44 | 1.28;1.63 | 1.23 | 1.07;1.41 | ||
| CEF | 1.25 | 1.13;1.38 | 1.31 | 1,16;1.49 | 1.01 | 0.84;1.20 | ||
| > 2.5 yrs | ||||||||
| CMF | 1.08 | 0.97;1.19 | 1.00 | 0.89;1.12 | 1.10 | 0.89;1.36 | ||
| CEF | 0.92 | 0.83;1.02 | 0.87 | 0.78;0.97 | 0.92 | 0.70;1.20 | ||
| Adjusted analysis | ||||||||
| 0.002 | ||||||||
| CMF | 1.16 | 1.08;1.26 | ||||||
| CEF | 1.00 | 0.93;.1.09 | ||||||
| ≤ 2.5 yrs | 0.008 | 0.009 | ||||||
| CMF | 1.27 | 1.15;1.40 | 1.35 | 1.18;1.55 | 1.13 | 0.98;1.30 | ||
| CEF | 1.12 | 1.02;1.24 | 1.25 | 1.10;1.42 | 0.95 | 0.82;1.10 | ||
| > 2.5 yrs | ||||||||
| CMF | 1.02 | 0.92;1.14 | 1.01 | 0.89;1.14 | 1.05 | 0.84;1.31 | ||
| CEF | 0.87 | 0.77;0.97 | 0.86 | 0.76;0.98 | 0.86 | 0.65;1.13 | ||
CMF cyclophosphamide, methotrexate and fluorouracil; CEF cyclophosphamide, epirubicin and fluorouracil; ROR, risk of recurrence; DR, distant recurrence; HR, hazard ratio; 95% CI, 95% confidence interval; Pinteraction, P derived from a Wald test for heterogeneity. *Adjusted for age, tumor size, nodal status, histological type and grade, estrogen receptor status and HER2 status.
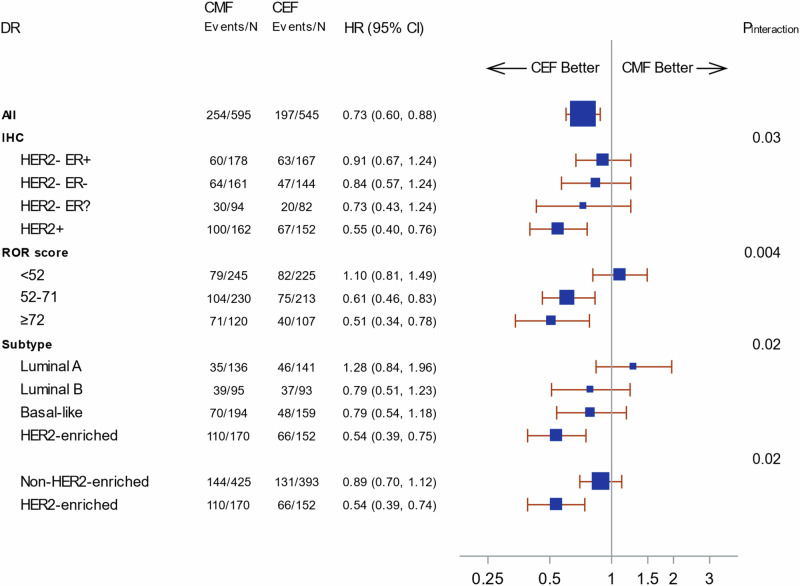
The ROR scores were, according to predefined cutpoints dived into three categories and 470 (41%) were in the low risk, 443 (39%) in the intermediate risk and 227 (20%) in the high-risk group, with a higher ROR score in patients with HER2-positive disease (Table 3). Figure 2 shows the cumulative incidence for distant recurrence according to the ROR score categories for each of the treatment regimens (Fig. 2A CMF and Fig. 2B CEF) and HER2 status. A clear distinction according to ROR score category within HER2-negative and HER2-positive patients is shown for the CMF but not for the CEF group. A statistically significant heterogeneity in treatment effect according to ROR score category is shown in the multivariable analysis (Fig. 3) for DR (Pinteraction = 0.004), and TR (Supplemental Fig. 1, Pinteraction = 0.002), but not for OS (Supplemental Fig. 2, Pinteraction = 0.20) although with a similar trend of better effect with higher ROR score. Univariable results are shown in Supplemental Fig. 3, 4 and 5. A subdivision of the patients according to HER2 status did not show a differential treatment versus ROR score category interaction comparing patients with HER2-negative disease and HER2-positive disease; an increasing treatment effect with increasing ROR score category is present for both HER2-negative and HER2-positive disease, and a better treatment effect across all 3 ROR groups for HER2-positive (data not shown), following the results for ROR continuous score.
Table 3.
Distribution of ROR score and PAM50 subtype according to HER2 status
| Total | HER2-negative | HER2-positive | ||||
|---|---|---|---|---|---|---|
| N | Col % | N | Col % | N | Col % | |
| 1140 | 826 | 314 | ||||
| ROR score | ||||||
| ≤ 51 | 470 | 41 | 404 | 49 | 66 | 21 |
| 52-71 | 443 | 39 | 322 | 39 | 121 | 39 |
| ≥ 72 | 227 | 20 | 100 | 12 | 127 | 40 |
| PAM50 subtype | ||||||
| HER2-enriched | 322 | 28 | 65 | 8 | 257 | 82 |
| Basal-like | 353 | 31 | 338 | 41 | 15 | 5 |
| Luminal B | 188 | 16 | 171 | 21 | 17 | 5 |
| Luminal A | 277 | 24 | 252 | 31 | 25 | 8 |
Fig. 2. Estimates of distant recurrence (DR) rate according to Prosigna ROR ≤51 (Low), 52–71 (IM), and ≥72 (High), and HER2 status.
HER2N HER2-negative, HER2P HER2-positive. 10-year estimates with 95% CI are included. A Patients in the CMF regimen and B Patients in the CEF regimen.
Fig. 3. Forest plot illustrating subdistributional hazard models for distant recurrence (DR) according to immunohistochemistry (IHC) HER2 and ER status, Risk of reccurence (ROR) score and intrinsic subtype.
- negative, + positive, ? unknown. Hazard ratios (HRs) refer to adjusted estimates obtained in multivariable analysis. Boxes represent the weight of data for each subgroup relative to the total data. Pinteraction derived from a Wald test for heterogeneity; for IHC: HER2 negative versus HER2 positive.
PAM50 subtypes
Among 826 patients with HER2-negative disease, 65 (8%) have a HER2-enriched PAM50 subtype, and among 314 patients with HER2-positive disease, 57 (18%) have a non-HER2-enriched subtype (Table 3).
For each of the four PAM50 subtypes, the DR rate over time is shown in Supplemental Fig. 6 according to treatment regimen. Patients with a HER2-enriched subtype had an improved outcome if treated with CEF as compared to CMF (Fig. 3, Supplemental Figs. 1 and 2), with a statistically significant interaction in treatment effect across the four subtypes (Pinteraction = 0.02, Pinteraction = 0.01 and Pinteraction = 0.03, for DR, TR and OS respectively), and with similar results for the HER2-enriched subtype compared with the non-HER2-enriched (Luminal A, Luminal B and Basal-like) and the HER2 status.
Among patients with a non-HER2-enriched subtype, the treatment effect does not differ significantly according to HER2 status. For patients with a HER2-enriched subtype, there was a clear differential outcome for the two treatment regimens, irrespective of HER2 status, with fewer events in the anthracycline (CEF) arm. The univariable analysis is presented in Supplemental Fig. 7 where the distinct pattern between HER2-enriched and non-HER2-enriched is evident.
Discussion
In breast cancer adjuvant chemotherapy regimens, benefit from exchanging methotrexate (in CMF) with an anthracycline (epirubicin, in CEF) has previously been demonstrated in the NCIC-CTG MA.5 and the DBCG 89D trials separately and is mirrored in this joint analysis of these two trials6,7. Furthermore, the risk of distant recurrence increased significantly with increasing ROR score. When broken down into predefined categories a differential benefit from anthracyclines was shown according to ROR score and intrinsic subtype.
The result of this MA.5 and DBCG 89D joint analysis highlights that breast cancer patients clinically classified as high-risk may not derive benefit from adjuvant anthracycline-based polychemotherapy if, by PAM50, they have a Luminal A subtype or a low ROR score. Notably, more than 80% of the patients included in this joint analysis were premenopausal, and almost 80% were node-positive (with 35% having ≥ 4 positive nodes). Further, the results from the DBCG 77B trial showing lack of benefit from CMF-based chemotherapy in high-risk predominantly node-positive patients whose breast tumors have a low ROR score or Luminal A subtype16, might further support sparing these patients chemotherapy16. Furthermore, we also previously validated the prognostic clinical utility of PAM50 in patients with zero to three positive nodes; a low ROR score or a Luminal A subtype in patients who, without chemotherapy, were allocated to 5 years of adjuvant endocrine therapy was associated with a low risk of recurrence, as has also been demonstrated in a joint analysis of the ATAC and ABCSG-8 trials17,18.
Treatment with anthracycline-containing adjuvant chemotherapy led to fewer recurrences in patients with an intermediate or high ROR score, although only those with a high ROR score obtained a significant survival benefit. While patients with a HER2-enriched subtype achieved a highly significant benefit from being assigned to CEF, this study cannot fully address to what extent those with a Basal-like or a Luminal B subtype benefitted. This study has some potential limitations. First, the two trials precede the era of HER2-directed therapies, and in the absence of trastuzumab a greater benefit from anthracycline-based adjuvant chemotherapy was observed in patients with HER2-positive than HER2-negative breast cancer. Second, results on the Prosigna assay were only available from 454 of the 716 participants in MA.5 and from 686 of the 980 Danish participants in 89D. Third, the subgroup analysis in our study was not adequately powered, and so moderate effects may not have been identified. Fourth, although the use of older mature clinical trial data has the advantage of providing long-term follow-up with many events, this comes with the limitation that extrapolation into the context of contemporary treatment practices (e.g. cdk4/6 inhibitors, immunotherapy) becomes more challenging. There was a considerable overlap between having a HER2-enriched subtype and being HER2-positive; however, in those without concurrence a HER2-enriched gene expression subtype was predictive of benefit from anthracyclines whereas clinically HER2-positive disease was not. Anthracycline-containing chemotherapy therefore could be considered in patients with a PAM50 HER2-enriched tumor irrespective of HER2 status.
Responsiveness to anthracyclines has not been evaluated by other gene expression assays in comparable randomized trials or similar high-risk populations19. Although many patients who require adjuvant chemotherapy may avoid anthracyclines, no clear subsets based on clinical factors have been identified. While advancements in other therapies may have reduced the utility of anthracyclines, gene expression assays have proved useful to guide treatment with anthracyclines and it is still debated how this should affect treatment decisions. The evidence of this study suggest that anthracyclines improve outcomes especially for patients with high genomic risk. In conclusion, this joint analysis provides evidence that with increasing ROR score the benefit of including an anthracycline increases while patients with a low ROR score or a Luminal A subtype breast cancer do not obtain any incremental benefit and may be spared anthracyclines.
Methods
Study population
The NCIC.CTG MA.5 trial and the DBCG 89D trial were included in this pooled analysis. Both are open-labeled randomized phase 3 trials comparing CMF (cyclophosphamide, methotrexate, 5-fluorouracil) with an anthracycline-based regimen; CEF including epirubicin. Inclusion criteria, patient characteristics, trial results and several biomarker studies have been published6–15. In brief, the MA.5 trial included premenopausal women with node-positive breast cancer, and in DBCG 89D women with invasive early-stage breast cancer were eligible if they were (I) premenopausal with node-negative and grade II-III tumors, (II) premenopausal with node-positive and hormone receptor-negative tumors, or (III) postmenopausal with node-positive and hormone receptor-negative tumors. The trials were conducted in accordance with the Helsinki Declaration and approved by ethical committees with jurisdiction for the participating institutions6,7. Informed consent was obtained before randomisation following oral and written information. Meta-analyses do not require submission to an ethics committee/IRB review.
Assay and HER2 status
The Predictor Analysis of Microarray 50 (PAM50) gene expression algorithm characterizes invasive breast cancers by assigning a risk of recurrence (ROR) score and an intrinsic subtype20. Assessment of the intrinsic subtypes, the ROR score and the IHC/ISH-based HER2 status have been described in detail previously8,9,13–15.
Study design
The study is a prospective-retrospective design following the REMARK criteria21. An analysis plan for the combined analysis was prespecified and agreed by the trial groups. Individual patient data previously published were used for this combined analysis14,15.
Endpoints
The primary endpoint is distant recurrence-free survival (DRFS). This is defined as the interval from randomization until distant recurrence or death due to breast cancer. Contralateral breast cancer, other secondary cancer and death due to causes other than breast cancer were treated as competing events. Analyses was performed restricted to 10-year follow-up data. Secondary endpoints are overall survival, defined as the interval from randomization until death from any cause, and time to recurrence.
Statistical methods
Patient-level data were collected and analyzed at the DBCG statistical office. The PAM50 subtype, the PAM50 subtype in combination with clinical HER2 status and the ROR score both continuous and categorical were analyzed in separate models. Kaplan-Meier estimates were calculated for OS and estimates of cumulative incidence for recurrence. The prognostic and predictive values were analyzed using univariable and multivariable models. Competing-risk analysis using Fine and Gray’s proportional sub-distribution hazards model to evaluate the recurrence endpoints and the Cox proportional hazards regression modelling to evaluate the endpoint of OS were applied, all stratified for trial. The multivariable models included age ( ≤ 50, > 50), tumor size ( ≤ 20 mm, > 20 mm; ≤ 50 mm or unknown, > 50 mm), nodal status (ln(number of positive nodes)), histological type and grade (I,II and not graded, III), ER status (ER negative, ER-positive/unknown) HER2 status (negative, positive), treatment regimen (CMF, CEF) and the specific marker. PAM50 molecular subtype was included as four categories (Luminal A, Luminal B, Basal-like, HER2-enriched) and as two categories (HER2-enriched, non-HER2-enriched), the latter alone and in combination with clinical HER2 status. The ROR score was included as a continuous measure (10-point change) and in categorical risk groups ( ≤ 51, 52-71, ≥ 72), based on previous work15,16. Proportional hazards assumptions were assessed using Schoenfeld residuals and by including a time-dependent component for each covariate. Time-dependent components for time ≥ 5 years were included for ER, grade, ROR score and subtype, and at 2.5 years for ROR score for recurrence endpoints, to fulfill the model assumption. The Wald test was used to assess heterogeneity in treatment effect, and applied for ROR continuous score, ROR score categories, ROR score combined with HER2 status, PAM50 four subtypes, PAM50 HER2-enriched vs PAM50 Non-HER2-enriched, and the latter combined with clinical HER2-negative vs HER2-positive. P-values are 2-tailed, unadjusted for the number of comparisons. Statistical analyses used the SAS Enterprise Guide v8.3 software program package (SAS Institute, Cary, NC).
Supplementary information
Acknowledgements
Not applicable.
Author contributions
Conception and design: M.J., T.O.N., B.E. Provision of study material or patients: A.V.L., T.O.N., J.B., L.S., B.E. Collection and assembly of data: M.J. Data analysis and interpretation: M.J., T.O.N., J.B., B.E. Manuscript writing: All authors. Final approval of manuscript: All authors. Accountable for all aspects of the work: All authors.
Data availability
Clinical data for the patients included in this study are not publicly available to protect patient privacy. Access to data used in this study can be made available to qualified researchers through application to the respective trial groups.
Competing interests
The authors declare no competing non-financial interests but the following competing financial interests: Advisory board, Novartis (MJ). Grants from Canadian Cancer Society during the conduct of the study as well as personal fees from Bioclassifier outside the submitted work, a patent for Prosigna issued, licensed, and with royalties paid from Veracyte (TON). Consultancies with Cerca Biotech and Biotheranostics as well as multiple patents in biomarkers, all outside the submitted work (JB). Institutional grant from AstraZeneca, personal grants from AstraZeneca, advisory board MSD and travel expenses from Daiichi Sankyo and Astra Zeneca (AVL). Institutional grants from the Danish Cancer Society, Astra Zeneca, Daiichi Sankyo, Eli Lilly, MSD, Novartis and Pfizer, and travel expenses from Daiichi Sankyo, MSD, Pfizer (BE).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41523-025-00738-7.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet Lond. Engl.365, 1687–1717 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Peto, R. et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet Lond. Engl.379, 432–444 (2012). [DOI] [PMC free article] [PubMed]
- 3.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Electronic address: bc.overview@ctsu.ox.ac.uk. Anthracycline-containing and taxane-containing chemotherapy for early-stage operable breast cancer: a patient-level meta-analysis of 100 000 women from 86 randomised trials. Lancet Lond. Engl.401, 1277–1292 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conforti, R. et al. Breast cancer molecular subclassification and estrogen receptor expression to predict efficacy of adjuvant anthracyclines-based chemotherapy: a biomarker study from two randomized trials. Ann. Oncol. J. Eur. Soc. Med Oncol.18, 1477–1483 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Hurvitz, S. A. et al. A careful reassessment of anthracycline use in curable breast cancer. NPJ Breast Cancer7, 134 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine, M. N. et al. Randomized trial comparing cyclophosphamide, epirubicin, and fluorouracil with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer: update of National Cancer Institute of Canada Clinical Trials Group Trial MA5. J. Clin. Oncol. J. Am. Soc. Clin. Oncol.23, 5166–5170 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Ejlertsen, B. et al. Improved outcome from substituting methotrexate with epirubicin: results from a randomised comparison of CMF versus CEF in patients with primary breast cancer. Eur. J. Cancer Oxf. Engl. 199043, 877–884 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Knoop, A. S. et al. Retrospective Analysis of Topoisomerase IIa Amplifications and Deletions As Predictive Markers in Primary Breast Cancer Patients Randomly Assigned to Cyclophosphamide, Methotrexate, and Fluorouracil or Cyclophosphamide, Epirubicin, and Fluorouracil: Danish Breast Cancer Cooperative Group. J. Clin. Oncol.23, 7483–7490 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Pritchard, K. I. et al. HER2 and Responsiveness of Breast Cancer to Adjuvant Chemotherapy. N. Engl. J. Med.354, 2103–2111 (2006). [DOI] [PubMed] [Google Scholar]
- 10.O’Malley, F. P. et al. Topoisomerase II alpha and responsiveness of breast cancer to adjuvant chemotherapy. J. Natl. Cancer Inst.101, 644–650 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartlett, J. M. S. et al. Predicting Anthracycline Benefit: TOP2A and CEP17-Not Only but Also. J. Clin. Oncol. J. Am. Soc. Clin. Oncol.33, 1680–1687 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Ejlertsen, B. et al. HER2, TOP2A, and TIMP-1 and responsiveness to adjuvant anthracycline-containing chemotherapy in high-risk breast cancer patients. J. Clin. Oncol. J. Am. Soc. Clin. Oncol.28, 984–990 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Di Leo, A. et al. HER2 and TOP2A as predictive markers for anthracycline-containing chemotherapy regimens as adjuvant treatment of breast cancer: a meta-analysis of individual patient data. Lancet Oncol.12, 1134–1142 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Cheang, M. C. U. et al. Responsiveness of intrinsic subtypes to adjuvant anthracycline substitution in the NCIC.CTG MA.5 randomized trial. Clin. Cancer Res J. Am. Assoc. Cancer Res.18, 2402–2412 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen, M. B. et al. The Prosigna 50-gene profile and responsiveness to adjuvant anthracycline-based chemotherapy in high-risk breast cancer patients. NPJ Breast Cancer6, 7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen, M. B. et al. The Prosigna gene expression assay and responsiveness to adjuvant cyclophosphamide-based chemotherapy in premenopausal high-risk patients with breast cancer. Breast Cancer Res.20, 79 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lænkholm, A. V. et al. PAM50 Risk of Recurrence Score Predicts 10-Year Distant Recurrence in a Comprehensive Danish Cohort of Postmenopausal Women Allocated to 5 Years of Endocrine Therapy for Hormone Receptor-Positive Early Breast Cancer. J. Clin. Oncol. J. Am. Soc. Clin. Oncol.36, 735–740 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Gnant, M. et al. Identifying clinically relevant prognostic subgroups of postmenopausal women with node-positive hormone receptor-positive early-stage breast cancer treated with endocrine therapy: a combined analysis of ABCSG-8 and ATAC using the PAM50 risk of recurrence score and intrinsic subtype. Ann. Oncol. J. Eur. Soc. Med Oncol.26, 1685–1691 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Andre, F. et al. Biomarkers for Adjuvant Endocrine and Chemotherapy in Early-Stage Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. J. Am. Soc. Clin. Oncol.40, 1816–1837 (2022). [DOI] [PubMed] [Google Scholar]
- 20.Parker, J. S. et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. J. Am. Soc. Clin. Oncol.27, 1160–1167 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altman, D. G., McShane, L. M., Sauerbrei, W. & Taube, S. E. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): explanation and elaboration. PLoS Med.9, e1001216 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Clinical data for the patients included in this study are not publicly available to protect patient privacy. Access to data used in this study can be made available to qualified researchers through application to the respective trial groups.