Abstract
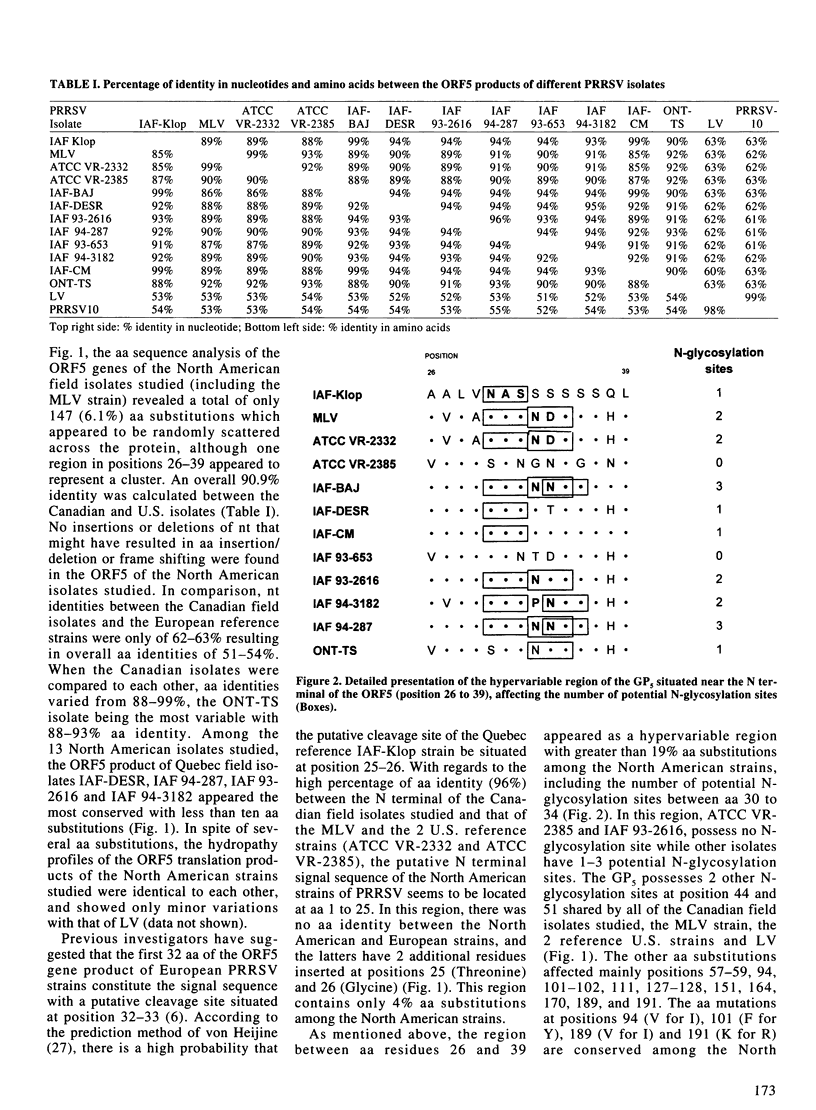
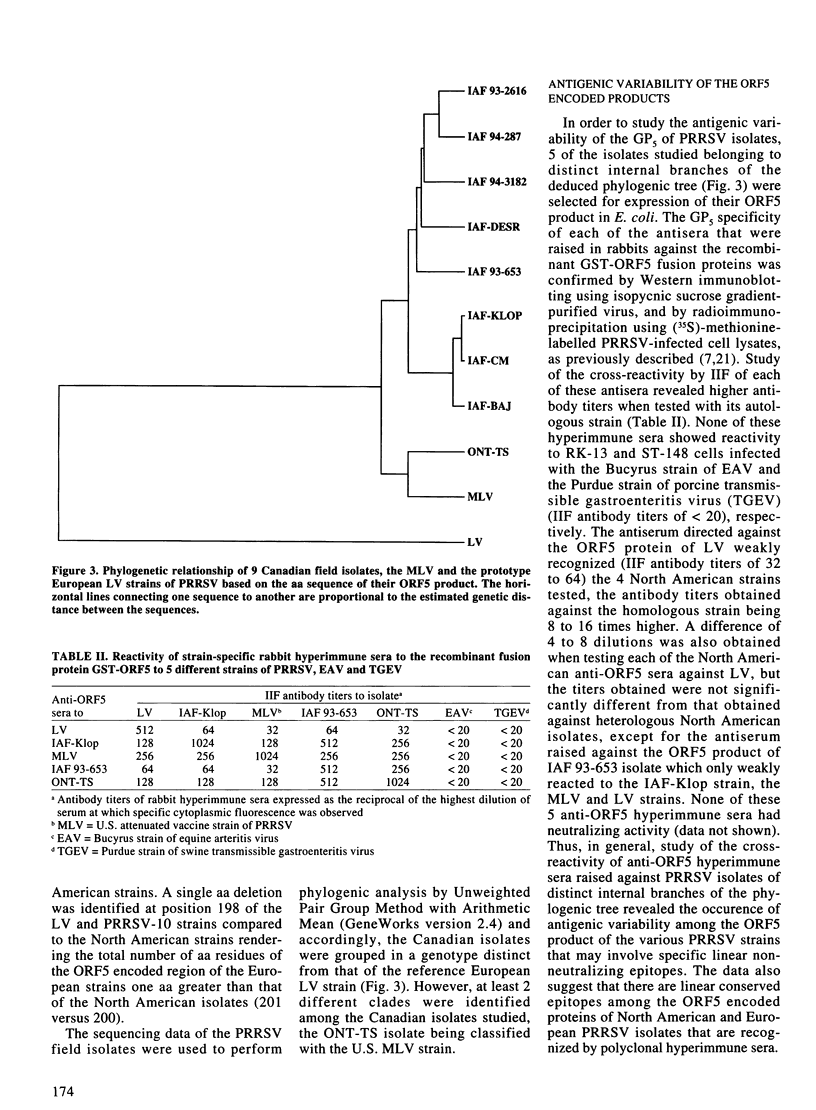
The objective of the present study was to evaluate the importance of genomic and antigenic variations which may have affected the major envelope glycoprotein GP5 of porcine reproductive and respiratory syndrome virus (PRRSV) isolates responsible for outbreaks in Quebec and Ontario, in comparison with the modified-live U.S. vaccine strain (MLV) and the European prototype strain from Lelystad (LV). Nucleotide sequence analyses of the open reading frame (ORF)5 genes showed that all of the isolates studied were heterogenous, amino acid (aa) identities varied from 88 to 99% with the MLV strain, and between 51 and 54% with the LV strain. The aa substitutions were randomly scattered across the protein, although one region between residues 26 and 39 was found to correspond to a hypervariable region which involved 0 to 3 potential N-glycosylation sites. The ORF5 encoded products of 5 of these isolates, including the MLV and LV strains, were expressed in E. coli as recombinant proteins fused to the glutathione S-transferase (GST) protein and used to raise hyperimmune anti-ORF5 sera in rabbits. The reactivity patterns of strain-specific hyperimmune anti-ORF5 sera and a panel of 4 monoclonal antibodies directed against the ORF5 gene product of the Quebec IAF-Klop strain of PRRSV, indicated that GP5 of field isolates also underwent antigenic variations. The data suggest that neutralizing epitopes, independent of conformation and glycosylation, are also associated with antigenic variability of the GP5 of PRRSV.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balasuriya U. B., Maclachlan N. J., De Vries A. A., Rossitto P. V., Rottier P. J. Identification of a neutralization site in the major envelope glycoprotein (GL) of equine arteritis virus. Virology. 1995 Mar 10;207(2):518–527. doi: 10.1006/viro.1995.1112. [DOI] [PubMed] [Google Scholar]
- Balasuriya U. B., Timoney P. J., McCollum W. H., MacLachlan N. J. Phylogenetic analysis of open reading frame 5 of field isolates of equine arteritis virus and identification of conserved and nonconserved regions in the GL envelope glycoprotein. Virology. 1995 Dec 20;214(2):690–697. doi: 10.1006/viro.1995.0087. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch Virol. 1997;142(3):629–633. [PubMed] [Google Scholar]
- Chirnside E. D., de Vries A. A., Mumford J. A., Rottier P. J. Equine arteritis virus-neutralizing antibody in the horse is induced by a determinant on the large envelope glycoprotein GL. J Gen Virol. 1995 Aug;76(Pt 8):1989–1998. doi: 10.1099/0022-1317-76-8-1989. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Conzelmann K. K., Visser N., Van Woensel P., Thiel H. J. Molecular characterization of porcine reproductive and respiratory syndrome virus, a member of the arterivirus group. Virology. 1993 Mar;193(1):329–339. doi: 10.1006/viro.1993.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dea S., Gagnon C. A., Mardassi H., Milane G. Antigenic variability among North American and European strains of porcine reproductive and respiratory syndrome virus as defined by monoclonal antibodies to the matrix protein. J Clin Microbiol. 1996 Jun;34(6):1488–1493. doi: 10.1128/jcm.34.6.1488-1493.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew T. W., Meulenberg J. J., Sands J. J., Paton D. J. Production, characterization and reactivity of monoclonal antibodies to porcine reproductive and respiratory syndrome virus. J Gen Virol. 1995 Jun;76(Pt 6):1361–1369. doi: 10.1099/0022-1317-76-6-1361. [DOI] [PubMed] [Google Scholar]
- Frorath B., Abney C. C., Berthold H., Scanarini M., Northemann W. Production of recombinant rat interleukin-6 in Escherichia coli using a novel highly efficient expression vector pGEX-3T. Biotechniques. 1992 Apr;12(4):558–563. [PubMed] [Google Scholar]
- Gagnon C. A., Dea S. Differentiation between porcine reproductive and respiratory syndrome virus isolates by restriction fragment length polymorphism of their ORFs 6 and 7 genes. Can J Vet Res. 1998 Apr;62(2):110–116. [PMC free article] [PubMed] [Google Scholar]
- Glaser A. L., de Vries A. A., Dubovi E. J. Comparison of equine arteritis virus isolates using neutralizing monoclonal antibodies and identification of sequence changes in GL associated with neutralization resistance. J Gen Virol. 1995 Sep;76(Pt 9):2223–2233. doi: 10.1099/0022-1317-76-9-2223. [DOI] [PubMed] [Google Scholar]
- Goyal S. M. Porcine reproductive and respiratory syndrome. J Vet Diagn Invest. 1993 Oct;5(4):656–664. doi: 10.1177/104063879300500435. [DOI] [PubMed] [Google Scholar]
- Kapur V., Elam M. R., Pawlovich T. M., Murtaugh M. P. Genetic variation in porcine reproductive and respiratory syndrome virus isolates in the midwestern United States. J Gen Virol. 1996 Jun;77(Pt 6):1271–1276. doi: 10.1099/0022-1317-77-6-1271. [DOI] [PubMed] [Google Scholar]
- Kim H. S., Kwang J., Yoon I. J., Joo H. S., Frey M. L. Enhanced replication of porcine reproductive and respiratory syndrome (PRRS) virus in a homogeneous subpopulation of MA-104 cell line. Arch Virol. 1993;133(3-4):477–483. doi: 10.1007/BF01313785. [DOI] [PubMed] [Google Scholar]
- Magar R., Larochelle R., Dea S., Gagnon C. A., Nelson E. A., Christopher-Hennings J., Benfield D. A. Antigenic comparison of Canadian and US isolates of porcine reproductive and respiratory syndrome virus using monoclonal antibodies to the nucleocapsid protein. Can J Vet Res. 1995 Jul;59(3):232–234. [PMC free article] [PubMed] [Google Scholar]
- Mardassi H., Massie B., Dea S. Intracellular synthesis, processing, and transport of proteins encoded by ORFs 5 to 7 of porcine reproductive and respiratory syndrome virus. Virology. 1996 Jul 1;221(1):98–112. doi: 10.1006/viro.1996.0356. [DOI] [PubMed] [Google Scholar]
- Mardassi H., Mounir S., Dea S. Molecular analysis of the ORFs 3 to 7 of porcine reproductive and respiratory syndrome virus, Québec reference strain. Arch Virol. 1995;140(8):1405–1418. doi: 10.1007/BF01322667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X. J., Paul P. S., Halbur P. G., Lum M. A. Phylogenetic analyses of the putative M (ORF 6) and N (ORF 7) genes of porcine reproductive and respiratory syndrome virus (PRRSV): implication for the existence of two genotypes of PRRSV in the U.S.A. and Europe. Arch Virol. 1995;140(4):745–755. doi: 10.1007/BF01309962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X. J., Paul P. S., Halbur P. G., Morozov I. Sequence comparison of open reading frames 2 to 5 of low and high virulence United States isolates of porcine reproductive and respiratory syndrome virus. J Gen Virol. 1995 Dec;76(Pt 12):3181–3188. doi: 10.1099/0022-1317-76-12-3181. [DOI] [PubMed] [Google Scholar]
- Meulenberg J. J., Hulst M. M., de Meijer E. J., Moonen P. L., den Besten A., de Kluyver E. P., Wensvoort G., Moormann R. J. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology. 1993 Jan;192(1):62–72. doi: 10.1006/viro.1993.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenberg J. J., Petersen-den Besten A., De Kluyver E. P., Moormann R. J., Schaaper W. M., Wensvoort G. Characterization of proteins encoded by ORFs 2 to 7 of Lelystad virus. Virology. 1995 Jan 10;206(1):155–163. doi: 10.1016/S0042-6822(95)80030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenberg J. J., Petersen-den Besten A. Identification and characterization of a sixth structural protein of Lelystad virus: the glycoprotein GP2 encoded by ORF2 is incorporated in virus particles. Virology. 1996 Nov 1;225(1):44–51. doi: 10.1006/viro.1996.0573. [DOI] [PubMed] [Google Scholar]
- Murtaugh M. P., Elam M. R., Kakach L. T. Comparison of the structural protein coding sequences of the VR-2332 and Lelystad virus strains of the PRRS virus. Arch Virol. 1995;140(8):1451–1460. doi: 10.1007/BF01322671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E. A., Christopher-Hennings J., Drew T., Wensvoort G., Collins J. E., Benfield D. A. Differentiation of U.S. and European isolates of porcine reproductive and respiratory syndrome virus by monoclonal antibodies. J Clin Microbiol. 1993 Dec;31(12):3184–3189. doi: 10.1128/jcm.31.12.3184-3189.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirzadeh B., Dea S. Monoclonal antibodies to the ORF5 product of porcine reproductive and respiratory syndrome virus define linear neutralizing determinants. J Gen Virol. 1997 Aug;78(Pt 8):1867–1873. doi: 10.1099/0022-1317-78-8-1867. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Laurent G., Lepage N., Carman S., Archambault D. Genetic and amino acid analysis of the GL protein of Canadian, American and European equine arteritis virus isolates. Can J Vet Res. 1997 Jan;61(1):72–76. [PMC free article] [PubMed] [Google Scholar]
- Wensvoort G., de Kluyver E. P., Luijtze E. A., den Besten A., Harris L., Collins J. E., Christianson W. T., Chladek D. Antigenic comparison of Lelystad virus and swine infertility and respiratory syndrome (SIRS) virus. J Vet Diagn Invest. 1992 Apr;4(2):134–138. doi: 10.1177/104063879200400203. [DOI] [PubMed] [Google Scholar]
- van Nieuwstadt A. P., Meulenberg J. J., van Essen-Zanbergen A., Petersen-den Besten A., Bende R. J., Moormann R. J., Wensvoort G. Proteins encoded by open reading frames 3 and 4 of the genome of Lelystad virus (Arteriviridae) are structural proteins of the virion. J Virol. 1996 Jul;70(7):4767–4772. doi: 10.1128/jvi.70.7.4767-4772.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]


