Abstract
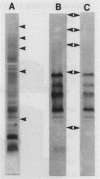
Outer sheath antigen from Leptospira interrogans serovar hardjo type hardjoprajitno and acetic acid extracted antigens from serovar hardjo types hardjoprajitno and hardjobovis were evaluated in an immunoassay for ability to detect hyperimmune rabbit serum to serovar hardjo. The degree of cross-reactivity with hyperimmune rabbit sera to L. interrogans serovars pomona, copenhageni, grippotyphosa, canicola and sejroe, and Leptospira biflexa serovar patoc was also measured for each antigen. All of the antigens reacted with the antiserum to L. interrogans serovar hardjo. The outer sheath antigen however, also showed wide cross-reactivity with the antisera to all of the serovars of L. interrogans tested and with the antiserum to L. biflexa serovar patoc. The acetic acid extracted antigen from either type hardjoprajitno, or type hardjobovis, showed a high degree of specificity for serovar hardjo antiserum. The hardjobovis acetic acid extracted antigen was characterized by sodium dodecyl sulfate polyacrylamide gel electrophoresis and immunoblotting, and was incorporated into an indirect ELISA for detection of anti-serovar hardjo antibodies in bovine serum. This ELISA showed a relative specificity of 100% with 156 bovine sera which were negative at a dilution of 1:100 in the microscopic agglutination test (MAT) for L. interrogans serovars hardjo, pomona, sejroe, icterohaemorrhagiae, copenhageni, canicola, and grippotyphosa. The relative sensitivity of this assay with 192 bovine sera which had serovar hardjo MAT titres of > or = 100 was 95.3% (95% confidence limit = 2.99%). The degree of cross-reactivity with 289 bovine sera which had serovar pomona MAT titres of > or = 100 (with no detectable serovar hardjo MAT titres) was approximately 1.0%. This assay was: easily standardized, scored objectively, repeatable, semi-automated and used a non-hazardous antigen that can be routinely prepared in gram amounts.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler B., Faine S. A pomona serogroup-specific, agglutinating antigen in Leptospira, identified by monoclonal antibodies. Pathology. 1983 Jul;15(3):247–250. doi: 10.3109/00313028309083501. [DOI] [PubMed] [Google Scholar]
- Adler B., Murphy A. M., Locarnini S. A., Faine S. Detection of specific anti-leptospiral immunoglobulins M and G in human serum by solid-phase enzyme-linked immunosorbent assay. J Clin Microbiol. 1980 May;11(5):452–457. doi: 10.1128/jcm.11.5.452-457.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auran N. E., Johnson R. C., Ritzi D. M. Isolation of the outer sheath of Leptospira and its immunogenic properties in hamsters. Infect Immun. 1972 Jun;5(6):968–975. doi: 10.1128/iai.5.6.968-975.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercovich Z., Taaijke R., Bokhout B. A. Evaluation of an ELISA for the diagnosis of experimentally induced and naturally occurring Leptospira hardjo infections in cattle. Vet Microbiol. 1990 Jan;21(3):255–262. doi: 10.1016/0378-1135(90)90036-u. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chappel R. J., Prime R. W., Millar B. D., Mead L. J., Jones R. T., Adler B. Comparison of diagnostic procedures for porcine leptospirosis. Vet Microbiol. 1992 Feb;30(2-3):151–163. doi: 10.1016/0378-1135(92)90110-f. [DOI] [PubMed] [Google Scholar]
- Cho H. J., Gale S. P., Masri S. A., Malkin K. L. Diagnostic specificity, sensitivity and cross-reactivity of an enzyme-linked immunosorbent assay for the detection of antibody against Leptospira interrogans serovars pomona, sejroe and hardjo in cattle. Can J Vet Res. 1989 Jul;53(3):285–289. [PMC free article] [PubMed] [Google Scholar]
- Cinco M., Banfi E., Panfili E. Leptospiral lipopolysaccharide presence in the outer envelope: electrophoretic evidence and immunological specificity. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Nov;269(3):277–283. doi: 10.1016/s0176-6724(88)80171-8. [DOI] [PubMed] [Google Scholar]
- Cole J. R., Jr, Sulzer C. R., Pursell A. R. Improved microtechnique for the leptospiral microscopic agglutination test. Appl Microbiol. 1973 Jun;25(6):976–980. doi: 10.1128/am.25.6.976-980.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins D. V., Robertson G. M., Hustas L. The use of the enzyme-linked immunosorbent assay (ELISA) to detect the IgM and IgG antibody response to Leptospira interrogans serovars hardjo, pomona and tarassovi in cattle. Vet Microbiol. 1985 Aug;10(5):439–450. doi: 10.1016/0378-1135(85)90026-4. [DOI] [PubMed] [Google Scholar]
- Goddard R. D., Luff P. R., Thornton D. H. The serological response of calves to Leptospira interrogans serovar hardjo vaccines and infection as measured by the microscopic agglutination test and anti-IgM and anti-IgG enzyme-linked immunosorbent assay. Vet Microbiol. 1991 Jan;26(1-2):191–201. doi: 10.1016/0378-1135(91)90055-k. [DOI] [PubMed] [Google Scholar]
- Jost B. H., Adler B., Faine S. Reaction of monoclonal antibodies with species specific determinants in Leptospira interrogans outer envelope. J Med Microbiol. 1988 Sep;27(1):51–57. doi: 10.1099/00222615-27-1-51. [DOI] [PubMed] [Google Scholar]
- Jost B. H., Adler B., Vinh T., Faine S. A monoclonal antibody reacting with a determinant on leptospiral lipopolysaccharide protects guinea pigs against leptospirosis. J Med Microbiol. 1986 Nov;22(3):269–275. doi: 10.1099/00222615-22-3-269. [DOI] [PubMed] [Google Scholar]
- Kawaoka Y., Naiki M., Yanagawa R. Radioimmunoassay system using a serovar-specific lipopolysaccharide antigen of Leptospira. J Clin Microbiol. 1979 Sep;10(3):313–316. doi: 10.1128/jcm.10.3.313-316.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nielsen K., Cherwonogrodzky J. W., Duncan J. R., Bundle D. R. Enzyme-linked immunosorbent assay for differentiation of the antibody response of cattle naturally infected with Brucella abortus or vaccinated with strain 19. Am J Vet Res. 1989 Jan;50(1):5–9. [PubMed] [Google Scholar]
- Ono E., Takase H., Naiki M., Yanagawa R. Purification, characterization and serological properties of a glycolipid antigen reactive with a serovar-specific monoclonal antibody against Leptospira interrogans serovar canicola. J Gen Microbiol. 1987 May;133(5):1329–1336. doi: 10.1099/00221287-133-5-1329. [DOI] [PubMed] [Google Scholar]
- Terpstra W. J., Ligthart G. S., Schoone G. J. ELISA for the detection of specific IgM and IgG in human leptospirosis. J Gen Microbiol. 1985 Feb;131(2):377–385. doi: 10.1099/00221287-131-2-377. [DOI] [PubMed] [Google Scholar]
- Thiermann A. B., Garrett L. A. Enzyme-linked immunosorbent assay for the detection of antibodies to Leptospira interrogans serovars hardjo and pomona in cattle. Am J Vet Res. 1983 May;44(5):884–887. [PubMed] [Google Scholar]
- Thiermann A. B. Leptospirosis: current developments and trends. J Am Vet Med Assoc. 1984 Mar 15;184(6):722–725. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueba G. A., Bolin C. A., Thoen C. O. Evaluation of an enzyme immunoassay for diagnosis of bovine leptospirosis caused by Leptospira interrogans serovar hardjo type hardjo-bovis. J Vet Diagn Invest. 1990 Oct;2(4):323–329. doi: 10.1177/104063879000200413. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Tsuji M., Kawaoka Y., Naiki M., Yanagawa R. Isolation of antigenically active components from leptospiral serovar-specific lipopolysaccharide antigen by alkaline treatment. Microbiol Immunol. 1981;25(9):949–957. doi: 10.1111/j.1348-0421.1981.tb00099.x. [DOI] [PubMed] [Google Scholar]
- Waltman W. D., 2nd, Dawe D. L. Enzyme-linked immunosorbent assay for the detection of antileptospiral antibodies in swine sera. Am J Vet Res. 1983 Jun;44(6):1120–1122. [PubMed] [Google Scholar]
- Wright P. Enzyme immunoassay: observations on aspects of quality control. Vet Immunol Immunopathol. 1987 Dec;17(1-4):441–452. doi: 10.1016/0165-2427(87)90160-7. [DOI] [PubMed] [Google Scholar]