Abstract
Eukaryotic translation initiation factor 4F (eIF4F), comprising subunits eIF4G, eIF4E, and eIF4A, plays a pivotal role in the 48S preinitiation complex assembly and ribosomal scanning. Additionally, eIF4B enhances the helicase activity of eIF4A. eIF4F also interacts with poly (A)-binding protein (PABP) bound to the poly (A) tail of messenger RNA (mRNA), thereby forming a closed-loop structure. PABP, in turn, interacts with eukaryotic release factor 3 (eRF3), stimulating translation termination. Here, we employed a reconstituted mammalian system to directly demonstrate that eIF4F potently enhances translation termination. Specifically, eIF4A and eIF4B promote the loading of eRF1 into the A site of the ribosome, while eIF4G1 stimulates the GTPase activity of eRF3 and facilitates the dissociation of release factors following peptide release. We also identified MIF4G as the minimal domain required for this activity and showed that eIF4G2/DAP5 can also promote termination. Our findings provide compelling evidence that the closed-loop mRNA structure facilitates translation termination, with PABP and eIF4F directly involved in this process.
Graphical Abstract
Graphical Abstract.

Introduction
Eukaryotic translation initiation factor 4F (eIF4F) is required for the preinitiation complex (PIC) assembly at capped messenger RNA (mRNA) and the subsequent scanning [1–4]. eIF4F consists of eIF4G, eIF4E, and eIF4A [5], each of them performs a specific function in translation initiation. eIF4G is a scaffold protein which binds the other two subunits [5]. In addition, it interacts with the eukaryotic translation initiation factor 3 (eIF3) [6] at the small 40S subunit of the ribosome and loads capped mRNA into 48S PIC [7]. eIF4E recognizes the cap structure at the 5′ end of mRNA [3, 8, 9]. eIF4A is an RNA helicase that unwinds the secondary structures of mRNA during scanning of the 5′ untranslated region (5′ UTR) by the PIC [10]. The eIF4F subunits co-stimulate each other’s activity during translation initiation [11–16]. eIF4A also associates with the eIF4B [17] which increases its helicase activity [12, 17, 18]. On the other hand, the poly (A)-binding protein (PABP) interacts with the N-terminal domain of eIF4G, stimulating its activity in translation initiation [19–24]. Additionally, interaction of eIF4B and PABP was observed [25–28]. As a result, the 5′ and 3′ ends of the mRNA get closer to each other and a closed-loop mRNA structure is formed, which has been shown to increase the translation rate [29–32].
In addition to the major isoform of eIF4G called eIF4G1 or eIF4GI, two other eIF4G family members, eIF4G2/DAP5 and eIF4G3/eIF4GII, have been found in mammals [33, 34]. eIF4G2/DAP5 (also known as p97 or Nat1) is involved in translation initiation along with eIF4G1. Although eIF4G2 does not bind eIF4E, it promotes cap-dependent translation via facilitating both the scanning through short upstream open reading frames (uORFs) and resumption of scanning after the translation thereof (so called translation reinitiation) [35–37].
Under some conditions, eIF4G1 undergoes proteolytic cleavage. For example, during infection by certain picornaviruses, e.g. poliovirus or rhinovirus, viral 2A protease cleaves eIF4G after 674–681 aa, which separates the N-terminal PABP- and eIF4E-binding part from the rest of the protein, thereby inhibiting cap-dependent translation [38]. The resulting core eIF4G1 fragment p100, which contains both the MIF4G and MA3 domains, can interact with the internal ribosome entry sites and drive the translation of viral mRNAs. At the same time, the N-terminal fragment of eIF4G1 is believed to remain bound to eIF4E, rendering it unresponsive to cap thus further inhibiting cap-dependent translation of cellular mRNAs [39, 40]. eIF4G1 is also cleaved by HIV protease, which cuts the protein at two sites—after 721 aa and after 1126 aa. As a result of the proteolytic cleavage three polypeptides are obtained, one of which is a p50 fragment containing the MIF4G domain, thereby also leading to inhibition of cap-dependent translation [41–43]. Since the p100 and p50 fragments of eIF4G, as well as eIF4G2, lack the PABP-binding domain, these proteins are thought to be unable to form a closed-loop mRNA structure.
PABP also interacts with eukaryotic release factor 3 (eRF3) [44–46]. This interaction becomes possible during the formation of a termination closed-loop mRNA structure, bringing the poly (A) tail of the mRNA into close proximity with the stop codon in the ribosome [47, 48]. It was proposed that termination closed-loop structure is formed due to the structuring of the 3′ UTR, bridging the ribosome-unbound 5′ and 3′ ends of mRNA [47]. By positioning itself near the ribosome at the stop codon, PABP stimulates translation termination [44].
Translation termination is performed by two eukaryotic release factors: eRF1 and eRF3. The termination process can be divided into three distinct stages: (i) recognition of the stop codons by the N domain of eRF1 [49–53]; (ii) GTP hydrolysis by eRF3 [54–57], which induces a conformational change in the complex of release factors and facilitates the accommodation of the M domain of eRF1 in the peptidyl transferase center (PTC) [57]; and (iii) peptidyl-transfer RNA (peptidyl-tRNA) hydrolysis, mediated by the GGQ loop of eRF1, with subsequent release of the newly synthesized polypeptide from the ribosome [56]. Poly (A) tail-bound PABP has been demonstrated to stimulate translation termination by loading eRF3 or its complex with eRF1 into the ribosome [44].
Termination is the final stage of translation that occurs just prior to the release of the synthesized peptide from the ribosome, making its regulation critical for cellular function. Nonsense mutations can introduce stop codons within the coding sequences (CDS), resulting in what are known as premature termination codons. The latter disrupt protein synthesis and may lead to the generation of toxic truncated proteins. Additionally, ribosomes may encounter stop codons in the uORFs within 5′ UTRs or in the small downstream open reading frames within 3′ UTRs, as well as in extensions of the CDSs within 3′ UTRs if the main CDS stop codon experiences readthrough. These scenarios highlight the importance of precise control over the termination stage of translation. We propose that these situations require auxiliary proteins to prevent undesirable translation termination or to support an appropriate peptide release. In addition to PABP, several other proteins have been implicated in translation termination, including the yeast ribosome recycling factor Rli1 [58], the human and yeast nuclear mRNA export factors DDX19 and Dbp5 [59, 60], the human and yeast translation initiation factors eIF3j and Hcr1 [61, 62], the human translation factor eIF5A [63], the human programmed cell death 4 protein (PDCD4) [64], and the SARS-CoV-2 viral protein Nsp1 [65]. Notably, the PABP-binding proteins PAIP1 and PAIP2 have emerged as critical regulators in inhibiting termination at premature termination codons [66].
However, the regulation of translation termination across various parts of mRNA and under different cellular conditions (such as apoptosis, cellular stress, virus infection, etc.) remains inadequately explored. A closed-loop structure formation can bring the initiation and termination ribosomal complexes close to each other [47, 48]. This raises the question: could initiation factors that interact with PABP also play a role in the regulation of translation termination?
To address this question, we examined the activities of release factors in the presence of the human eIF4F complex and its individual subunits. Using a reconstituted mammalian translation system and pretermination complexes isolated from rabbit reticulocyte lysate (RRL), we show that eIF4F, eIF4G1, eIF4A, eIF4B, eIF4G2, as well as the eIF4G1 and eIF4G2 deletion variants (p100, p50, and p86) affect various stages of translation termination. Furthermore, we investigated the ability of initiation factors to bind release factors and pretermination or termination ribosomal complexes. Based on these findings, we propose a model for the regulation of translation termination by eIF4F and eIF4B, highlighting their potential roles in coordinating the termination process.
Materials and methods
Ribosomal subunits and translation factors
The 40S and 60S ribosomal subunits and eukaryotic translation factors eIF2, eIF3, eEF1H, and eEF2 were purified from RRL and HeLa cell lysates as described previously [56]. The human translation factors eIF1, eIF1A, eIF4A, eIF4B, p50 (MIF4G domain of eIF4G1), ΔeIF5B, eIF5, eRF1, eRF1 (AGQ) variant, and eRF3c lacking the N-terminal domain (138 amino acid residues including PAM2 domain) were expressed in Escherichia coli strain BL21 (DE3) and purified via Ni-NTA agarose followed by ion-exchange chromatography [56]. Human eRF3a was expressed in insect cells Sf21 using the EMBacY baculovirus from the MultiBac Expression system and purified as described [44, 67, 68].
DNA fragments coding for eIF4F (6×His-eIF4G1, eIF4A, and eIF4E), 6×His-eIF4G1, 6×His-eIF4G2, and its C-terminally truncated form 6×His-p86 (1–792) were obtained from human complementary DNA (cDNA) and cloned into pACEBac2 vector for MultiBac Expression system. The expression followed the previously described protocol [68]. The cells expressing 6×His-eIF4G1 or eIF4F complex were harvested from 800 ml of liquid culture 24 h after the day of proliferation arrest (DPA). The cells expressing 6×His-eIF4G2 or 6×His-tag-p86 were harvested from 400 ml of liquid culture 72 h after DPA. Cells were lysed by sonication in 20 mM Tris–HCl (pH 7.5), 500 mM KCl, 1 mM DTT, 0.1% Tween-20, and 10% glycerol supplemented with Halt™ Protease Inhibitor Cocktail (Thermo Fisher Scientific, Waltham, USA). eIF4F, eIF4G1, and eIF4G2 were purified by affinity chromatography using a HisTrap HP column (GE Healthcare, Chicago, USA) followed by anion-exchange chromatography using a MonoQ column in 100–500 mM gradient of KCl (GE Healthcare, Chicago, USA). eIF4E was obtained from the eIF4F preparation also using a MonoQ column in 100–500 mM gradient of KCl. p86 was purified by affinity chromatography using a HisTrap HP column (GE Healthcare, Chicago, USA) followed by anion-exchange chromatography using a MonoS column in 100–500 mM gradient of KCl (GE Healthcare, Chicago, USA).
DNA fragments coding for truncated variants of the human eIF4G1 — p100 (701–1600 aa), MA3 domain (1234–1437 aa), and W2 domain (1437–1600 aa) were cloned to pGEX vector by BamHI/XhoI sites. All proteins were produced in E. coli strain Rosetta and subsequently purified via Glutathione–Sepharose with elution by PreScission protease (Cytiva, Marlborough, USA), followed by ion-exchange chromatography on HiTrap Q (GE Healthcare, Chicago, USA).
In vitro transcription of mRNA
Nanoluciferase (Nluc) poly (A) mRNA, containing 50-nt poly (A) tail, and nonadenylated Nluc mRNA, both containing UAA stop codon at the end of Nluc coding sequence, were in vitro transcribed using the T7 RiboMAX Express Large Scale RNA Production System (Promega, Madison, USA) from the Nluc template, obtained via polymerase chain reaction as described [65] and capped using Anti-Reverse Cap Analog (ARCA) (NEB, S1411) as described [47]. The MVHL mRNA was transcribed in vitro using T7 RNA polymerase from the plasmid, which contained a T7 promoter, four CAA repeats, the β-globin 5′ UTR, a short open reading frame coding for the peptide Met-Val-His-Leu (MVHL), followed by the UAA stop codon, and a 3′ UTR comprising the rest of the natural β-globin coding sequence. For run-off transcription, the MVHL-stop plasmid was linearized with XhoI endonuclease.
Termi-luc assay
Peptide release rate during translation termination was determined using Termi-luc assay as described previously [69] with minor modifications. To obtain preterminaton complexes (preTCs) at the Nluc mRNA, a reaction mixture containing 70% nuclease-treated RRL (Green Hectares, Oregon, USA) was supplemented with 20 mM HEPES–KOH (pH 7.5), 80 mM KOAc, 0.5 mM Mg(OAc)2, 0.36 mM ATP, 0.2 mM GTP, 0.05 mM each of 20 amino acids (Promega, Madison, USA), 0.5 mM spermidine, 5 ng/μl total rabbit tRNA, 10 mM creatine phosphate, 0.003 U/μl creatine kinase (Sigma–Aldrich, Burlington, USA), 1 mM DTT, and 0.2 U/μl RiboLock (Thermo Fisher Scientific, Waltham, USA) in a total volume of 200 μl. The mixture was preincubated with 2 μM eRF1 (AGQ) at 30°C for 10 min, followed by the addition of the NLuc mRNA to a final concentration of 8 μg/ml. Next, the mixture was incubated for 1 h to obtain the TCs with translated Nluc, and, after that, KOAc concentration was adjusted to 300 mM and the mixture was layered on 10%–35% linear sucrose density gradient (SDG) in a buffer containing 50 mM HEPES–KOH (pH 7.5), 7.5 mM Mg(OAc)2, 300 mM KOAc, and 1 mM DTT. The gradients were centrifuged in a SW55 Ti rotor (Beckman Coulter, Brea, USA) at 55 000 rpm for 65 min. Fractions enriched with preTCs were collected by optical density and peptide release activity in the presence of excessed amounts of eRFs. PreTC was aliquoted, flash-frozen in liquid nitrogen, and stored at −80°C.
The peptide release assay was performed in a solution containing preTC as described [47]: 20 mM Tris–HCl (pH 7.5), 0.25 mM spermidine, 100 mM KOAc, 1 mM DTT, 0.2 mM GTP, and 1% Nluc substrate in the presence of release factors (50 nM of eRF1 alone, 5 nM eRF1 with 5 nM eRF3a, or 2.5 nM eRF1 with 2.5 nM eRF3c, as indicated) and 2.5–100 nM protein of interest (eIF4F, eIF4G1, eIF4G2, eIF4A, eIF4E, eIF4B, p86, p100, p50, and MA3 or W2 domains of eIF4G1) or Glutathione S-transferase (GST), Y box-binding protein 1 (YB-1), eukaryotic elongation factor 1 (eEF1), and eIF5 as the negative controls. Luminescence was measured at 30°C using a Tecan Infinite 200 Pro (Tecan, Männedorf, Switzerland).
Analysis of formation of termination complexes by fluorescent toe-printing assay
PreTCs on MVHL were assembled as described previously [56, 70] and purified by centrifugation in a linear SDG [10%–30% (w/w), prepared in buffer containing 20 mM Tris–HCl (pH 7.5), 300 mM KCl, 1 mM DTT, and 5 mM MgCl2] for 75 min at 50 000 rpm and 4°C using a SW55 Ti rotor (Beckman Coulter, Brea, USA). Conformation rearrangements in the ribosome during TC formation were detected by a fluorescent toe-print assay as described [70, 71] with modifications. eIF4F, eIF4G1, or eIF4G2 (final concentrations 125 nM), combined with eRF1 (AGQ) and eRF3a (final concentrations 3 nM each) were added to preTC assembled on MVHL mRNA (final volume 10 μl) with 0.2 mM GTP. The mixture was incubated for 15 min at 37°C. Then 2 μl of a buffer [containing 2.5 mM of each dNTPs, 25 mM Tris–HCl (pH 7.5), 50 mM KAc, 2 mM DDT, and 42.5 mM MgCl2], 250 nM 5′-6-FAM-labeled primer 5′-GCATGTGCAGAGGACAGG-3′ (Syntol, Moscow, Russian Federation), and 0.625 U AMV reverse transcriptase (NEB, USA) were added. Reverse transcription was performed for 40 min at 37°C, then FAM-labeled cDNA products were purified by phenol–chloroform extraction. Obtained cDNAs were separated by capillary electrophoresis using standard GeneScan® conditions on an Applied Biosystems® Sanger Sequencing 3500xL Genetic Analyzers (Thermo Fisher Scientific, Waltham, USA).
GTPase assay
The 40S and 60S ribosomal subunits were associated at 37°C for 10 min, then were dissolved by a GTPase buffer [containing 10 mМ Tris–HCl (pH 8.0), 12 mM NH4Cl, 30 mM KCl, and 6 mM MgCl2] and 2 μM [γ – 32P]GTP with specific radioactive activity 2.16 μCu/pmol, to the final concentration 250 nM. Then eRF1 was added to the final concentration 166 nM with eIF4F/p50/eIF4G2 to the final concentration 78-312 nM. The reaction was started by addition of a GTPase eRF3a to 166 nM, followed by incubation at 37°C for 10 min. Then 500 μl of 5% charcoal in 50 mM Na2HPO4 were added to stop the reaction. 380 μl of liquid fraction after centrifugation at 12 000 × g was applied in Perkin-Elmer scintillator counter.
Ribosomal complexes binding assay
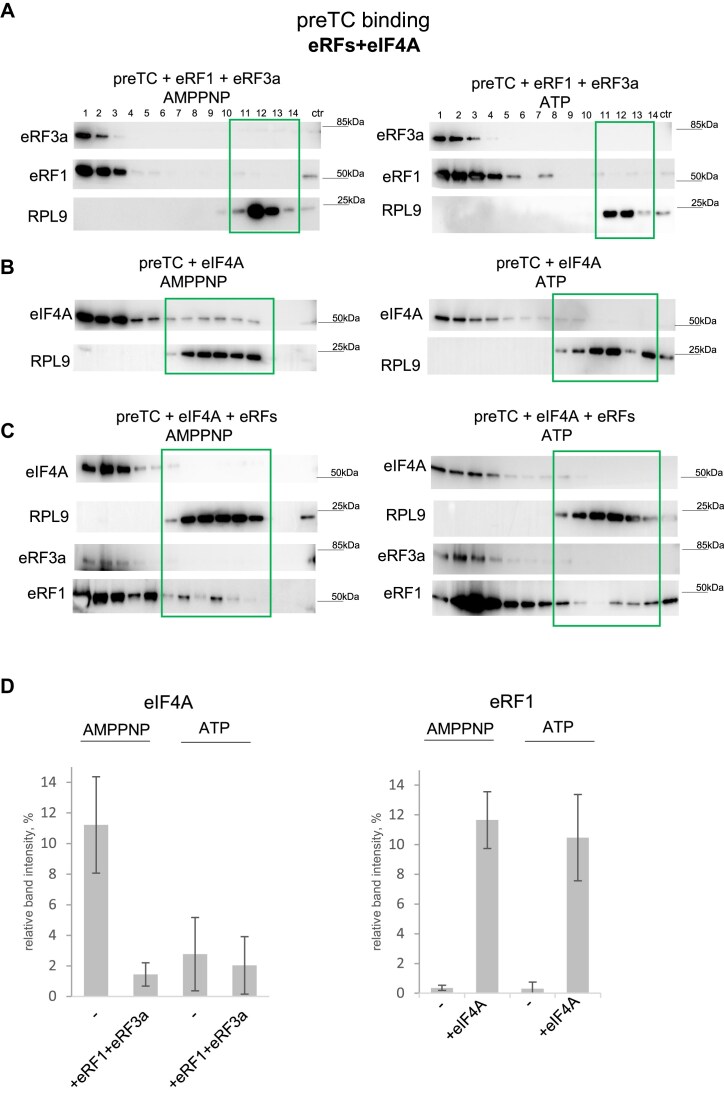
Purified preTCs with MVHL peptide were obtained, as described [61], and purified through linear SDG [10%–30% (w/w)] centrifugation using a SW55 Ti rotor (Beckman Coulter, Brea, USA) for 75 min at 50 000 rpm and 4°C in buffer containing either 20 mM Tris–HCl (pH 7.5), 300 mM KCl, 1 mM DTT, and 5 mM MgCl2. Purified preTCs were incubated with 10 pmol of eRF3a, 10 pmol of eRF1, and 10 pmol of p100 or eIF4A in a buffer containing 20 mM Tris–HCl (pH 7.0), 2.5 mM MgCl2, 150 mM KAc, 2 mM DTT, and 0.2 mM GTP supplemented with 0.2 mM MgCl2 at 37°C for 15 min in the volume of 100 μl. eIF4A was additionally incubated with 0.2 mM ATP, AMPNP, or without a nucleotide. The obtained complexes were centrifuged through a 10%–30% (w/w) linear SDG in a buffer containing 20 mM Tris–HCl (pH 7.5), 5 mM MgCl2, 150 mM KAc, and 1 mM DTT. The whole gradient was fractionated into 14 equal fractions (385 μl), followed by precipitation with 10% (v/v) trichloroacetic acid in the presence of 2% (v/v) casamino acids at 4°C overnight. The protein pellets were dried, dissolved in a loading buffer, and analyzed using western blotting with corresponding antibodies raised against eIF4G1 (Cell Signaling, Danvers, USA, 2858S), eIF4A (Cell Signaling, Danvers, USA, 2013S), eRF1 (Abcam, Cambridge, UK, 153731), eRF3 (Cell Signaling, Danvers, USA, 14980S), or RPL9 (Abcam, Cambridge, UK, 182556). The amount of eIF4A or eRF1 in preTC was normalized to the total amount of these proteins on each membrane.
Pull-down assay
HisSUMO-tagged eRF3a and eRF1 or His-tagged eIF4G1, eIF4A, and eIF4B were preincubated for 15 min at room temperature with Ni-NTA Sepharose (GE Healthcare, Chicago, USA) pre-equilibrated by wash buffer (WB; 25 mM Tris–HCl, 150 mM KCl, 10 mM imidazole) at a ratio of 20 pmol of protein per 1 μl of packed resin and washed once with WB. HisSUMO tag or His-Acetate Kinase (Ack) bound to resin were used as a negative control. After that, 1 μl of this resin was added to 20 pmol of the tested protein in a buffer containing 25 mM Tris–HCl (pH 7.5), 150 mM KCl, 2.5 mM MgCl2, 2 mM DTT, 0.25 mM spermidine, and 0.2 mM nucleotide (GDP/GTP/GDPCP) supplemented with MgCl2. The final volume was 20 μl. The mixture was incubated for 10 min at 37°C with constant shaking. After that, the resin was washed thrice with 500 μl of WB. The washed resin was incubated in WB with either 0.2 pmol Ulp1 protease (for His-tag removal from HisSUMO-eRF1 or HisSumo-eRF3a) in a volume of 20 μl, or 300 mM imidazole (for elution from Ni-NTA of His-eIF4G1, His-eIF4A, His-eIF4B, and His-Ack). After centrifugation, the supernatant was analysed in PAAG.
Quantification and statistical analysis
All experiments were carried out on at least three replicates (the exact number of replicates is indicated in the legends). The data are presented as mean ± standard deviation (SD), when analyzing luminescence signals, or mean ± standard error of mean (SE), when analyzing parameters (rate of peptide release). A two-tailed t-test was used to compare mean values between two groups. The Holm–Bonferroni method was used to counteract the problem of multiple comparisons.
Results
eIF4F promotes termination of translation
To investigate the activity of eIF4F in translation termination, we co-expressed all three subunits of human eIF4F (eIF4G1, eIF4A, and eIF4E) in a baculovirus expression system and purified the trimer by affinity and ion exchange chromatographies. The eIF4F preparation contained all the subunits and was free of human release factors eRF1 and eRF3a (Supplementary Fig. S1A). The functional activity in translation initiation of purified eIF4F was confirmed by the assembly of the 48S initiation complex in a reconstituted translation system (Supplementary Fig. S2).
First of all, we studied whether eIF4F affects peptidyl-tRNA hydrolysis induced by release factors. For this purpose, we employed the Termi–Luc assay, which allowed us to analyze the efficiency of release of the Nluc during termination of translation from preTC isolated from RRL [69]. PreTCs were assembled at uncapped but polyadenylated Nluc mRNA at the UAA stop codon and then purified by ultracentrifugation in the linear SDG. As we previously showed, such preTCs contain PABP associated with the poly (A) tail and ribosomal proteins but not initiation factors [47]. This allowed us to study the functions of eIF4F exclusively in translation termination. eIF4F was added to Nluc preTC in the presence of either eRF1 alone or eRF1 with eRF3a-GTP (Fig. 1A and B and Supplementary Fig. S1C). We have shown that eIF4F significantly (up to 3-fold) increased the rate of Nluc release from the ribosome in the presence of both eRF1 and eRF3a (we observed this effect even at a molar ratio of as low as 1:1 for eRFs to eIF4F) (Fig. 1A). eIF4F also stimulated translation termination in the presence of eRF1 alone, but to a smaller extent (Fig. 1B). An increase in peptide release was detected in the eRF1:eIF4F ratio of 1:2. In the absence of release factors, eIF4F did not induce peptidyl-tRNA hydrolysis on its own (Supplementary Fig. S1D). GST, RNA-binding YB-1, eEF1, eIF5 were used as a negative controls and had no significant positive effect on translation termination (Supplementary Fig. S1E–H). Collectively, these findings indicate that human eIF4F stimulates peptide release both in the presence of eRF1 alone and in the presence of the eRF1–eRF3 complex.
Figure 1.
Translation termination is stimulated by eIF4F. (A) Rate of peptide release (v0) at Nluc preTC induced by eRF1 and eRF3a (5 nM each) in the presence of various concentrations of eIF4F (left panel) and difference in peptide release rates at selected concentrations: eRFs 5 nM each and eIF4F 5 nM (1:1) (right panel). (B) Rate of peptide release (v0) at Nluc preTC induced by eRF1 (50 nM) in the presence of various concentrations of eIF4F (left panel) and difference in peptide release rates at selected concentrations: eRF1 50 nM and eIF4F 100 nM (1:2) (right panel). RLU, relative luminescent units. (C) Toe-print analysis of TC formation in the reconstituted mammalian translation system in the presence of eRF1 (AGQ) and eIF4F or eIF4G1. ru, relative units. (D) GTPase activity of eRF3a in the presence of various concentrations of eIF4F (left panel) and difference in the amount of the released 32Pi at selected concentrations: eRFs 166 nM each and eIF4F 312 nM (1:1.8) (right panel). The data are shown as the mean ± standard error, number of repeats, n = 3. Asterisks indicate statistically significant differences between the values (*, P < .05; **, P < .01).
Translation termination consists of three consecutive stages (see “Introduction” section). To further elucidate the specific stage at which eIF4F exerts its functions, we conducted experiments in a reconstituted in vitro translation system [56]. First, using a fluorescent toe-print assay [70, 71], we examined the effect of eIF4F on the stop codon recognition by eRF1. Toe-print analysis was performed using preTC assembled at MVHL-UAA mRNA, which was subsequently purified through equilibrium ultracentrifugation in a linear SDG under high ionic strength conditions. Centrifugation at high ionic strength was necessary to wash preTC from eIFs, since at least eIF3 remains associated with preTC assembled on short CDS in the reconstituted translation system. Increasing KCl concentration during centrifugation to 300 mM facilitated the separation of ribosomes from most eIFs [61].
After the accommodation of eRF1, the ribosome protects additional nucleotides on the mRNA, which can be detected in a toe-print assay as a 1–2 nucleotide “shift” of the ribosomal complex [56]. In this particular experiment, we used the mutant eRF1 (AGQ), which binds to the stop codon but is incapable of hydrolyzing peptidyl-tRNA [72]. Thus, we focused on stages 1 and 2 of translation termination, i.e. the binding of eRF1 to the stop codon and GTP hydrolysis by eRF3, excluding the stage of peptidyl-tRNA hydrolysis. The addition of eIF4F to the reaction significantly promoted preTC to TC transition in the presence of eRF1 (AGQ)–eRF3a–GTP (Fig. 1C). Thus, eIF4F promotes either stop codon recognition by eRF1, or GTP hydrolysis by eRF3, or both. Given that eIF4F can stimulate the activity of eRF1 even in the absence of eRF3a (Fig. 1B), it appears to stimulate at least the first step of translation termination which can be performed by eRF1 alone.
To address the possibility that eIF4F affects the second step of the termination, we studied whether eIF4F alters eRF3a GTPase activity in the presence of eRF1 and ribosomal subunits. Indeed, eIF4F stimulated the GTPase activity of eRF3a by 3.5-fold at 1:1.8 ratio (Fig. 1D). Thus, we can conclude that eIF4F stimulates translation termination both at the stage of eRF1 binding to the ribosome and stop codon recognition, and at the stage of GTP hydrolysis by eRF3a and further accommodation of the GGQ loop in the PTC. However, it cannot be excluded that stimulation of the second stage of termination by eIF4F may be indirect effect resulting from its enhancement of the first stage.
eIF4G1 and its truncated forms stimulate translation termination enhancing GTPase activity of eRF3
eIF4F consists of three subunits, i.e. eIF4G1, eIF4A, and eIF4E. The functional activity in translation initiation of reconstructed eIF4F from individual recombinant subunits was confirmed by the assembly of the 48S initiation complex at capped and uncapped mRNAs (Supplementary Fig. S2). To investigate the contributions of these subunits to translation termination, we assessed the activity of each subunit individually, along with the eIF4G1 homologue eIF4G2 and various truncated forms of eIF4G1 (Fig. 2A). eIF4G1 increased the rate of peptidyl-tRNA hydrolysis by 3.5-fold at a 1:10 ratio of eRFs to eIF4G1 (Fig. 2B and Supplementary Fig. S3A), while in the absence of release factors it did not induce this reaction (Supplementary Fig. S1D). Thus, individual eIF4G1 also stimulates translation termination, albeit 10 times less efficiently than the complete eIF4F complex. Furthermore, toe-printing also showed that, in the presence of eRF1 (AGQ), eIF4G1 stimulated TC formation to a much lesser extent compared with the full eIF4F complex (Fig. 1C). In addition, eIF4G1 failed to stimulate translation termination in the presence of eRF1 alone (Supplementary Fig. S3B). Therefore, eIF4G1 probably functions at the second stage of translation termination, in which GTP is hydrolyzed by eRF3. Interestingly, eIF4G1 also stimulated activity of the N-terminally truncated eRF3c, lacking the PABP-binding N-domain, and it was almost three-fold more active than full-length eRF3a in the presence of eIF4G1 (Supplementary Fig. S3C and Fig. 2B). Consequently, the activity of eIF4G1 in translation termination was partially suppressed by the N-domain of eRF3. Considering that PABP was present in our system associated with the poly (A) tail, it can be assumed that a complex network of protein–protein interactions governs the interplay among these three proteins in facilitating translation termination.
Figure 2.
eIF4G1 and its truncated forms stimulate peptide release and GTPase activity of eRF3. (A) Schematic representation of eIF4G1, eIF4G2, their truncated forms, and domain organization. Regions of interaction with other proteins are indicated. (B) Rates of peptide release (v0) at Nluc preTC induced by eRF1 and eRF3a (5 nM each) in the presence of various concentrations of eIF4G1, p100, and p50 (left panel) and difference in peptide release rates at selected concentrations: eRFs 5 nM each and eIF4G1/p100/p50 50 nM (1:10) (right panel). RLU, relative luminescent units. (C) GTPase activity of eRF3a in the presence of various concentrations of p50 (left panel) and difference in the amount of the released 32Pi at selected concentrations: eRFs 166 nM each and p50 312 nM (1:1.8) (right panel). The data are shown as the mean ± standard error, number of repeats, n = 3. Asterisks indicate statistically significant differences between the values (*, P < .05; **, P < .01).
Human eIF4G1 consists of five domains that coordinate the translation factors to effectively execute their functions. The spatial arrangement of these domains within eIF4G1, along with the proteins interacting with them, is shown in the scheme (Fig. 2A). To identify the region of eIF4G1 responsible for stimulation of translation termination, we generated truncated forms of the factor: p100, p50, MA3, and W2 (Fig. 2A). The activities of the resulting proteins were tested in the Termi–Luc assay. p100 or p50 stimulated translation termination, and p50 showed even higher activity in translation termination than the full-length eIF4G1 (Fig. 2B). Notably, p100 and p50 lack the PABP-binding region (Fig. 2A). In addition, the p50 variant lacks MA3 and W2 domains, either of which may also be involved in the partial suppression of the activity of full-length eIF4G1 in termination. Importantly, the individual MA3 and W2 domains failed to affect the translation termination (Supplementary Fig. S4A). In the presence of eRF1 alone, p100 showed some activity in translation termination, which distinguishes it from full-length eIF4G1, which is inactive under these conditions (Supplementary Fig. S4B).
The activity of p50 was evaluated in a GTPase assay. We found stimulation of eRF3a GTP hydrolase activity by p50, although weaker than by eIF4F (Figs 1D and 2C). Given that p50 comprises solely the MIF4G domain, we concluded that the GTPase-stimulating activity of eIF4G1 is confined to its MIF4G domain. Therefore, the active forms of eIF4G1 involved in translation termination invariably include the MIF4G domain, and it is the minimum fragment of eIF4G1 required to stimulate translation termination at the second stage—GTP hydrolysis and accommodation of the GGQ loop in the PTC. It is important to note that the N-terminus of eIF4G1, which interacts with PABP in the absence of the eRF3–PABP complex, increases the activity of the release factors in translation termination, and the C-terminus of eIF4G1 partially suppresses the activity of peptide release.
eIF4G2 stimulates translation termination more strongly than the p100 fragment of eIF4G1
Like eIF4G1, eIF4G2 contains MIF4G, MA3, and W2 domains, all of which share high similarity with those of eIF4G1 (Fig. 2A). We obtained recombinant eIF4G2, as well as its truncated form p86 (Fig. 2A), which is generated in cells as a result of proteolytic cleavage during apoptosis. By testing their activity in translation termination using the Termi–Luc assay, we found that full-length eIF4G2 significantly stimulated the activity of the release factors (Fig. 3A). The activity of p86 in peptide release was lower than that of eIF4G2, but still pronounced. eIF4G2 appears to be an analogue of the p100 fragment of eIF4G1 as these proteins contain a similar set of homologous domains. We compared the activities of eIF4G2 and p100 in translation termination directly (Fig. 3B). The activity of p100 was much lower than that of eIF4G2. Therefore, we can conclude that both proteins are able to activate translation termination, but eIF4G2 is more effective. Subsequent toe-printing and GTPase analyses (Fig. 3C and D) showed that eIF4G2, similarly to eIF4G1, operated at the stage preceding peptidyl-tRNA hydrolysis and stimulated the GTPase activity of eRF3a.
Figure 3.
eIF4G2 stimulates translation termination more strongly than the p100 fragment of eIF4G1. (A) Rates of peptide release (v0) at Nluc preTC induced by eRF1 and eRF3a (5 nM each) in the presence of various concentrations of eIF4G2 and p86 (left panel) and difference in peptide release rates at selected concentrations: eRFs 5 nM each, and eIF4G1/p86 50 nM (1:10) (right panel). RLU, relative luminescent units. (B) Nluc release induced by eRF1 and eRF3a in the presence of eIF4G2 and p100 at selected concentrations: eRFs 5 nM each and eIF4G2 and p100 50 nM (1:10) (left panel) and difference in peptide release rates (right panel). RLU, relative luminescent units. (C) Toe-print analysis of TC formation in the reconstituted mammalian translation system in the presence of eRF1 (AGQ) and eIF4G2. ru, relative units. (D) GTPase activity of eRF3a in the presence of various concentrations of eIF4G2 (left panel) and difference in the amount of the released 32Pi at selected concentrations: eRFs 166 nM each and eIF4G2 312 nM (1:1.8) (right panel). The data are shown as the mean ± standard error, number of repeats, n = 3. Asterisks indicate statistically significant differences between the values (*, P < .05; **, P < .01).
eIF4A stimulates stop codon recognition by eRF1
The eIF4F complex stimulates translation termination much more efficiently than its core subunit, eIF4G1 (Figs 1A and 2B). In addition, eIF4G1 only operates in stage 2 of translation termination (Supplementary Fig. S3B), while eIF4F is active in both stages (stage 1 and 2; Fig. 1). This suggests that eIF4A and/or eIF4E could also be involved in translation termination. To study their contribution to translation termination, we obtained recombinant eIF4A and eIF4E. The functional activity in translation initiation of purified eIF4A and eIF4E was confirmed by the assembly of the 48S initiation complex in reconstituted translation system at capped mRNA (Supplementary Fig. S2). The ATP-dependent RNA-helicase activity of eIF4A preparation was confirmed through ATPase assay (Supplementary Fig. S5). Cap-dependence of the 48S complex assembly in the presence of recombinant eIF4E was confirmed by the assembly at uncapped mRNA (Supplementary Fig. S2). We then assessed the activities of these proteins in the peptidyl-tRNA hydrolysis reaction in the absence of eIF4G1 using the Termi–Luc assay, using ADP, ATP, or its nonhydrolysable analogue AMPPNP in the reaction. In the absence of ATP, eIF4A significantly enhanced the release of Nluc from the ribosome in the presence of both release factors, achieving a 12-fold increase in the rate of peptidyl-tRNA hydrolysis at a 1:10 ratio (Fig. 4A and Supplementary Fig. S6A). However, the activity of eIF4A in the presence of both release factors at a 1:1 ratio achieving only a 1.5-fold increase in the rate of peptidyl-tRNA hydrolysis. This is lower than the activity of the entire eIF4F complex in the same conditions (Fig. 1B). Interestingly, eIF4A exhibit a similar stimulating activity in the presence of AMPPNP (Fig. 4A and Supplementary Fig. S6B), while in the presence of ATP or ADP the factor stimulated termination only half as efficient (Fig. 4A and Supplementary Fig. S6A). We concluded that during the assay, ATP may have been hydrolyzed to ADP on the ribosome, which decreased the activity of eIF4A. Therefore, eIF4A promotes translation termination independently of eIF4G1, operating in either an ATP-bound or free form, i.e. in its closed conformation. Conversely, the open conformation of eIF4A-ADP form resulting from ATP hydrolysis or ADP binding is less effective in translation termination.
Figure 4.
eIF4A, eIF4E, and eIF4B activities in translation termination. (A) Rates of peptide release (v0) at Nluc preTC induced by eRF1 and eRF3a (5 nM each) in the presence of various concentrations of eIF4A (left panel). eIF4A was pre-incubated with ATP, ADP, AMPPNP, or without a nucleotide. The right panel shows difference in peptide release rates at selected concentrations: eRFs 5 nM each and eIF4A 50 nM (1:10). (B) Rate of peptide release (v0) at Nluc preTC induced by eRF1 (50 nM) in the presence of various concentrations of eIF4A (left panel) and difference in peptide release rates at selected concentrations: eRF1 50 nM and eIF4A 75 nM (1:1.5) (right panel). (C) Rate of peptide release (v0) at Nluc preTC induced by eRF1 and eRF3a (5 nM each) in the presence of various concentrations of eIF4E (left panel) and difference in peptide release rates at selected concentrations: eRFs 5 nM each and eIF4E 50 nM (1:10) (right panel). (D) Rate of peptide release (v0) at Nluc preTC induced by eRF1 and eRF3a (5 nM) in the presence of various concentrations of eIF4B (left panel) and difference in peptide release rates at selected concentrations: eRFs 5 nM each and eIF4B 25 nM (1:5) (right panel). RLU, relative luminescent units. The data are shown as the mean ± standard error, number of repeats, n = 3. Asterisks indicate statistically significant differences between the values (*, P < .05; **, P < .01; n.s., not significant).
In the presence of eRF1 alone, eIF4A, unlike eIF4G1, stimulated peptide release to a degree similar to the complete eIF4F complex, indicating its contribution to the overall eIF4F activity (Fig. 4B and Supplementary Fig. S6C). Since eIF4A activated eRF1 in the absence of eRF3, it is likely that it stimulates the step of translation termination associated with the binding of eRF1 to the stop codon.
We further evaluated eIF4A activity in the presence of the truncated version of the release factor eRF3—eRF3c (Supplementary Figs S6D and S7A). We found that eIF4A enhances the efficiency of peptide release to a similar extent as observed in the presence of the full-length eRF3a, suggesting that the activity of eIF4A is independent of eRF3 binding to PABP.
The activity of the remaining subunit of the eIF4F complex, eIF4E, in promoting peptide release was also examined across various concentrations. However, no significant effect was observed (Fig. 4C and Supplementary Fig. S8A). Therefore, eIF4E is unlikely to contribute to the stimulating activity of the eIF4F complex in translation termination.
In summary, our results indicate that two of the three subunits of eIF4F are involved in translation termination, albeit at different steps: eIF4A promotes stop codon recognition by eRF1, while eIF4G1 promotes GTP hydrolysis by eRF3a and the accommodation of the GGQ loop in the PTC.
eIF4B stimulates translation termination
The activity of eIF4F in translation initiation, particularly that of its subunit eIF4A, is stimulated by eIF4B. It was shown that this factor interacts with both eIF4A [73–76], and PABP [25–28]. It was demonstrated that eIF4B and eIF4G1 cooperate to enhance the ATPase activity of eIF4A (reviewed in [10, 13]). Thus, eIF4B associates with the proteins bound to both the 5′ and 3′ ends of mRNA and may also play a role in translation termination at the closed-loop mRNA structure.
The functional activity of purified eIF4B in translation initiation was confirmed through the assembly of the 48S initiation complex in reconstituted translation system (Supplementary Fig. S2). Then, we evaluated the activity of purified eIF4B in the peptide release reaction in the presence of eRF1, eRF3a, and PABP (Fig. 4D and Supplementary Fig. S8B). Remarkably, eIF4B exhibited a pronounced stimulatory effect on translation termination, resulting in enhancements exceeding an order of magnitude. Notably, the saturating concentrations of eIF4B required for this effect were lower compared with other individual initiation factors. Specifically, at an eRF–to–eIF4B ratio of 1:5, eIF4B increased the rate of peptidyl-tRNA hydrolysis by 12-fold (Fig. 4D). When eRF3a was substituted for eRF3c, the rate of translation termination decreased by approximately two-fold (Supplementary Figs S7B and S8C). This suggests that the interaction between eRF3 and PABP may be important for the activity of eIF4B in translation termination. Additionally, we found that eIF4B stimulated translation termination in the presence of eRF1 alone, achieving levels comparable to those observed with eIF4A (Supplementary Figs S7C and S8D). This indicates that eIF4B may participate in translation termination at the stage of stop codon recognition. However, since its activity significantly increases in the presence of eRF3a, we suppose that it may also be involved in subsequent stages of termination or contribute to overall stability and conformational dynamics of the termination complex.
eIF4F subunits increase each other’s activity during translation termination
To investigate a mutual influence of eIF4F subunits during translation termination, we performed experiments to assess the stimulation of peptidyl-tRNA hydrolysis by various combinations of these proteins, both in the absence and presence of ATP (Fig. 5). We found that the activity of the combination of eIF4G1 and eIF4A was significantly higher than their individual activities. On the contrary, the addition of eIF4E to the mixture of eIF4G1 and eIF4A resulted in a reduction of the activity (Fig. 5A). This suggests that eIF4E, through its interaction with the capped mRNA, may shift eIF4F activity from termination to the initiation of translation. Notably, as the functional activity of recombinant eIF4E was confirmed in translation initiation (Supplementary Fig. S2), we assume that inhibitory effect of eIF4E is detected only in translation termination. In the presence of ATP, the coactivation of eIF4G1 and eIF4A was markedly reduced (Fig. 5B), which aligns well with the data obtained earlier for eIF4A in the presence of ATP (Fig. 4A).
Figure 5.
Influence of reconstituted eIF4F on translation termination. Relative peptide release rates (v0) at Nluc preTC induced by eRFs (8 nM each) in the presence of different combinations of eIF4F complex components eIF4G1, eIF4A, and eIF4E (35 nM each) (A) in the absence of ATP and (B) in the presence of ATP. p100, eIF4A, and eIF4E (20 nM each) (C) in the absence of ATP and (D) in the presence of ATP. (E) Relative peptide release rates (v0) at Nluc preTC induced by eRFs (8 nM each) in the absence and presence of eIF4B (15 nM) and eIF4G1 (35 nM)/eIF4F (5 nM). The data are shown as the mean ± standard error, number of repeats, n = 3. Asterisks indicate statistically significant differences between the values (*, P < .05; **, P < .01).
To further elucidate the influence of distinct regions of eIF4G1 on the activity of reconstituted eIF4F in translation termination, we compared the results obtained for eIF4G1 with those for p100. This comparison revealed no synergistic interaction between p100 and eIF4A in translation termination (Fig. 5C and D). Although p100 contains eIF4A-binding domains, its conformation appears to preclude eIF4A from exerting a synergistic effect within the complex. Furthermore, the addition of eIF4E, which lacks a binding site for p100, slightly diminished its stimulatory activity both in the presence and absence of eIF4A (Fig. 5C), suggesting some slight nonspecific inhibitory effect of eIF4E on translation termination in this context.
Additionally, we investigated the potential mutual influence of the factors eIF4F/eIF4G1 and eIF4B during translation termination. Combinations of these factors exhibited greater activity than the individual proteins, indicating probable additive effects (Fig. 5E). This suggests that the mechanisms by which eIF4F and eIF4B stimulate translation termination operate independently. Interestingly, eIF4B equally well stimulated both the eIF4F ternary complex and eIF4G1 alone.
In conclusion, our findings indicate that the eIF4G1–eIF4A–eIF4B complex, or their combination, is more active in translation termination than its individual components, with the N-terminal region of eIF4G1 being essential for it to function full-throttle. This region is capable of binding both PABP and eIF4E, but inasmuch as eIF4E does not promote the activity of the eIF4G1–eIF4A complex, we tend to think that it is PABP, associated with the poly (A) tail of the mRNA, that plays a crucial role in the eIF4F-mediated enhancement of translation termination. Furthermore, as eIF4B can also bind to PABP, it is possible that the co-stimulation by eIF4G1–eIF4B may be mediated by the poly (A) tail as well.
Poly (A) tail of mRNA increases stimulation of translation termination by eIF4G1, eIF4A, and eIF4B
To assess the effect of PABP bound in cis to the poly (A) tail on the activities of eIF4G1, eIF4A, and eIF4B during translation termination, we assembled preTCs in RRL on both polyadenylated and nonpolyadenylated mRNAs, as well as capped and uncapped mRNAs, and purified them through the linear SDG centrifugation. We have previously shown that PABP remains associated with preTC assembled on polyadenylated mRNA, and PABP is not detected in preTC without a poly (A) tail [47]. At a ratio of 2:5 to eRFs, eIF4F effectively stimulated peptide release across all tested mRNAs (Supplementary Fig. S9A–D), exhibiting slight variations among the four conditions, ranging from 1.5- to 3-fold (Supplementary Fig. S9E). Therefore, eIF4F is capable of activating translation termination in both the presence and absence of PABP.
We further assayed the potential dependence of the activity of eIF4B and the individual subunits of eIF4F on the presence of a poly (A) tail in translation termination (Fig. 6). Each of the three factors — eIF4G1, eIF4A, and eIF4B — exhibited enhanced stimulation of translation termination in the presence of the poly (A) tail, while, eIF4E, as expected, showed no effect on peptide release regardless of the presence of the poly (A) (Fig. 6). The effect of the poly (A) tail on the activity of eIF4B and eIF4G1 can be attributed to their direct interactions with PABP. In contrast, eIF4A, which does not directly interact with PABP, may exert its poly (A) tail-dependent stimulatory activity through a complex network of interactions involving PABP, eRF3a, and eRF1. As we have shown above, eIF4A promotes the ribosome loading of eRF1, which also depends on eRF3a and PABP.
Figure 6.
Effect of poly (A) tail, bound with PABP, on the activity of eIF4F components in translation termination. (A) The rates of peptide release (v0) at preTCs assembled on Nluc mRNA without poly (A) tail or with poly (A) tail. Peptide release was induced by eRF1 and eRF3a (8 nM each) in the presence of various concentrations of eIF4G1, eIF4A, eIF4E, and eIF4B. (B) Differences in peptide release rates at selected concentrations: eRFs 8 nM each, eIF4G1, eIF4A, eIF4E, and eIF4B 50 nM (1:6). RLU, relative luminescent units. The data are shown as the mean ± standard error, number of repeats, n = 3. Asterisks indicate statistically significant differences between the values (*, P < .05; **, P < .01; n.s., not significant).
The results presented here clearly demonstrate that while PABP enhances the function of eIF4F and eIF4B in promoting translation termination, it is not an obligatory factor in this process.
eIF4A promotes the loading of eRF1 on the ribosome, while eIF4G1 enhances the dissociation of eRF1–eRF3a from the ribosome
The involvement of the eIF4F complex in translation termination implies that its subunits may physically interact with release factors or the terminating ribosome, thereby stimulating their activities. To elucidate how initiation factors participate in translation termination, we examined if these proteins bind to preTC or the release factors.
Since the interaction of the eIF4F complex with the terminating ribosomes has not been studied previously in a reconstituted system, we conducted a series of experiments to assess the binding of eIF4A, eIF4G1, and p100 to preTCs. For this purpose, preTCs were assembled on the MVHL-UAA mRNA in the reconstituted mammalian translation system and subsequently purified using equilibrium ultracentrifugation in a linear SDG under high ionic strength conditions. The purified preTCs were then incubated with eIF4F subunits, followed by a second round of centrifugation at 150 mM KCl. Proteins from the gradient fractions were precipitated and analyzed via western blotting using specific antibodies.
In the presence of GTP, the release factors were barely detectable in the ribosomal fractions. This observation suggests that following GTP hydrolysis, they likely dissociate from the ribosomes during the second round of ultracentrifugation, as no cross-linking agents were used to stabilize protein interactions in these experiments (Fig. 7A and D). However, eIF4A was found in preTC when AMPPNP was included, but was nearly undetectable when ATP was present (Fig. 7B and D). This suggests that eIF4A interacts with preTC in the ATP/AMPPNP-bound form, while subsequent ATP hydrolysis and conformational change of eIF4A-ADP weaken the interaction with the ribosome promoting eIF4A-ADP dissociation during sucrose gradient centrifugation. When both eRFs and eIF4A were added to preTC, we observed an increase in the amount of eRF1 associated with the ribosomal complex in the presence of both ATP and AMPPNP (Fig. 7C and D). Importantly, in the presence of eRFs, eIF4A dissociated from the ribosome even in the presence of AMPPNP (Fig. 7C and D), which confirms the authentic binding of eIF4A to preTC. Notably, eRF3a was not detected in the ribosomal complex even in the presence of eIF4A. We suppose that eIF4A binding to a preTC in an ATP-bound state promotes the loading of eRF1 into the ribosome, after which eIF4A hydrolyzes ATP and dissociates from the ribosome in an ADP-bound form. Control experiments without ribosomes ruled out potential oligomerization of the proteins under the reaction and centrifugation conditions (Supplementary Fig. S10A).
Figure 7.
eIF4A promotes the loading of eRF1 to the ribosome. (A) Western blot analysis of SDG fractions obtained after incubation of preTC with eRF1 and eRF3a in the presence of ATP or AMPPNP. (B) Western blot analysis of SDG fractions obtained after incubation of preTC with eIF4A in the presence of ATP or AMPPNP. (C) Western blot analysis of SDG fractions obtained after incubation of preTC with eRFs and eIF4A in the presence of ATP or AMPPNP. Fraction numbering: from 1 to 14, from top to bottom of SDG. PreTC-associated fractions are marked with frames. ctr - control recombinant protein. Antibodies raised against the proteins of interest were used. (D) Intensities of eIF4A (left panel) or eRF1 (right panel) bands in preTC-bound fractions relative to total eIF4A or eRF1 on each membrane. Number of repeats: n = 2 (for AMPNP), n = 3 (for ATP).
Since individual eIF4A and eIF4G1 both are able to independently operate in translation termination, we also performed experiments on the binding of eIF4G1 or p100 to the purified preTCs or TCs. We found that eIF4G1 is unstable under these conditions: it likely aggregated during the incubation and then precipitated, as confirmed by control ultracentrifugation of eIF4G1 without ribosomal complexes. p100, on the other hand, did not bind stably to preTC. However, we were able to detect small quantities of release factors associated with the ribosomes, which allowed us to ascertain that p100 promotes the dissociation of the eRF1–eRF3a complex from the ribosome in the presence of GTP, while not affecting the dissociation of individual eRF1 (Supplementary Fig. S10B). In the presence of a nonhydrolysable analogue of GTP, GDPCP, no binding of the release factors to the ribosome could be detected. Thus, we have shown that p100 promotes the dissociation of release factors from the ribosome after GTP hydrolysis, acting specifically in the presence of eRF3a. This is consistent with our previous observations of the stimulatory activity of eIF4G1 in the second stage of translation termination during GTP hydrolysis by eRF3a (see Fig. 2).
Release factors are unable to bind eIF4F or eIF4B in solution
Next, we tested binding of the release factors to His-tagged eIF4A, eIF4E, eIF4B, and eIF4G1 domains (p100, p50, and MA3). The release factors without the His-tag were mixed with the proteins of interest, which were pre-bound to an affinity resin. After incubation and washes, bound complexes were eluted with imidazole and analyzed by western blotting with antibodies against release factors or His-tag. The acetate kinase from E. coli, which also carried His-tag, was used as a negative control. Neither eIF4A nor eIF4B interacted with eRF1 and eRF3a in solution, since the amounts of factors eluted from the putative complexes were comparable to those in the negative control (Supplementary Fig. S11A). Thus, we could only determine the nonspecific binding of eRFs to the resin.
To evaluate the binding interactions between eIF4E, eIF4G1, and its domains with release factors eRF1 and eRF3a, we conducted a series of co-precipitation assays using recombinant His-SUMO-tagged release factors and His-tagged eIF4G1. Our results demonstrated that neither eIF4E, nor full-length eIF4G1, nor its individual domains exhibited binding to the release factors in solution (Supplementary Figs S11B and C and S12). Importantly, we found no detectable interactions between eIF4G1 domains and eRF3a, even in the presence of GTP, GDP, or GDPCP in the reaction mixtures. These findings suggest that the initiation factors investigated do not interact directly with the release factors in solution. Rather, it appears that their role in translation termination is mediated through modulation of the release factors’ activity on the ribosome.
Discussion
Upon the formation of the mRNA closed-loop structure, the cap-binding initiation complex located at the mRNA 5′ end is brought into close proximity with the termination complex due to the simultaneous interaction of PABP, which is bound to the poly (A) tail of mRNA, with initiation factor eIF4F and release factors. In this study, we explored the role of eukaryotic translation initiation factors, particularly eIF4F, in the process of translation termination in mammals. The results regarding the activities of different initiation factors and their truncated forms at various stages of translation termination are summarized in Table 1.
Table 1.
Activities of eIFs in different stages of translation termination
| eIF | Forms | Peptide release | TC complex formation | GTPase activity | ||
|---|---|---|---|---|---|---|
| eRF1 + eRF3a | eRF1 | eRF1 + eRF3c | ||||
| eIF4F | +++ | ++ | +++ | ++ | ||
| eIF4G1 | + | − | + | + | ++ | |
| P100 | + | + | ||||
| P50 | + | |||||
| MA3 | − | |||||
| W2 | − | |||||
| eIF4G2 | +++ | ++ | ++ | |||
| P86 | ++ | |||||
| eIF4A | ++ | ++ | ++ | |||
| AMPPNP | ++ | |||||
| ATP | + | |||||
| ADP | + | |||||
| eIF4E | − | |||||
| eIF4B | +++ | ++ | ++ | |||
+++, high activity; ++, medium activity; +, low activity; −, inactive.
Our findings indicate that eIF4F effectively promotes translation termination. We found that individual subunits of the eIF4F complex stimulate the activity of different release factors: eIF4A promotes loading of eRF1 into the ribosome, while eIF4G1 enhances the GTPase activity of eRF3a. Notably, our data demonstrate that both eIF4G1 and eIF4G2 exhibit this activity in translation termination, even despite eIF4G2 does not bind PABP. Importantly, we identified MIF4G domain as the minimal functional unit capable of stimulating translation termination. Furthermore, our findings extend to eIF4B, another initiation factor associated with PABP, which also displays a stimulatory effect on translation termination.
It has previously been shown that the helicase involved in the transport of mRNA from the nucleus, DDX19 in human (Dbp5 in yeast), is also involved in translation termination [59, 60], facilitating the loading of eRF1 into the ribosome [60]. Notably, DDX19/Dbp5 shares structural similarities with eIF4A. In its ATP-bound form, DDX19 interacts with preTC and, likely by changing the conformation of the A site, enhances the binding of eRF1 to the stop codon. Following ATP hydrolysis, DDX19 subsequently dissociates from the ribosome [60]. In this study, we show a similar stimulatory effect on termination by eIF4A (Figs 4 and 7), which implies that the two proteins may perform analogous functions in this process.
Other proteins that share structural homology with eIF4G1, which have a hand both in translation initiation and termination, include PDCD4 and PAIP1. Tumor suppressor PDCD4 contains two MA3 [77] domains, which bind to eIF4A, and the N-terminal domain of this protein interacts with PABP [78]. We showed previously that PDCD4 binds to preTC and stimulates translation termination via promoting the GTPase activity of eRF3 and its subsequent dissociation from the ribosome [64]. Therefore, PDCD4 may serve as a functional analogue of eIF4G1 in the context of translation termination, with the potential to displace it from the termination complex through interactions with preTC and PABP. PAIP1 contains a MIF4G domain homologous to that of eIF4G1 [66], and this protein, as its name implies, also binds to PABP. PAIP1 facilitates the formation of termination complexes in the presence of eRF3 and binds to the eRF3–eRF1 complex but does not stimulate the hydrolysis of peptidyl-tRNA and inhibits the activity of PABP in translation termination [66]. We proposed that this protein can reduce the efficiency of translation termination via binding with PABP in solution. Therefore, while the MIF4G domain of PAIP1 retains some functional properties of its eIF4G1 counterpart, it lacks the capacity to stimulate the GTPase activity of eRF3. This feature allows PAIP1 to function as an inhibitor of translation termination in some instances, e.g. at premature termination codons, or under some conditions. Collectively, these data suggest that the mechanism we have described for the stimulation of translation termination by eIF4F is fundamentally conserved and is frequently used by the cell as a target for regulating various processes, including readthrough of premature termination codons, translation pausing, and the induction of apoptosis under stress.
The eukaryotic initiation factor eIF4B interacts with eIF4A, as well as with the ribosome, to promote translation initiation [10, 13, 73–76, 79]. However, in yeast, deletion of eIF4B reduces the relative translation efficiencies of many more mRNAs than does inactivation of eIF4A [17]. This might be related to the activity of eIF4B in translation termination, as we have shown in this study (Fig. 4D). According to recent structural data [75], eIF4B is bound in the vicinity of the mRNA entry channel and thus unlikely can interact with release factors directly. However, it may contribute to translation termination through enhancing the eIF4A activity and by stabilizing the termination complex indirectly by changing the conformation of the 40S subunit.
The minimal domain of eIF4G1 that is active in translation termination, MIF4G (Fig. 2B), is capable of interacting with eIF4A, eIF3, and RNA. eIF4G2, which also promotes the termination (Fig. 3), binds eIF4A and, likely, eIF3, but not eIF4E or PABP. In addition, eIF4G1 and eIF4A stimulate translation termination in the presence of eRF3c, which is unable to bind to PABP (Supplementary Figs S3C and S7A). Therefore, the presence of PABP and eIF4E is not required for the eIF4F complex to promote translation termination. However, we also showed that, unlike the full-length eIF4G1, p100 does not increase the activity of eIF4A in termination (Fig. 5A and C). This means that the N-terminal region of eIF4G1 is important for efficient activation of translation termination. In addition, the presence of a poly (A) tail increases the activities of all tested eIFs in termination (Fig. 6). These observations point to a more intricate regulatory mechanism governing translation termination, which encompasses the individual contributions of eIF4A and eIF4G1, as well as their interactions with one another. Importantly, these interactions are likely mediated by the poly (A) tail-bound PABP and the ribosome, suggesting a multifaceted framework for the regulation of translation termination.
Based on the obtained data, we propose a comprehensive model for the eIF4F and eIF4B function in translation termination (Fig. 8). In this model, PABP, which is bound to the poly (A) tail of the mRNA and may be represented by multiple molecules, serves as the platform for the assembly of several factors involved in translation termination. eIF4F and eIF4B associate with PABP molecule(s), resulting in the formation of a closed-loop mRNA structure. The eRF1–eRF3–GTP complex then associates with either the same or a neighboring PABP molecule on the poly (A) tail. As the elongating ribosome encounters the stop codon and stalls while waiting for eRF1, eIF4A and eIF4B from the nearby PABP–eIF4F complex bind to preTC and induce its conformational changes. PABP promotes the binding of eRF3-GTP to preTC, while ATP-bound eIF4A accelerates the loading of eRF1 into the A site. eIF4B stimulates these processes and stabilizes the optimal ribosome conformation. The MIF4G domain of eIF4G1 enhances the GTPase activity of eRF3a on the ribosome. As a result, the release factor complex undergoes conformational changes, accommodating the GGQ loop of eRF1 in the PTC. This induces the hydrolysis of peptidyl-tRNA and the release of the synthesized peptide from the ribosome. Subsequently, eIF4G1 promotes the dissociation of release factors from the ribosome, while eIF4A hydrolyzes ATP and also dissociates. Notably, eIF4A is the most abundant eukaryotic translation initiation factor, suggesting that it may participate in processes beyond initiation. It is possible that eIF4F subunits reassemble into a complex on PABP, enabling their further participation in both translation termination and initiation. After translation termination, eRFs may also bind to PABP awaiting the next ribosome to arrive at the stop codon. It is also important to note here that cap-bound eIF4E in the initiation complex appears to switch the activity of the other two eIF4F subunits between participation in initiation and termination.
Figure 8.
The model of eIF4F functioning in translation termination. The figure is created in BioRender. Alkalaeva, E. (2025) https://BioRender.com/p54r922.
In addition to the eIF4F molecules recruited to the termination complex by the poly (A)-bound PABP, translation termination may also be affected by eIF4F present in the solution. However, PABP facilitates the interaction of all the participants at once by bringing initiation factors closer to the terminating ribosome, thereby increasing the likelihood of rapid translation termination (Fig. 6). In addition, data regarding the activity of truncated forms of eIF4G1 and eRF3 (Fig. 2, S3) indicate that these proteins compete for interaction with PABP with their N domains, although they bind to different regions of PABP (Fig. 2A). Thus, the activity of full-length eIF4G1 is diminished compared with that of p100, as well as the activity of eRF3a is reduced compared with that of eRF3c. This competition was not observed in the presence of eIF4A as a part of the eIF4F complex, likely due to a conformational change in eIF4G1. Similarly, the high activity of eIF4G2 in translation termination may result from a lack of competition with eRF3a for binding to PABP, as eIF4G2 does not have a PABP binding site (Fig. 3).
eIF4G1 has long been implicated in reinitiation on mRNA with uORFs [80, 81]. Both eIF4G1 and eIF4A are present in elongating and terminating ribosomes on reinitiation-permissive uORFs in yeast [82], while eIF4G1 is retained on elongating ribosomes in human [83]. Considering that eIF4G2 can substitute for eIF4G1 and that it is involved in scanning and reinitiation after translation of uORFs [36, 37], we hypothesize that eIF4G2 may also directly participate in translation termination at the uORFs’ stops. eIF4G2 interacts with eIF4A [84] and thus may form an eIF4F-like complex capable of affecting translation termination. In line with this notion, the mutant eRF1 (AGQ) suppressed eIF4G2-dependent translation of both uORF and subsequent CDS [37]. The binding of eIF4G2 to the terminating ribosome is probably enhanced by the W2 domain, since the truncated form of this protein demonstrates a two-fold reduction in stimulation of translation termination compared with the full-length protein (Fig. 3A). Collectively, our data suggest eIF4G1 and eIF4G2 not only facilitate the resumption of ribosome scanning, but also promote efficient termination, which may be a prerequisite for efficient reinitiation.
Apart from viral infection, eIF4G1 and eIF4G3 are also cleaved by caspase-3 to a 76-kDa fragment (M-FAG) that lacks PABP-binding site and MA3-W2 domains (reviewed in [85]), and eIF4G2 is also cleaved by caspase-3 to remove its W2 domain [86]. The differential activities of eIF4G1 fragments described here suggest that apoptotic and viral reprogramming of the translational machinery may not be limited to translation initiation, but may affect translation termination as well.
Finally, effective translation termination is also a critical issue for mRNA stability, particularly in preventing nonsense-mediated decay (NMD) [87]. The role of PABP in inhibiting NMD is well-established (reviewed in [88, 89]). Notably, some studies have also highlighted the significant involvement of eIF4G in regulating this process. A series of investigations conducted by the Romão group demonstrated that mRNAs harboring premature termination codons in close proximity to the translation initiation codon can evade NMD through a mechanism that involves the interaction of PABP with the translation initiation complex (see [90] and references therein). This finding aligns with the established notion that NMD majorly tolerates translation termination at the stop codon of uORFs, despite the fact that such stop codons may be recognized as premature termination codons according to NMD principles. Furthermore, in 2014, two independent research groups reported that the interaction between PABP and eIF4G is essential for suppressing NMD, and that the direct tethering of eIF4G to an NMD reporter is sufficient to antagonize NMD factor activity [91, 92]. The authors proposed that eIF4G may play a role in both translation termination and NMD suppression. Additionally, eIF4B was recently shown to interact with UPF1, the key NMD factor [93]. We suggest that it is the close proximity to initiation complex that allows both authentic CDS stop codons and uORF ones to evade NMD.
In summary, our results suggest that during the formation of the closed-loop mRNA structure, the translation rate increases not only due to stimulation of initiation but also due to stimulation of termination, with the factors that form the closed-loop structure, eIF4F and PABP, playing critical roles in both processes.
Supplementary Material
Acknowledgements
We are grateful to Ludmila Frolova for providing us with plasmids encoding eRF1, eRF1 (AGQ), and eRF3c, to Tatyana Pestova and Christopher Hellen who provided us with plasmids encoding translation initiation factors, to Boris Eliseev and Christian Schaffitzel who provided us with plasmid encoding eRF3a and offered consulting and assistance with the expression of eIF4F, eIF4G, and eRF3a in baculovirus expression system, and to Dmitry Lyabin who provided us with YB-1. Sequencing of plasmids and cDNA fragment analyses were performed by the center of the collective use “Genome” of EIMB RAS. We are thankful to the Center for Precision Genome Editing and Genetic Technologies for Biomedicine for access to the facilities necessary for this study. The graphical abstract is created in BioRender. Alkalaeva, E. (2025) https://BioRender.com/l62j090.
Author contributions: E.S., A.S., W.A., A.I., N.B., and T.E. conducted the experiments; E.A. designed the experiments; and E.A, S.D., and I.T. wrote the paper.
Contributor Information
Ekaterina Shuvalova, Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, 119991 Moscow, Russia; Center for Precision Genome Editing and Genetic Technologies for Biomedicine, Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, 119991 Moscow, Russia.
Alexey Shuvalov, Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, 119991 Moscow, Russia; Center for Precision Genome Editing and Genetic Technologies for Biomedicine, Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, 119991 Moscow, Russia.
Walaa Al Sheikh, Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, 119991 Moscow, Russia.
Alexander V Ivanov, Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, 119991 Moscow, Russia.
Nikita Biziaev, Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, 119991 Moscow, Russia.
Tatiana V Egorova, Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, 119991 Moscow, Russia.
Sergey E Dmitriev, Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, 119234 Moscow, Russia.
Ilya M Terenin, Belozersky Institute of Physico-Chemical Biology, Lomonosov Moscow State University, 119234 Moscow, Russia.
Elena Alkalaeva, Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, 119991 Moscow, Russia; Center for Precision Genome Editing and Genetic Technologies for Biomedicine, Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, 119991 Moscow, Russia.
Supplementary data
Supplementary data is available at NAR online.
Conflict of interest
None declared.
Funding
The study was supported by the Russian Science Foundation (Grant No. 22-14-00279). Funding to pay the Open Access publication charges for this article was provided by Russian Science Foundation.
Data availability
The data that support the findings of this study are contained within the article and the supporting information. All source data generated for this study are available from the corresponding author upon reasonable request.
References
- 1. Brito Querido J, Díaz-López I, Ramakrishnan V The molecular basis of translation initiation and its regulation in eukaryotes. Nat Rev Mol Cell Biol. 2024; 25:168–86. 10.1038/s41580-023-00624-9. [DOI] [PubMed] [Google Scholar]
- 2. Shirokikh NE, Preiss T Translation initiation by cap-dependent ribosome recruitment: recent insights and open questions. Wiley Interdiscip Rev RNA. 2018; 9:e1473. 10.1002/wrna.1473. [DOI] [PubMed] [Google Scholar]
- 3. Pelletier J, Schmeing TM, Sonenberg N The multifaceted eukaryotic cap structure. Wiley Interdiscip Rev RNA. 2021; 12:e1636. 10.1002/wrna.1636. [DOI] [PubMed] [Google Scholar]
- 4. Kumar P, Hellen CUT, Pestova TV Toward the mechanism of eIF4F-mediated ribosomal attachment to mammalian capped mRNAs. Genes Dev. 2016; 30:1573–88. 10.1101/gad.282418.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grifo JA, Tahara SM, Morgan MA et al. New initiation factor activity required for globin mRNA translation. J Biol Chem. 1983; 258:5804–10. 10.1016/S0021-9258(20)81965-6. [DOI] [PubMed] [Google Scholar]
- 6. Villa N, Do A, Hershey JWB et al. Human eukaryotic initiation factor 4G (eIF4G) protein binds to eIF3c, -d, and -e to promote mRNA recruitment to the ribosome. J Biol Chem. 2013; 288:32932–40. 10.1074/jbc.M113.517011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brito Querido J, Sokabe M, Kraatz S et al. Structure of a human 48 S translational initiation complex. Science. 2020; 369:1220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sonenberg N, Rupprecht KM, Hecht SM et al. Eukaryotic mRNA cap binding protein: purification by affinity chromatography on sepharose-coupled m7GDP. Proc Natl Acad Sci USA. 1979; 76:4345–9. 10.1073/pnas.76.9.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sonenberg N, Morgan MA, Merrick WC et al. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5′-terminal cap in mRNA. Proc Natl Acad Sci USA. 1978; 75:4843–7. 10.1073/pnas.75.10.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parsyan A, Svitkin Y, Shahbazian D et al. mRNA helicases: the tacticians of translational control. Nat Rev Mol Cell Biol. 2011; 12:235–45. 10.1038/nrm3083. [DOI] [PubMed] [Google Scholar]
- 11. Yanagiya A, Svitkin YV, Shibata S et al. Requirement of RNA binding of mammalian eukaryotic translation initiation factor 4GI (eIF4GI) for efficient interaction of eIF4E with the mRNA cap. Mol Cell Biol. 2009; 29:1661–9. 10.1128/MCB.01187-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. García-García C, Frieda KL, Feoktistova K et al. Factor-dependent processivity in human eIF4A DEAD-box helicase. Science. 2015; 348:1486–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rogers GW, Komar AA, Merrick WC eIF4A: the godfather of the DEAD box helicases. Prog Nucleic Acid Res Mol Biol. 2002; 72:307–31. 10.1016/S0079-6603(02)72073-4. [DOI] [PubMed] [Google Scholar]
- 14. Rogers GW, Richter NJ, Lima WF et al. Modulation of the helicase activity of eIF4A by eIF4B, eIF4H, and eIF4F. J Biol Chem. 2001; 276:30914–22. 10.1074/jbc.M100157200. [DOI] [PubMed] [Google Scholar]
- 15. Grifo JA, Abramson RD, Satler CA et al. RNA-stimulated ATPase activity of eukaryotic initiation factors. J Biol Chem. 1984; 259:8648–54. 10.1016/S0021-9258(17)39779-X. [DOI] [PubMed] [Google Scholar]
- 16. Ray BK, Lawson TG, Kramer JC et al. ATP-dependent unwinding of messenger RNA structure by eukaryotic initiation factors. J Biol Chem. 1985; 260:7651–8. 10.1016/S0021-9258(17)39658-8. [DOI] [PubMed] [Google Scholar]
- 17. Sen ND, Zhou F, Harris MS et al. eIF4B stimulates translation of long mRNAs with structured 5′ UTRs and low closed-loop potential but weak dependence on eIF4G. Proc Natl Acad Sci USA. 2016; 113:10464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dmitriev SE, Terenin IM, Dunaevsky YE et al. Assembly of 48S translation initiation complexes from purified components with mRNAs that have some base pairing within their 5′ untranslated regions. Mol Cell Biol. 2003; 23:8925–33. 10.1128/MCB.23.24.8925-8933.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Safaee N, Kozlov G, Noronha AM et al. Interdomain allostery promotes assembly of the poly (A) mRNA complex with PABP and eIF4G. Mol Cell. 2012; 48:375–86. 10.1016/j.molcel.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 20. Kahvejian A, Svitkin YV, Sukarieh R et al. Mammalian poly (A)-binding protein is a eukaryotic translation initiation factor, which acts via multiple mechanisms. Genes Dev. 2005; 19:104–13. 10.1101/gad.1262905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gallie DR A tale of two termini: a functional interaction between the termini of an mRNA is a prerequisite for efficient translation initiation. Gene. 1998; 216:1–11. 10.1016/S0378-1119(98)00318-7. [DOI] [PubMed] [Google Scholar]
- 22. Imataka H, Gradi A, Sonenberg N A newly identified N-terminal amino acid sequence of human eIF4G binds poly (A)-binding protein and functions in poly (A)-dependent translation. EMBO J. 1998; 17:7480–9. 10.1093/emboj/17.24.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tarun SZ, Sachs AB Association of the yeast poly (A) tail binding protein with translation initiation factor eIF-4G. EMBO J. 1996; 15:7168–77. 10.1002/j.1460-2075.1996.tb01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gallie DR The cap and poly (A) tail function synergistically to regulate mRNA translational efficiency. Genes Dev. 1991; 5:2108–16. 10.1101/gad.5.11.2108. [DOI] [PubMed] [Google Scholar]
- 25. Le H, Tanguay RL, Balasta ML et al. Translation initiation factors eIF-iso4G and eIF-4B interact with the poly (A)-binding protein and increase its RNA binding activity. J Biol Chem. 1997; 272:16247–55. 10.1074/jbc.272.26.16247. [DOI] [PubMed] [Google Scholar]
- 26. Cheng S, Gallie DR Eukaryotic initiation factor 4B and the poly (A)-binding protein bind eIF4G competitively. Translation. 2013; 1:e24038. 10.4161/trla.24038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheng S, Gallie DR eIF4G, eIFiso4G, and eIF4B bind the poly (A)-binding protein through overlapping sites within the RNA recognition motif domains. J Biol Chem. 2007; 282:25247–58. 10.1074/jbc.M702193200. [DOI] [PubMed] [Google Scholar]
- 28. Bushell M, Wood W, Carpenter G et al. Disruption of the interaction of mammalian protein synthesis eukaryotic initiation factor 4B with the poly (A)-binding protein by caspase- and viral protease-mediated cleavages. J Biol Chem. 2001; 276:23922–8. 10.1074/jbc.M100384200. [DOI] [PubMed] [Google Scholar]
- 29. Wells SE, Hillner PE, Vale RD et al. Circularization of mRNA by eukaryotic translation initiation factors. Mol Cell. 1998; 2:135–40. 10.1016/S1097-2765(00)80122-7. [DOI] [PubMed] [Google Scholar]
- 30. Lai W-JC, Zhu M, Belinite M et al. Intrinsically unstructured sequences in the mRNA 3′ UTR reduce the ability of poly (A) tail to enhance translation. J Mol Biol. 2022; 434:167877. 10.1016/j.jmb.2022.167877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Amrani N, Ghosh S, Mangus DA et al. Translation factors promote the formation of two states of the closed-loop mRNP. Nature. 2008; 453:1276–80. 10.1038/nature06974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alekhina OM, Terenin IM, Dmitriev SE et al. Functional cyclization of eukaryotic mRNAs. Int J Mol Sci. 2020; 21:1677. 10.3390/ijms21051677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamanaka S Essential role of NAT1/p97/DAP5 in embryonic differentiation and the retinoic acid pathway. EMBO J. 2000; 19:5533–41. 10.1093/emboj/19.20.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gradi A, Imataka H, Svitkin YV et al. A novel functional human eukaryotic translation initiation factor 4G. Mol Cell Biol. 1998; 18:334–42. 10.1128/MCB.18.1.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smirnova VV, Shestakova ED, Nogina DS et al. Ribosomal leaky scanning through a translated uORF requires eIF4G2. Nucleic Acids Res. 2022; 50:1111–27. 10.1093/nar/gkab1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shestakova ED, Tumbinsky RS, Andreev DE et al. The roles of eIF4G2 in leaky scanning and reinitiation on the human dual-coding POLG mRNA. Int J Mol Sci. 2023; 24:17149. 10.3390/ijms242417149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weber R, Kleemann L, Hirschberg I et al. DAP5 enables main ORF translation on mRNAs with structured and uORF-containing 5′ leaders. Nat Commun. 2022; 13:7510. 10.1038/s41467-022-35019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Etchison D, Milburn SC, Edery I et al. Inhibition of HeLa cell protein synthesis following poliovirus infection correlates with the proteolysis of a 220, 000-dalton polypeptide associated with eucaryotic initiation factor 3 and a cap binding protein complex. J Biol Chem. 1982; 257:14806–10. 10.1016/S0021-9258(18)33352-0. [DOI] [PubMed] [Google Scholar]
- 39. Lamphear BJ, Kirchweger R, Skern T et al. Mapping of functional domains in eukaryotic protein synthesis initiation factor 4G (eIF4G) with picornaviral proteases. J Biol Chem. 1995; 270:21975–83. 10.1074/jbc.270.37.21975. [DOI] [PubMed] [Google Scholar]
- 40. Lloyd RE, Grubman MJ, Ehrenfeld E Relationship of p220 cleavage during picornavirus infection to 2A proteinase sequencing. J Virol. 1988; 62:4216–23. 10.1128/jvi.62.11.4216-4223.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ohlmann T, Prévôt D, Décimo D et al. In vitro cleavage of eIF4GI but not eIF4GII by HIV-1 protease and its effects on translation in the rabbit reticulocyte lysate system. J Mol Biol. 2002; 318:9–20. 10.1016/S0022-2836(02)00070-0. [DOI] [PubMed] [Google Scholar]
- 42. Álvarez E, Menéndez-Arias L, Carrasco L The eukaryotic translation initiation factor 4GI is cleaved by different retroviral proteases. J Virol. 2003; 77:12392–400. 10.1128/JVI.77.23.12392-12400.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ventoso I, Blanco R, Perales C et al. HIV-1 protease cleaves eukaryotic initiation factor 4G and inhibits cap-dependent translation. Proc Natl Acad Sci USA. 2001; 98:12966–71. 10.1073/pnas.231343498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ivanov A, Mikhailova T, Eliseev B et al. PABP enhances release factor recruitment and stop codon recognition during translation termination. Nucleic Acids Res. 2016; 44:7766–76. 10.1093/nar/gkw635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cosson B, Berkova N, Couturier A et al. Poly (A)-binding protein and eRF3 are associated in vivo in human and Xenopus cells. Biol Cell. 2002; 94:205–16. 10.1016/S0248-4900(02)01194-2. [DOI] [PubMed] [Google Scholar]
- 46. Uchida N, Hoshino S, Imataka H et al. A novel role of the mammalian GSPT/eRF3 associating with poly (A)-binding protein in cap/poly (A)-dependent translation. J Biol Chem. 2002; 277:50286–92. 10.1074/jbc.M203029200. [DOI] [PubMed] [Google Scholar]
- 47. Biziaev N, Shuvalov A, Salman A et al. The impact of mRNA poly (A) tail length on eukaryotic translation stages. Nucleic Acids Res. 2024; 52:7792–808. 10.1093/nar/gkae510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Biziaev NS, Egorova TV, Alkalaeva EZ Dynamics of eukaryotic mRNA structure during translation. Mol Biol. 2022; 56:382–94. 10.1134/S0026893322030037. [DOI] [PubMed] [Google Scholar]
- 49. Song H, Mugnier P, Das AK et al. The crystal structure of human eukaryotic release factor eRF1—mechanism of stop codon recognition and peptidyl-tRNA hydrolysis. Cell. 2000; 100:311–21. 10.1016/S0092-8674(00)80667-4. [DOI] [PubMed] [Google Scholar]
- 50. Matheisl S, Berninghausen O, Becker T et al. Structure of a human translation termination complex. Nucleic Acids Res. 2015; 43:8615–26. 10.1093/nar/gkv909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brown A, Shao S, Murray J et al. Structural basis for stop codon recognition in eukaryotes. Nature. 2015; 524:493–6. 10.1038/nature14896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Frolova L, Le Goff X, Rasmussen HH et al. A highly conserved eukaryotic protein family possessing properties of polypeptide chain release factor. Nature. 1994; 372:701–3. 10.1038/372701a0. [DOI] [PubMed] [Google Scholar]
- 53. Kryuchkova P, Grishin A, Eliseev B et al. Two-step model of stop codon recognition by eukaryotic release factor eRF1. Nucleic Acids Res. 2013; 41:4573–86. 10.1093/nar/gkt113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhouravleva G, Frolova L, Le Goff X et al. Termination of translation in eukaryotes is governed by two interacting polypeptide chain release factors, eRF1 and eRF3. EMBO J. 1995; 14:4065–72. 10.1002/j.1460-2075.1995.tb00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Frolova L, Le Goff X, Zhouravleva G et al. Eukaryotic polypeptide chain release factor eRF3 is an eRF1- and ribosome-dependent guanosine triphosphatase. RNA. 1996; 2:334–41. [PMC free article] [PubMed] [Google Scholar]
- 56. Alkalaeva EZ, Pisarev AV, Frolova LY et al. In vitro reconstitution of eukaryotic translation reveals cooperativity between release factors eRF1 and eRF3. Cell. 2006; 125:1125–36. 10.1016/j.cell.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 57. Cheng Z, Saito K, Pisarev AV et al. Structural insights into eRF3 and stop codon recognition by eRF1. Genes Dev. 2009; 23:1106–18. 10.1101/gad.1770109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shoemaker CJ, Green R Kinetic analysis reveals the ordered coupling of translation termination and ribosome recycling in yeast. Proc Natl Acad Sci USA. 2011; 108:E1392–8. 10.1073/pnas.1113956108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gross T, Siepmann A, Sturm D et al. The DEAD-box RNA helicase Dbp5 functions in translation termination. Science. 2007; 315:646–9. [DOI] [PubMed] [Google Scholar]
- 60. Mikhailova T, Shuvalova E, Ivanov A et al. RNA helicase DDX19 stabilizes ribosomal elongation and termination complexes. Nucleic Acids Res. 2017; 45:18–1307. 10.1093/nar/gkw1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Egorova T, Biziaev N, Shuvalov A et al. eIF3j facilitates loading of release factors into the ribosome. Nucleic Acids Res. 2021; 49:11181–96. 10.1093/nar/gkab854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Beznosková P, Cuchalová L, Wagner S et al. Translation initiation factors eIF3 and HCR1 control translation termination and stop codon read-through in yeast cells. PLoS Genet. 2013; 9:e1003962. 10.1371/journal.pgen.1003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schuller AP, Wu CC-C, Dever TE et al. eIF5A Functions globally in translation elongation and termination. Mol Cell. 2017; 66:194–205. 10.1016/j.molcel.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shuvalova E, Egorova T, Ivanov A et al. Discovery of a novel role of tumor suppressor PDCD4 in stimulation of translation termination. J Biol Chem. 2021; 297:101269. 10.1016/j.jbc.2021.101269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shuvalov A, Shuvalova E, Biziaev N et al. Nsp1 of SARS-CoV-2 stimulates host translation termination. RNA Biology. 2021; 18:804–17. 10.1080/15476286.2021.1999103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ivanov A, Shuvalova E, Egorova T et al. Polyadenylate-binding protein-interacting proteins PAIP1 and PAIP2 affect translation termination. J Biol Chem. 2019; 294:8630–9. 10.1074/jbc.RA118.006856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fitzgerald DJ, Schaffitzel C, Berger P et al. Multiprotein expression strategy for structural biology of eukaryotic complexes. Structure. 2007; 15:275–9. 10.1016/j.str.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 68. Vijayachandran LS, Viola C, Garzoni F et al. Robots, pipelines, polyproteins: enabling multiprotein expression in prokaryotic and eukaryotic cells. J Struct Biol. 2011; 175:198–208. 10.1016/j.jsb.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Susorov D, Egri S, Korostelev AA Termi–Luc: a versatile assay to monitor full-protein release from ribosomes. RNA. 2020; 26:2044–50. 10.1261/rna.076588.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Egorova T, Sokolova E, Shuvalova E et al. Fluorescent toeprinting to study the dynamics of ribosomal complexes. Methods. 2019; 162–163:9–54. 10.1016/j.ymeth.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 71. Shirokikh NE, Alkalaeva EZ, Vassilenko KS et al. Quantitative analysis of ribosome–mRNA complexes at different translation stages. Nucleic Acids Res. 2009; 38:e15. 10.1093/nar/gkp1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Seit-Nebi A, Frolova L, Justesen J et al. Class-1 translation termination factors: invariant GGQ minidomain is essential for release activity and ribosome binding but not for stop codon recognition. Nucleic Acids Res. 2001; 29:3982–7. 10.1093/nar/29.19.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Methot N, Pickett G, Keene JD et al. In vitro RNA selection identifies RNA ligands that specifically bind to eukaryotic translation initiation factor 4B: the role of the RNA remotif. RNA. 1996; 2:38–50. [PMC free article] [PubMed] [Google Scholar]
- 74. Rozovsky N, Butterworth AC, Moore MJ Interactions between eIF4AI and its accessory factors eIF4B and eIF4H. RNA. 2008; 14:2136–48. 10.1261/rna.1049608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Brito Querido J, Sokabe M, Díaz-López I et al. The structure of a human translation initiation complex reveals two independent roles for the helicase eIF4A. Nat Struct Mol Biol. 2024; 31:455–64. 10.1038/s41594-023-01196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Andreou AZ, Harms U, Klostermeier D eIF4B stimulates eIF4A ATPase and unwinding activities by direct interaction through its 7-repeats region. RNA Biol. 2017; 14:113–23. 10.1080/15476286.2016.1259782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yang H-S, Jansen AP, Komar AA et al. The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol Cell Biol. 2003; 23:26–37. 10.1128/MCB.23.1.26-37.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fehler O, Singh P, Haas A et al. An evolutionarily conserved interaction of tumor suppressor protein Pdcd4 with the poly (A)-binding protein contributes to translation suppression by Pdcd4. Nucleic Acids Res. 2014; 42:11107–18. 10.1093/nar/gku800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Walker SE, Zhou F, Mitchell SF et al. Yeast eIF4B binds to the head of the 40S ribosomal subunit and promotes mRNA recruitment through its N-terminal and internal repeat domains. RNA. 2013; 19:191–207. 10.1261/rna.035881.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pöyry TAA, Kaminski A, Jackson RJ What determines whether mammalian ribosomes resume scanning after translation of a short upstream open reading frame?. Genes Dev. 2004; 18:62–75. 10.1101/gad.276504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Watanabe R, Murai MJ, Singh CR et al. The eukaryotic initiation factor (eIF) 4G HEAT domain promotes translation re-initiation in yeast both dependent on and independent of eIF4A mRNA helicase. J Biol Chem. 2010; 285:21922–33. 10.1074/jbc.M110.132027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mohammad MP, Smirnova A, Gunišová S et al. eIF4G is retained on ribosomes elongating and terminating on short upstream ORFs to control reinitiation in yeast. Nucleic Acids Res. 2021; 49:8743–56. 10.1093/nar/gkab652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bohlen J, Fenzl K, Kramer G et al. Selective 40S footprinting reveals cap-tethered ribosome scanning in human cells. Mol Cell. 2020; 79:561–74. 10.1016/j.molcel.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 84. Virgili G, Frank F, Feoktistova K et al. Structural analysis of the DAP5 MIF4G domain and its interaction with eIF4A. Structure. 2013; 21:517–27. 10.1016/j.str.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Clemens MJ, Bushell M, Jeffrey IW et al. Translation initiation factor modifications and the regulation of protein synthesis in apoptotic cells. Cell Death Differ. 2000; 7:603–15. 10.1038/sj.cdd.4400695. [DOI] [PubMed] [Google Scholar]
- 86. Henis-Korenblit S, Strumpf NL, Goldstaub D et al. A novel form of DAP5 protein accumulates in apoptotic cells as a result of caspase cleavage and internal ribosome entry site-mediated translation. Mol Cell Biol. 2000; 20:496–506. 10.1128/MCB.20.2.496-506.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Amrani N, Dong S, He F et al. Aberrant termination triggers nonsense-mediated mRNA decay. Biochem Soc Trans. 2006; 34:39–42. 10.1042/BST0340039. [DOI] [PubMed] [Google Scholar]
- 88. Kervestin S, Jacobson A NMD: a multifaceted response to premature translational termination. Nat Rev Mol Cell Biol. 2012; 13:700–12. 10.1038/nrm3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nogueira G, Fernandes R, García-Moreno JF et al. Nonsense-mediated RNA decay and its bipolar function in cancer. Mol Cancer. 2021; 20:72. 10.1186/s12943-021-01364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Peixeiro I, Inacio A, Barbosa C et al. Interaction of PABPC1 with the translation initiation complex is critical to the NMD resistance of AUG-proximal nonsense mutations. Nucleic Acids Res. 2012; 40:1160–73. 10.1093/nar/gkr820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fatscher T, Boehm V, Weiche B et al. The interaction of cytoplasmic poly (A)-binding protein with eukaryotic initiation factor 4G suppresses nonsense-mediated mRNA decay. RNA. 2014; 20:1579–92. 10.1261/rna.044933.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Joncourt R, Eberle AB, Rufener SC et al. Eukaryotic initiation factor 4G suppresses nonsense-mediated mRNA decay by two genetically separable mechanisms. PLoS One. 2014; 9:e104391. 10.1371/journal.pone.0104391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Quintas A, Harvey RF, Horvilleur E et al. Eukaryotic initiation factor 4B is a multi-functional RNA binding protein that regulates histone mRNAs. Nucleic Acids Res. 2024; 52:12039–54. 10.1093/nar/gkae767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are contained within the article and the supporting information. All source data generated for this study are available from the corresponding author upon reasonable request.