Abstract
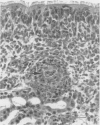
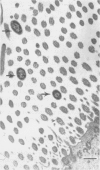
Twenty-three cesarean derived, colostrum deprived pigs were obtained at 5 wk of age and inoculated intranasally with either 1.4 x 10(8) colony forming units of Haemophilus parasuis or sterile phosphate buffered saline. Pigs were euthanized at 4, 8, 12, 18, 26, or 36 h post-inoculation and tissues from the oropharynx and respiratory tract were obtained for qualitative bacterial culture, immunohistochemistry for H. parasuis antigens, and light and transmission electron microscopy. Haemophilus parasuis was consistently isolated from the nasal cavity (17/17, 100%) and trachea (13/17, 76%) and rarely isolated from the lung (3/17, 18%) and blood stream (1/17, 6%) of infected pigs. Antigens of H. parasuis were sporadically detected on the nasal mucosa (6/17, 35%) and trachea (8/17, 47%). Light microscopic lesions included submucosal and intraepithelial infiltrates of neutrophils and infrequent, patchy loss of cilia. Ultrastructural changes in nasal mucosal epithelial cells included cell protrusion, loss of cilia, and dilation of the cytocavitary network. Bacteria were infrequently identified and were either within an amorphous material at the apical surface of the cilia or were between individual cilia. These results suggest H. parasuis associates with the nasal mucosa and can induce a suppurative rhinitis with nasal mucosal epithelial cell degeneration. This process may represent an initial event in the pathogenesis of H. parasuis infection of swine.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M. R., Cheville N. F., Gallagher J. E. Colonization of the pharyngeal tonsil and respiratory tract of the gnotobiotic pig by a toxigenic strain of Pasteurella multocida type D. Vet Pathol. 1991 Jul;28(4):267–274. doi: 10.1177/030098589102800402. [DOI] [PubMed] [Google Scholar]
- Ackermann M. R., DeBey M. C., Register K. B., Larson D. J., Kinyon J. M. Tonsil and turbinate colonization by toxigenic and nontoxigenic strains of Pasteurella multocida in conventionally raised swine. J Vet Diagn Invest. 1994 Jul;6(3):375–377. doi: 10.1177/104063879400600318. [DOI] [PubMed] [Google Scholar]
- Adams D. R. Epithelium lining the rostral portion of the porcine nasal mucosa. Res Vet Sci. 1990 Jul;49(1):61–65. [PubMed] [Google Scholar]
- Amano H., Shibata M., Kajio N., Morozumi T. Pathologic observations of pigs intranasally inoculated with serovar 1, 4 and 5 of Haemophilus parasuis using immunoperoxidase method. J Vet Med Sci. 1994 Aug;56(4):639–644. doi: 10.1292/jvms.56.639. [DOI] [PubMed] [Google Scholar]
- Chung W. B., Collins M. T., Bäckström L. R. Adherence of Bordetella bronchiseptica and Pasteurella multocida to swine nasal ciliated epithelial cells in vitro. APMIS. 1990 May;98(5):453–461. [PubMed] [Google Scholar]
- Dugal F., Girard C., Jacques M. Adherence of Bordetella bronchiseptica 276 to porcine trachea maintained in organ culture. Appl Environ Microbiol. 1990 Jun;56(6):1523–1529. doi: 10.1128/aem.56.6.1523-1529.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley M. M., Stephens D. S., Mulks M. H., Cooper M. D., Bricker J. V., Mirra S. S., Wright A. Pathogenesis of IgA1 protease-producing and -nonproducing Haemophilus influenzae in human nasopharyngeal organ cultures. J Infect Dis. 1986 Nov;154(5):752–759. doi: 10.1093/infdis/154.5.752. [DOI] [PubMed] [Google Scholar]
- Goiś M., Farrington D. O., Barnes H. J., Ross R. F. Long-acting oxytetracycline for control of induced Pasteurella multocida rhinitis in swine. J Am Vet Med Assoc. 1983 Aug 15;183(4):445–447. [PubMed] [Google Scholar]
- Inzana T. J. Capsules and virulence in the HAP group of bacteria. Can J Vet Res. 1990 Apr;54 (Suppl):S22–S27. [PubMed] [Google Scholar]
- Jacques M., Gottschalk M., Foiry B., Higgins R. Ultrastructural study of surface components of Streptococcus suis. J Bacteriol. 1990 Jun;172(6):2833–2838. doi: 10.1128/jb.172.6.2833-2838.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell E. M., Trump B. F. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med. 1976 Aug;100(8):405–414. [PubMed] [Google Scholar]
- Mebus C. A., Underdahl N. R. Scanning electron microscopy of trachea and bronchi from gnotobiotic pigs inoculated with Mycoplasma hyopneumoniae. Am J Vet Res. 1977 Aug;38(8):1249–1254. [PubMed] [Google Scholar]
- Morozumi T., Hiramune T., Kobayashi K. Glässer's disease in piglets produced by intraperitoneal inoculation with Haemophilus parasuis. Natl Inst Anim Health Q (Tokyo) 1981 Fall;21(3):121–128. [PubMed] [Google Scholar]
- Morozumi T., Nicolet J. Morphological variations of Haemophilus parasuis strains. J Clin Microbiol. 1986 Jan;23(1):138–142. doi: 10.1128/jcm.23.1.138-142.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon E. R. Molecular basis of invasive Haemophilus influenzae type b disease. J Infect Dis. 1992 Jun;165 (Suppl 1):S77–S81. doi: 10.1093/infdis/165-supplement_1-s77. [DOI] [PubMed] [Google Scholar]
- Moxon E. R., Wilson R. The role of Haemophilus influenzae in the pathogenesis of pneumonia. Rev Infect Dis. 1991 May-Jun;13 (Suppl 6):S518–S527. doi: 10.1093/clinids/13.supplement_6.s518. [DOI] [PubMed] [Google Scholar]
- Møller K., Andersen L. V., Christensen G., Kilian M. Optimalization of the detection of NAD dependent Pasteurellaceae from the respiratory tract of slaughterhouse pigs. Vet Microbiol. 1993 Sep;36(3-4):261–271. doi: 10.1016/0378-1135(93)90093-m. [DOI] [PubMed] [Google Scholar]
- Münch S., Grund S., Krüger M. Fimbriae and membranes on Haemophilus parasuis. Zentralbl Veterinarmed B. 1992 Feb;39(1):59–64. doi: 10.1111/j.1439-0450.1992.tb01138.x. [DOI] [PubMed] [Google Scholar]
- Neil D. H., McKay K. A., L'Ecuyer C., Corner A. H. Glasser's disease of swine produced by the intracheal inoculation of haemophilus suis. Can J Comp Med. 1969 Jul;33(3):187–193. [PMC free article] [PubMed] [Google Scholar]
- Rapp-Gabrielson V. J., Gabrielson D. A. Prevalence of Haemophilus parasuis serovars among isolates from swine. Am J Vet Res. 1992 May;53(5):659–664. [PubMed] [Google Scholar]
- Rapp-Gabrielson V. J., Gabrielson D. A., Schamber G. J. Comparative virulence of Haemophilus parasuis serovars 1 to 7 in guinea pigs. Am J Vet Res. 1992 Jun;53(6):987–994. [PubMed] [Google Scholar]
- Read R. C., Wilson R., Rutman A., Lund V., Todd H. C., Brain A. P., Jeffery P. K., Cole P. J. Interaction of nontypable Haemophilus influenzae with human respiratory mucosa in vitro. J Infect Dis. 1991 Mar;163(3):549–558. doi: 10.1093/infdis/163.3.549. [DOI] [PubMed] [Google Scholar]
- Riley M. G., Russell E. G., Callinan R. B. Haemophilus parasuis infection in swine. J Am Vet Med Assoc. 1977 Oct 1;171(7):649–651. [PubMed] [Google Scholar]
- Smart N. L., Miniats O. P., Rosendal S., Friendship R. M. Glasser's disease and prevalence of subclinical infection with Haemophilus parasuis in swine in southern Ontario. Can Vet J. 1989 Apr;30(4):339–343. [PMC free article] [PubMed] [Google Scholar]
- Stephens D. S., Farley M. M. Pathogenic events during infection of the human nasopharynx with Neisseria meningitidis and Haemophilus influenzae. Rev Infect Dis. 1991 Jan-Feb;13(1):22–33. doi: 10.1093/clinids/13.1.22. [DOI] [PubMed] [Google Scholar]
- Thomson R. G., Ruhnke H. L. Haemophilus Septicemia in Piglets. Can Vet J. 1963 Oct;4(10):271–275. [PMC free article] [PubMed] [Google Scholar]
- Vahle J. L., Haynes J. S., Andrews J. J. Experimental reproduction of Haemophilus parasuis infection in swine: clinical, bacteriological, and morphologic findings. J Vet Diagn Invest. 1995 Oct;7(4):476–480. doi: 10.1177/104063879500700409. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Lawson G. H., Rowland A. C. Streptococcal infection in piglets: the palatine tonsils as portals of entry for Streptococcus suis. Res Vet Sci. 1973 Nov;15(3):352–362. [PubMed] [Google Scholar]
- Yokomizo Y., Shimizu T. Adherence of Bordetella bronchiseptica to swine nasal epithelial cells and its possible role in virulence. Res Vet Sci. 1979 Jul;27(1):15–21. [PubMed] [Google Scholar]