Abstract
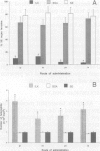
We have previously reported that the prophylactic administration of factor(s) from T-cell supernatants derived from Salmonella enteritidis-immune chickens (ILK) have a favorable effect in controlling or eliminating salmonellosis in neonatal poultry. Experimentally, we have used the intraperitoneal injection as the standard method of administering ILK to neonatal poultry. However, this method is neither easy, practical, nor economical for the poultry industry. In the present study, we evaluated the effectiveness of oral (p.o.), intranasal (i.n.), and subcutaneous (s.c.) administration of ILK for ease of delivery, induction of protective resistance against Salmonella enteritidis (Se) organ invasion, and the ability to activate peripheral blood heterophils in day-old chickens. In the first experiments, delivery of ILK p.o., i.n., and s.c. significantly (P < 0.01) increased the resistance of day-old chickens to Se organ invasion. The level of protection was equivalent to that induced by the i.p. route. Administration of a comparable protein control (bovine serum albumin, BSA) by the 3 routes induced no protective effect against Se organ invasion. Likewise, a significant increase was found in the number of circulating heterophils within 4 h of administration of the ILK by all routes. In the 2nd experiment, the function of the heterophils from ILK-treated birds was compared with that of the control cells in adherence, chemotaxis, and phagocytosis assays. The heterophils from birds given ILK i.p., s.c., p.o., or i.n. had significantly (P < 0.01) increased functional activities when compared to the activities of the heterophils from the control birds. These studies indicate that the delivery of ILK either orally or parenterally, routes which can be used by the poultry industry, can confer protection to chickens against a localized enteric Se organ invasion by potentiating the systemic heterophilic innate response.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baqar S., Pacheco N. D., Rollwagen F. M. Modulation of mucosal immunity against Campylobacter jejuni by orally administered cytokines. Antimicrob Agents Chemother. 1993 Dec;37(12):2688–2692. doi: 10.1128/aac.37.12.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bober L. A., Grace M. J., Pugliese-Sivo C., Rojas-Triana A., Waters T., Sullivan L. M., Narula S. K. The effect of GM-CSF and G-CSF on human neutrophil function. Immunopharmacology. 1995 Mar;29(2):111–119. doi: 10.1016/0162-3109(94)00050-p. [DOI] [PubMed] [Google Scholar]
- Cairo M. S. Cytokines: a new immunotherapy. Clin Perinatol. 1991 Jun;18(2):343–359. [PubMed] [Google Scholar]
- Coe N. E., Frank D. E., Roth J. A. Effect of recombinant human cytokines on porcine neutrophil function. Vet Immunol Immunopathol. 1993 Jun;37(1):39–47. doi: 10.1016/0165-2427(93)90014-u. [DOI] [PubMed] [Google Scholar]
- Cooper G. L., Venables L. M., Woodward M. J., Hormaeche C. E. Vaccination of chickens with strain CVL30, a genetically defined Salmonella enteritidis aroA live oral vaccine candidate. Infect Immun. 1994 Nov;62(11):4747–4754. doi: 10.1128/iai.62.11.4747-4754.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogut M. H., McGruder E. D., Hargis B. M., Corrier D. E., DeLoach J. R. Dynamics of avian inflammatory response to Salmonella-immune lymphokines. Changes in avian blood leukocyte populations. Inflammation. 1994 Aug;18(4):373–388. doi: 10.1007/BF01534435. [DOI] [PubMed] [Google Scholar]
- Kogut M. H., McGruder E. D., Hargis B. M., Corrier D. E., DeLoach J. R. In vivo activation of heterophil function in chickens following injection with Salmonella enteritidis-immune lymphokines. J Leukoc Biol. 1995 Jan;57(1):56–62. doi: 10.1002/jlb.57.1.56. [DOI] [PubMed] [Google Scholar]
- Kogut M. H., Moyes R., Deloach J. R. Neutralization of G-CSF inhibits ILK-induced heterophil influx: granulocyte-colony stimulating factor mediates the Salmonella enteritidis-immune lymphokine potentiation of the acute avian inflammatory response. Inflammation. 1997 Feb;21(1):9–25. doi: 10.1023/a:1027382523535. [DOI] [PubMed] [Google Scholar]
- Lowenthal J. W., Connick T. E., McWaters P. G., York J. J. Development of T cell immune responsiveness in the chicken. Immunol Cell Biol. 1994 Apr;72(2):115–122. doi: 10.1038/icb.1994.18. [DOI] [PubMed] [Google Scholar]
- Lowry V. K., Genovese K. J., Bowen L. L., Kogut M. H. Ontogeny of the phagocytic and bactericidal activities of turkey heterophils and their potentiation by Salmonella enteritidis-immune lymphokines. FEMS Immunol Med Microbiol. 1997 Sep;19(1):95–100. doi: 10.1111/j.1574-695X.1997.tb01077.x. [DOI] [PubMed] [Google Scholar]
- McCorkle F. M., Thaxton J. P. Ontogeny of antibody response to Brucella abortus in turkeys. Dev Comp Immunol. 1988 Summer;12(3):621–627. doi: 10.1016/0145-305x(88)90078-x. [DOI] [PubMed] [Google Scholar]
- McGruder E. D., Ramirez G. A., Kogut M. H., Moore R. W., Corrier D. E., Deloach J. R., Hargis B. M. In ovo administration of Salmonella enteritidis-immune lymphokines confers protection to neonatal chicks against Salmonella enteritidis organ infectivity. Poult Sci. 1995 Jan;74(1):18–25. doi: 10.3382/ps.0740018. [DOI] [PubMed] [Google Scholar]
- McGruder E. D., Ray P. M., Tellez G. I., Kogut M. H., Corrier D. E., DeLoach J. R., Hargis B. M. Salmonella enteritidis immune leukocyte-stimulated soluble factors: effects on increased resistance to Salmonella organ invasion in day-old Leghorn chicks. Poult Sci. 1993 Dec;72(12):2264–2271. doi: 10.3382/ps.0722264. [DOI] [PubMed] [Google Scholar]
- Powell P. C. Immune mechanisms in infections of poultry. Vet Immunol Immunopathol. 1987 May;15(1-2):87–113. doi: 10.1016/0165-2427(87)90107-3. [DOI] [PubMed] [Google Scholar]
- Rezabek G. B., Donahue J. M., Giles R. C., Petrites-Murphy M. B., Poonacha K. B., Rooney J. R., Smith B. J., Swerczek T. W., Tramontin R. R. Histoplasmosis in horses. J Comp Pathol. 1993 Jul;109(1):47–55. doi: 10.1016/s0021-9975(08)80239-3. [DOI] [PubMed] [Google Scholar]
- Schaefer A. E., Scafuri A. R., Fredericksen T. L., Gilmour D. G. Strong suppression by monocytes of T cell mitogenesis in chicken peripheral blood leukocytes. J Immunol. 1985 Sep;135(3):1652–1660. [PubMed] [Google Scholar]
- Seto F. Early development of the avian immune system. Poult Sci. 1981 Sep;60(9):1981–1995. doi: 10.3382/ps.0601981. [DOI] [PubMed] [Google Scholar]
- Steinbeck M. J., Roth J. A. Neutrophil activation by recombinant cytokines. Rev Infect Dis. 1989 Jul-Aug;11(4):549–568. doi: 10.1093/clinids/11.4.549. [DOI] [PubMed] [Google Scholar]
- Suresh M., Sharma J. M., Belzer S. W. Studies on lymphocyte subpopulations and the effect of age on immune competence in turkeys. Dev Comp Immunol. 1993 Nov-Dec;17(6):525–535. doi: 10.1016/s0145-305x(05)80008-4. [DOI] [PubMed] [Google Scholar]
- Tellez G. I., Kogut M. H., Hargis B. M. Immunoprophylaxis of Salmonella enteritidis infection by lymphokines in Leghorn chicks. Avian Dis. 1993 Oct-Dec;37(4):1062–1070. [PubMed] [Google Scholar]
- Toth T. E., Veit H., Gross W. B., Siegel P. B. Cellular defense of the avian respiratory system: protection against Escherichia coli airsacculitis by Pasteurella multocida-activated respiratory phagocytes. Avian Dis. 1988 Oct-Dec;32(4):681–687. [PubMed] [Google Scholar]
- Ziprin R. L., Corrier D. E., Elissalde M. H. Maturation of resistance to salmonellosis in newly hatched chicks: inhibition by cyclosporine. Poult Sci. 1989 Dec;68(12):1637–1642. doi: 10.3382/ps.0681637. [DOI] [PubMed] [Google Scholar]