Abstract
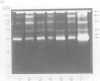
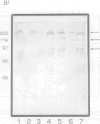
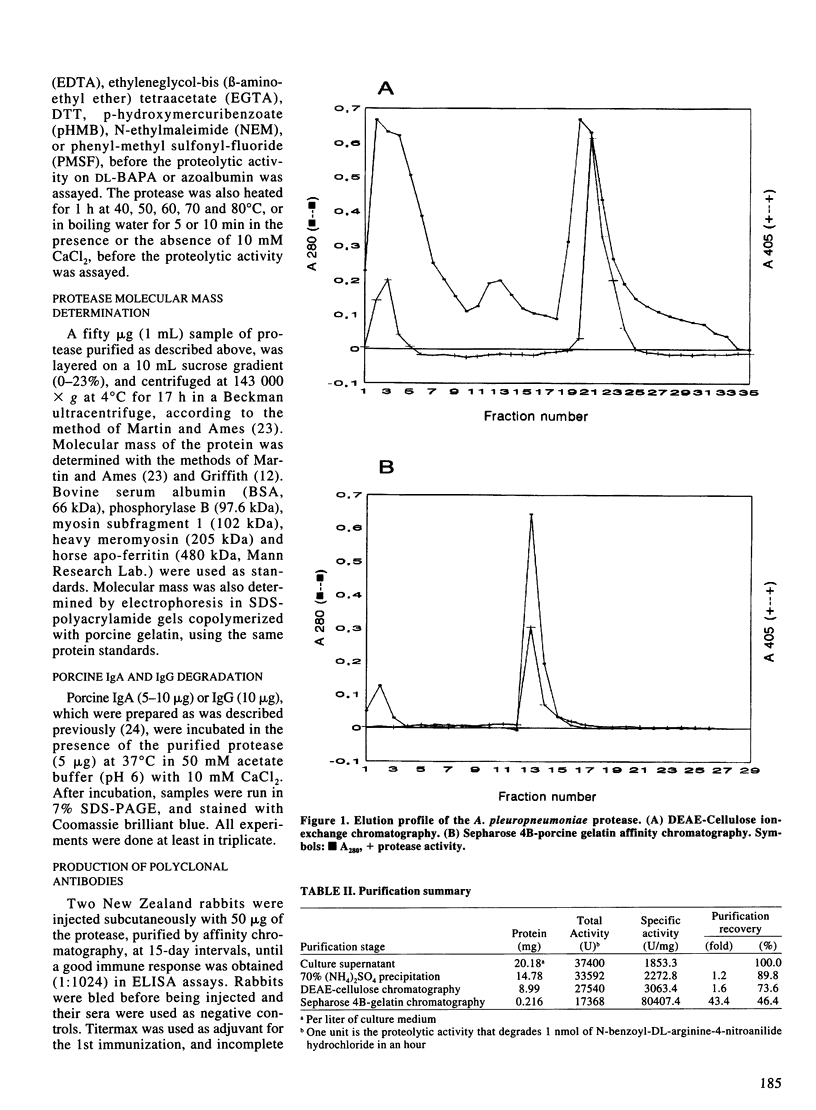
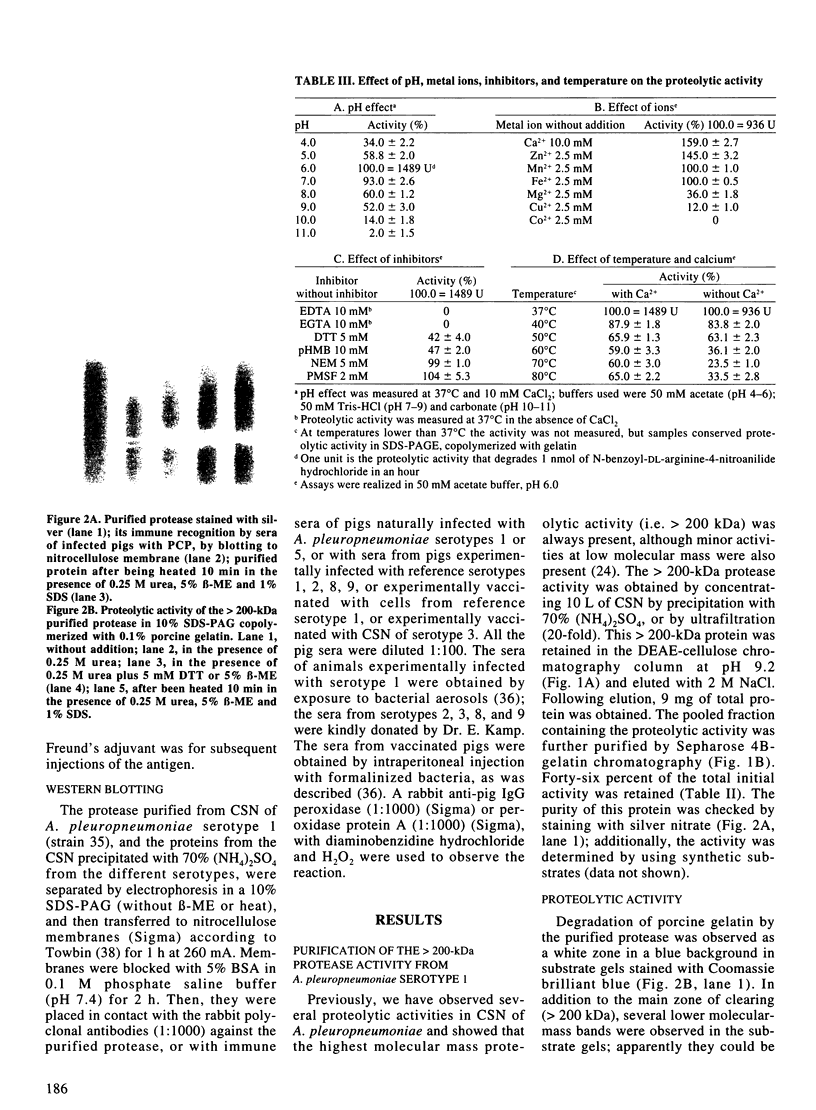
A high molecular-mass proteolytic enzyme of Actinobacillus pleuropneumoniae serotype 1, was purified from culture supernatants (CSN) by using DEAE-cellulose and sepharose-4B-gelatin chromatography. In 10% SDS-polyacrylamide gels copolymerized with porcine gelatin, the protease showed a single band of activity of > 200 kDa. However, minor molecular-mass proteolytic bands were observed when the protease was electrophoresed in the presence of either 5% beta-mercaptoethanol, 50 mM dithiothreitol, or 0.25 M urea. Furthermore, when the > 200-kDa purified protein was passed through a sucrose gradient, several bands with proteolytic activity were found: 62, 90, 190, and 540 kDa. The proteolytic activity was increased in the presence of calcium or zinc and was not affected after being heated at 90 degrees C for 5 min. Proteolytic activities were also observed in CSN from all A. pleuropneumoniae serotypes and biotypes. The purified protease hydrolyzed porcine IgA and IgG in vitro. In addition, by immunoblot the protease was recognized by serum of naturally infected pigs with serotypes 1 and 5, and by serum of pigs experimentally infected with serotypes 1, 2, 8, or 9. Serum of a pig vaccinated with CSN of a serotype 3 strain also recognized the protease, but not sera of pigs vaccinated with a bacterin (serotype 1). Proteins from CSN of all the serotypes, which were precipitated with 70% (NH4)2SO4, were recognized by a polyclonal antibody raised against the purified protease. Taken together these results indicate that an antigenic protease is produced in vivo by all the serotypes of A. pleuropneumoniae. The results indicate that proteases could have a role in the disease and in the immune response of pigs infected with A. pleuropneumoniae.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohach G. A., Snyder I. S. Composition of affinity-purified alpha-hemolysin of Escherichia coli. Infect Immun. 1986 Aug;53(2):435–437. doi: 10.1128/iai.53.2.435-437.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeau G., Lapointe H., Péloquin P., Mayrand D. Cloning, expression, and sequencing of a protease gene (tpr) from Porphyromonas gingivalis W83 in Escherichia coli. Infect Immun. 1992 Aug;60(8):3186–3192. doi: 10.1128/iai.60.8.3186-3192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chevalier G., Duclohier H., Thomas D., Shechter E., Wróblewski H. Purification and characterization of protein H, the major porin of Pasteurella multocida. J Bacteriol. 1993 Jan;175(1):266–276. doi: 10.1128/jb.175.1.266-276.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolbear T., Whittaker J. M., Daniel R. M. The effect of metal ions on the activity and thermostability of the extracellular proteinase from a thermophilic Bacillus, strain EA.1. Biochem J. 1992 Oct 15;287(Pt 2):367–374. doi: 10.1042/bj2870367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz W. T., Nedialkov Y. A., Thacker B. J., Mulks M. H. Molecular characterization of a common 48-kilodalton outer membrane protein of Actinobacillus pleuropneumoniae. Infect Immun. 1996 Jan;64(1):83–90. doi: 10.1128/iai.64.1.83-90.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorka-Cray P. J., Stine D. L., Greenwald J. M., Gray J. T., Huether M. J., Anderson G. A. The importance of secreted virulence factors in Actinobacillus pleuropneumoniae bacterin preparation: a comparison. Vet Microbiol. 1993 Oct;37(1-2):85–100. doi: 10.1016/0378-1135(93)90184-9. [DOI] [PubMed] [Google Scholar]
- Fenwick B. W., Osburn B. I. Immune responses to the lipopolysaccharides and capsular polysaccharides of Haemophilus pleuropneumoniae in convalescent and immunized pigs. Infect Immun. 1986 Nov;54(2):575–582. doi: 10.1128/iai.54.2.575-582.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Boesman-Finkelstein M., Chang Y., Häse C. C. Vibrio cholerae hemagglutinin/protease, colonial variation, virulence, and detachment. Infect Immun. 1992 Feb;60(2):472–478. doi: 10.1128/iai.60.2.472-478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey J. Virulence in Actinobacillus pleuropneumoniae and RTX toxins. Trends Microbiol. 1995 Jul;3(7):257–261. doi: 10.1016/s0966-842x(00)88939-8. [DOI] [PubMed] [Google Scholar]
- Galloway D. R. Pseudomonas aeruginosa elastase and elastolysis revisited: recent developments. Mol Microbiol. 1991 Oct;5(10):2315–2321. doi: 10.1111/j.1365-2958.1991.tb02076.x. [DOI] [PubMed] [Google Scholar]
- Haga Y., Ogino S., Ohashi S., Ajito T., Hashimoto K., Sawada T. Protective efficacy of an affinity-purified hemolysin vaccine against experimental swine pleuropneumonia. J Vet Med Sci. 1997 Feb;59(2):115–120. doi: 10.1292/jvms.59.115. [DOI] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Häse C. C., Finkelstein R. A. Bacterial extracellular zinc-containing metalloproteases. Microbiol Rev. 1993 Dec;57(4):823–837. doi: 10.1128/mr.57.4.823-837.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idris U. E., Harmon B. G., Udeze F. A., Kadis S. Pulmonary lesions in mice inoculated with Actinobacillus pleuropneumoniae hemolysin and lipopolysaccharide. Vet Pathol. 1993 May;30(3):234–241. doi: 10.1177/030098589303000303. [DOI] [PubMed] [Google Scholar]
- Inzana T. J., Ma J., Workman T., Gogolewski R. P., Anderson P. Virulence properties and protective efficacy of the capsular polymer of Haemophilus (Actinobacillus) pleuropneumoniae serotype 5. Infect Immun. 1988 Aug;56(8):1880–1889. doi: 10.1128/iai.56.8.1880-1889.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J., Todd J., Ma J. N., Veit H. Characterization of a non-hemolytic mutant of Actinobacillus pleuropneumoniae serotype 5: role of the 110 kilodalton hemolysin in virulence and immunoprotection. Microb Pathog. 1991 Apr;10(4):281–296. doi: 10.1016/0882-4010(91)90012-y. [DOI] [PubMed] [Google Scholar]
- Kilian M., Mestecky J., Schrohenloher R. E. Pathogenic species of the genus Haemophilus and Streptococcus pneumoniae produce immunoglobulin A1 protease. Infect Immun. 1979 Oct;26(1):143–149. doi: 10.1128/iai.26.1.143-149.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M., Reinholdt J., Lomholt H., Poulsen K., Frandsen E. V. Biological significance of IgA1 proteases in bacterial colonization and pathogenesis: critical evaluation of experimental evidence. APMIS. 1996 May;104(5):321–338. doi: 10.1111/j.1699-0463.1996.tb00724.x. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- McDermid A. S., McKee A. S., Marsh P. D. Effect of environmental pH on enzyme activity and growth of Bacteroides gingivalis W50. Infect Immun. 1988 May;56(5):1096–1100. doi: 10.1128/iai.56.5.1096-1100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKevitt A. I., Bajaksouzian S., Klinger J. D., Woods D. E. Purification and characterization of an extracellular protease from Pseudomonas cepacia. Infect Immun. 1989 Mar;57(3):771–778. doi: 10.1128/iai.57.3.771-778.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrete-Abascal E., Tenorio V. R., Serrano J. J., Garcia C., de la Garza M. Secreted proteases from Actinobacillus pleuropneumoniae serotype 1 degrade porcine gelatin, hemoglobin and immunoglobulin A. Can J Vet Res. 1994 Apr;58(2):83–86. [PMC free article] [PubMed] [Google Scholar]
- Norqvist A., Norrman B., Wolf-Watz H. Identification and characterization of a zinc metalloprotease associated with invasion by the fish pathogen Vibrio anguillarum. Infect Immun. 1990 Nov;58(11):3731–3736. doi: 10.1128/iai.58.11.3731-3736.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi E., Kitajima T., Koyama Y., Ohgitani T., Katayama S., Okabe T. Protective effect of the combined vaccine prepared from cell-free-antigen of Actinobacillus pleuropneumoniae serotypes 1, 2 and 5 in pigs. J Vet Med Sci. 1995 Dec;57(6):1125–1128. doi: 10.1292/jvms.57.1125. [DOI] [PubMed] [Google Scholar]
- Pacaud M. Purification and characterization of two novel proteolytic enzymes in membranes of Escherichia coli. Protease IV and protease V. J Biol Chem. 1982 Apr 25;257(8):4333–4339. [PubMed] [Google Scholar]
- Plaut A. G. Microbial IgA proteases. N Engl J Med. 1978 Jun 29;298(26):1459–1463. doi: 10.1056/NEJM197806292982608. [DOI] [PubMed] [Google Scholar]
- Potempa J., Pike R., Travis J. The multiple forms of trypsin-like activity present in various strains of Porphyromonas gingivalis are due to the presence of either Arg-gingipain or Lys-gingipain. Infect Immun. 1995 Apr;63(4):1176–1182. doi: 10.1128/iai.63.4.1176-1182.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn F. D., Tompkins L. S. Analysis of a cloned sequence of Legionella pneumophila encoding a 38 kD metalloprotease possessing haemolytic and cytotoxic activities. Mol Microbiol. 1989 Jun;3(6):797–805. doi: 10.1111/j.1365-2958.1989.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Rosendal S., Boyd D. A., Gilbride K. A. Comparative virulence of porcine Haemophilus bacteria. Can J Comp Med. 1985 Jan;49(1):68–74. [PMC free article] [PubMed] [Google Scholar]
- Rycroft A. N., Williams D., Cullen J. M., Macdonald J. The cytotoxin of Actinobacillus pleuropneumoniae (pleurotoxin) is distinct from the haemolysin and is associated with a 120 kDa polypeptide. J Gen Microbiol. 1991 Mar;137(3):561–568. doi: 10.1099/00221287-137-3-561. [DOI] [PubMed] [Google Scholar]
- Sebunya T. N., Saunders J. R. Haemophilus pleuropneumoniae infection in swine: a review. J Am Vet Med Assoc. 1983 Jun 15;182(12):1331–1337. [PubMed] [Google Scholar]
- Sojar H. T., Lee J. Y., Bedi G. S., Genco R. J. Purification and characterization of a protease from Porphyromonas gingivalis capable of degrading salt-solubilized collagen. Infect Immun. 1993 Jun;61(6):2369–2376. doi: 10.1128/iai.61.6.2369-2376.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teufel P., Götz F. Characterization of an extracellular metalloprotease with elastase activity from Staphylococcus epidermidis. J Bacteriol. 1993 Jul;175(13):4218–4224. doi: 10.1128/jb.175.13.4218-4224.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Potempa J., Maeda H. Are bacterial proteinases pathogenic factors? Trends Microbiol. 1995 Oct;3(10):405–407. doi: 10.1016/s0966-842x(00)88988-x. [DOI] [PubMed] [Google Scholar]
- Udeze F. A., Latimer K. S., Kadis S. Role of haemophilus pleuropneumoniae lipopolysaccharide endotoxin in the pathogenesis of porcine Haemophilus pleuropneumonia. Am J Vet Res. 1987 May;48(5):768–773. [PubMed] [Google Scholar]
- Uitto V. J., Grenier D., Chan E. C., McBride B. C. Isolation of a chymotrypsinlike enzyme from Treponema denticola. Infect Immun. 1988 Oct;56(10):2717–2722. doi: 10.1128/iai.56.10.2717-2722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utrera V., Pijoan C. Fimbriae in A pleuropneumoniae strains isolated from pig respiratory tracts. Vet Rec. 1991 Apr 13;128(15):357–358. doi: 10.1136/vr.128.15.357. [DOI] [PubMed] [Google Scholar]
- Wasylewski Z., Stryjewski W., Waśniowska A., Potempa J., Baran K. Effect of calcium binding on conformational changes of staphylococcal metalloproteinase measured by means of intrinsic protein fluorescence. Biochim Biophys Acta. 1986 Jun 5;871(2):177–181. doi: 10.1016/0167-4838(86)90171-8. [DOI] [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]