Abstract
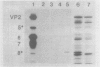
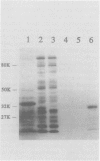
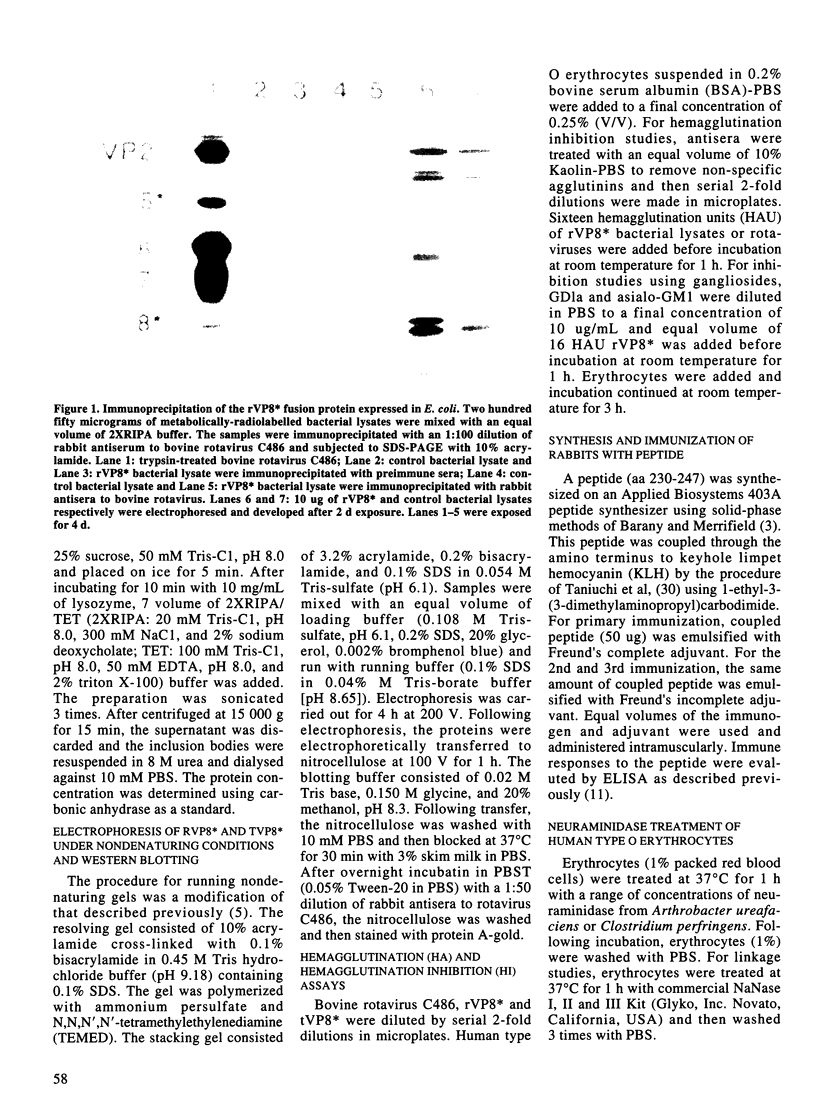
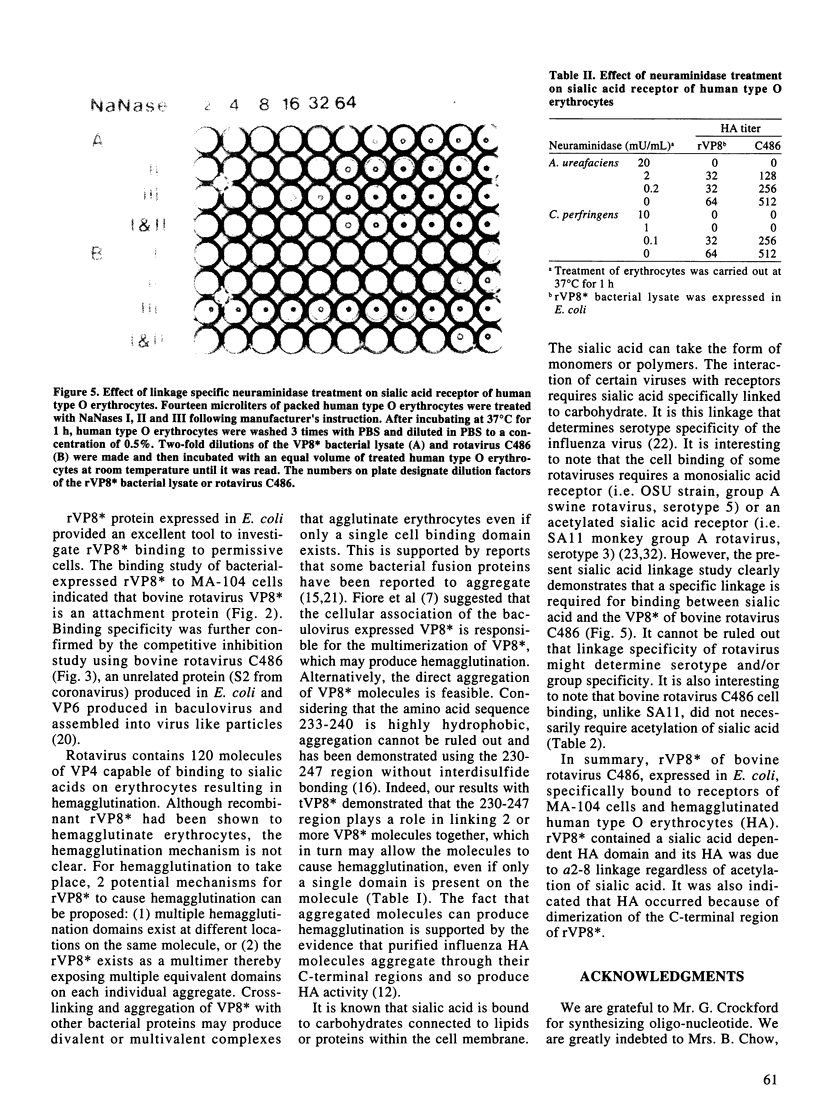
Rotavirus VP8*, the N-terminal trypsin cleavage product of VP4, has been shown to bind to MA-104 cells and human O type erythrocytes. To examine whether bacterially expressed VP8* binds to cellular components of MA-104 cells, the VP8* (aa 1-247) was expressed in E. coli and radiolabelled with 35S-methionine. The radiolabelled rVP8* was immunoprecipitated with antiserum to bovine rotavirus C486 (BRV). The rVP8* was found to bind to MA-104 cells and its binding was competed by BRV. To study the interaction between VP8* and receptors of erythrocytes, hemagglutination (HA) and hemagglutination inhibition (HI) assays were carried out using solubilized rVP8*. rVP8* showed HA which could be inhibited by antiserum to BRV. This interaction was also inhibited by gangliosides, demonstrating a sialic acid dependent interaction. To study the contribution of the C-terminal region of VP8* to HA, a number of approaches were used. First, a peptide spanning aa 230-247 was synthesized and antisera was raised against the peptide to see whether it could inhibit HA of rVP8*. Second, a truncated form of VP8* (tVP8*: aa 1-229) was expressed to examine its hemagglutinating activity. Third, the dimerization of rVP8* and tVP8* was compared by Western-blotting following electrophoresis using native SDS-PAGE. The results indicated that antibody to aa 230-247 inhibits hemagglutination by preventing dimerization of VP8* which in turn allows the molecule to cause HA. To characterize the interaction between the HA domain and sialic acid receptors, erythrocytes were treated with sialidases of different specificities. Arthrobacter ureafaciens, Clostridium perfringens and alpha 2-8 linkage-specific neuraminidase destroyed the ability of sialic acid of erythrocytes to interact with rVP8*, indicating that bovine rotavirus C486 binding requires an alpha 2-8 linkage but acetylation of the sialic acid is not necessary.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Babiuk L. A., Mohammed K., Spence L., Fauvel M., Petro R. Rotavirus isolation and cultivation in the presence of trypsin. J Clin Microbiol. 1977 Dec;6(6):610–617. doi: 10.1128/jcm.6.6.610-617.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastardo J. W., McKimm-Breschkin J. L., Sonza S., Mercer L. D., Holmes I. H. Preparation and characterization of antisera to electrophoretically purified SA11 virus polypeptides. Infect Immun. 1981 Dec;34(3):641–647. doi: 10.1128/iai.34.3.641-647.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Isola V. J., Kuhns J., Berman P. W., Eisenberg R. J. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing ("native" gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J Virol. 1986 Oct;60(1):157–166. doi: 10.1128/jvi.60.1.157-166.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford S. E., Labbé M., Cohen J., Burroughs M. H., Zhou Y. J., Estes M. K. Characterization of virus-like particles produced by the expression of rotavirus capsid proteins in insect cells. J Virol. 1994 Sep;68(9):5945–5952. doi: 10.1128/jvi.68.9.5945-5952.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore L., Greenberg H. B., Mackow E. R. The VP8 fragment of VP4 is the rhesus rotavirus hemagglutinin. Virology. 1991 Apr;181(2):553–563. doi: 10.1016/0042-6822(91)90888-i. [DOI] [PubMed] [Google Scholar]
- Fuentes-Pananá E. M., López S., Gorziglia M., Arias C. F. Mapping the hemagglutination domain of rotaviruses. J Virol. 1995 Apr;69(4):2629–2632. doi: 10.1128/jvi.69.4.2629-2632.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara N., Yoshie O., Kitaoka S., Konno T. Role of VP3 in human rotavirus internalization after target cell attachment via VP7. J Virol. 1988 Jul;62(7):2209–2218. doi: 10.1128/jvi.62.7.2209-2218.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijaz M. K., Attah-Poku S. K., Redmond M. J., Parker M. D., Sabara M. I., Frenchick P., Babiuk L. A. Heterotypic passive protection induced by synthetic peptides corresponding to VP7 and VP4 of bovine rotavirus. J Virol. 1991 Jun;65(6):3106–3113. doi: 10.1128/jvi.65.6.3106-3113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver W. G., Valentine R. C. Morphology of the isolated hemagglutinin and neuraminidase subunits of influenza virus. Virology. 1969 May;38(1):105–119. doi: 10.1016/0042-6822(69)90132-9. [DOI] [PubMed] [Google Scholar]
- Lee J., Babiuk L. A., Harland R., Gibbons E., Elazhary Y., Yoo D. Immunological response to recombinant VP8* subunit protein of bovine roravirus in pregnant cattle. J Gen Virol. 1995 Oct;76(Pt 10):2477–2483. doi: 10.1099/0022-1317-76-10-2477. [DOI] [PubMed] [Google Scholar]
- Lizano M., López S., Arias C. F. The amino-terminal half of rotavirus SA114fM VP4 protein contains a hemagglutination domain and primes for neutralizing antibodies to the virus. J Virol. 1991 Mar;65(3):1383–1391. doi: 10.1128/jvi.65.3.1383-1391.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri S. A., Nagata L., Mah D. C., Lee P. W. Functional expression in Escherichia coli of cloned reovirus S1 gene encoding the viral cell attachment protein sigma 1. Virology. 1986 Feb;149(1):83–90. doi: 10.1016/0042-6822(86)90089-9. [DOI] [PubMed] [Google Scholar]
- Patton J. T., Hua J., Mansell E. A. Location of intrachain disulfide bonds in the VP5* and VP8* trypsin cleavage fragments of the rhesus rotavirus spike protein VP4. J Virol. 1993 Aug;67(8):4848–4855. doi: 10.1128/jvi.67.8.4848-4855.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter A. A., Cox G., Parker M., Babiuk L. A. The complete nucleotide sequence of bovine rotavirus C486 gene 4 cDNA. Nucleic Acids Res. 1987 May 26;15(10):4361–4361. doi: 10.1093/nar/15.10.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad B. V., Burns J. W., Marietta E., Estes M. K., Chiu W. Localization of VP4 neutralization sites in rotavirus by three-dimensional cryo-electron microscopy. Nature. 1990 Feb 1;343(6257):476–479. doi: 10.1038/343476a0. [DOI] [PubMed] [Google Scholar]
- Redmond M. J., Ijaz M. K., Parker M. D., Sabara M. I., Dent D., Gibbons E., Babiuk L. A. Assembly of recombinant rotavirus proteins into virus-like particles and assessment of vaccine potential. Vaccine. 1993;11(2):273–281. doi: 10.1016/0264-410x(93)90029-w. [DOI] [PubMed] [Google Scholar]
- Rekosh D., Lindenbaum J., Brewster J., Mertz L. M., Hurwitz J., Prestine L. Expression in Escherichia coli of a fusion protein product containing a region of the adenovirus DNA polymerase. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2354–2358. doi: 10.1073/pnas.82.8.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers G. N., Paulson J. C. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology. 1983 Jun;127(2):361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- Rolsma M. D., Gelberg H. B., Kuhlenschmidt M. S. Assay for evaluation of rotavirus-cell interactions: identification of an enterocyte ganglioside fraction that mediates group A porcine rotavirus recognition. J Virol. 1994 Jan;68(1):258–268. doi: 10.1128/jvi.68.1.258-268.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri F. M., Greenberg H. B. Antibodies to the trypsin cleavage peptide VP8 neutralize rotavirus by inhibiting binding of virions to target cells in culture. J Virol. 1991 May;65(5):2211–2219. doi: 10.1128/jvi.65.5.2211-2219.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz M. C., Alonso-Torre S. R., Charpilienne A., Vasseur M., Michelangeli F., Cohen J., Alvarado F. Rotavirus interaction with isolated membrane vesicles. J Virol. 1994 Jun;68(6):4009–4016. doi: 10.1128/jvi.68.6.4009-4016.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabara M., Gilchrist J. E., Hudson G. R., Babiuk L. A. Preliminary characterization of an epitope involved in neutralization and cell attachment that is located on the major bovine rotavirus glycoprotein. J Virol. 1985 Jan;53(1):58–66. doi: 10.1128/jvi.53.1.58-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saulsbury F. T., Winkelstein J. A., Yolken R. H. Chronic rotavirus infection in immunodeficiency. J Pediatr. 1980 Jul;97(1):61–65. doi: 10.1016/s0022-3476(80)80131-4. [DOI] [PubMed] [Google Scholar]
- Shaw A. L., Rothnagel R., Chen D., Ramig R. F., Chiu W., Prasad B. V. Three-dimensional visualization of the rotavirus hemagglutinin structure. Cell. 1993 Aug 27;74(4):693–701. doi: 10.1016/0092-8674(93)90516-S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi M., Clark H. B., Johnson E. M., Jr Induction of nerve growth factor receptor in Schwann cells after axotomy. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4094–4098. doi: 10.1073/pnas.83.11.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby R. E., Yolken R. H. SA11 rotavirus is specifically inhibited by an acetylated sialic acid. J Infect Dis. 1990 Jan;161(1):116–119. doi: 10.1093/infdis/161.1.116. [DOI] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., Parker M. D., Massie B., van den Hurk J. V., Harland R., Babiuk L. A., Zamb T. J. Protection of cattle from BHV-1 infection by immunization with recombinant glycoprotein gIV. Vaccine. 1993;11(1):25–35. doi: 10.1016/0264-410x(93)90336-v. [DOI] [PubMed] [Google Scholar]