Abstract
Since the discovery of the palladium/norbornene (Pd/NBE)-catalyzed ortho amination in 2013, escaping the limitation of only yielding tertiary anilines has been a long-standing challenge in the past decade. Here, we describe that, by carefully choosing the phosphine ligand and NBE mediator, installation of a N-mono-alkylamino group becomes feasible. The reaction tolerates a wide range of aryl iodide substrates and various N-mono-tertiary alkylamine-derived electrophiles. Both ipso alkenylation and alkynylation can be realized. The synthetic utility of this method is exemplified in the formation of primary amino group via selective deprotection and streamlined access to N-heterocycles. Preliminary success of installing a bulky N-secondary alkylamino group and mechanistic understanding of the decomposition pathways of mono N-alkylamine electrophiles have been obtained.
Keywords: Amination, Palladium/norbornene catalysis, C–H activation, Vicinal difunctionalization, Heterocycle formation
Graphical Abstract

The first introduction of secondary amines at the aryl iodide ortho position by the palladium/norbornene (Pd/NBE) catalysis has been developed, which leads to versatile synthetic applications, such as primary amino group installation and concise heterocycle formations.
Owing to the prevalence of arylamines in bioactive molecules (Figure 1), such as natural products, pharmaceuticals, and agrochemicals,[1] amination of aromatic compounds has been one of the pivotal tools for synthetic chemists. The most widely used approach is the ipso amination of arenes (Scheme 1a), in which an existing functional group is replaced by an amino group, such as SNAr,[2] Buchwald–Hartwig amination,[3] Ullman–Goldberg[4] and Chan–Evans–Lam[5] couplings, etc.[6] While direct substitution of a C(aryl)–H bond with a C(aryl)–N bond, namely C–H amination,[7] is a very attractive approach, control of site-selectivity for electronically unbiased substrates mainly relies on the use of directing groups (Scheme 1b).[8] On the other hand, the palladium/norbornene (Pd/NBE)-catalyzed ortho amination provides an alternative strategy to introduce amino groups to the ortho position of aryl halides (Scheme 1c).[9] In this reaction, through the key aryl norbornyl palladacycle (ANP) intermediate,[10] an amine electrophile, i.e., N-benzoyloxyamines,[11] reacts with ANP to furnish ortho C–H amination and the catalytic cycle is completed by coupling a nucleophile (including alkenes) at the ipso position. As a result, it offers a complementary site-selectivity to the ipso amination approaches.[9a]
Figure 1.

Selected pharmaceuticals that contain arylamines with secondary and primary amino groups.
Scheme 1.

Site-selective amination of arenes. a) Ipso amination via SNAr or cross couplings; b) C–H amination often uses a directing group; c) Ortho C–H amination via the Pd/NBE catalysis; d) This work introduces secondary and primary amino groups via ortho C–H amination. Nu, nucleophile; Bz, benzoyl.
Since the Pd/NBE-catalyzed ortho amination was discovered in 2013,[9a] a large variety of nucleophiles have been identified to be compatible for the ortho amination/ipso functionalization reactions.[12] Its utility has been found in synthesizing indole derivatives,[13] simplifying small-molecule drug preparation,[12e,14] and enabling carbonyl 1,2-transposition.[15] However, the main limitation lasting in the past decade is that this ortho amination approach can only introduce tertiary amino moieties, i.e., –NR2 (R: alkyl). In particular, amino groups embedded in a six-membered ring, such as morpholinyl, are most common. Installation of secondary (–NHR) or primary (–NH2) amino groups via the Pd/NBE catalysis remains unachieved to date (Scheme 1c). We envision that, by overcoming the limitation of tertiary amino groups, applications of the ortho amination can be further extended. For example, acylation of the resulting secondary and primary anilines can give amide products, and intramolecular cyclization with –NHR or –NH2 groups can offer diverse heterocycles. Both applications are not easily realizable with the current ortho amination approach. Herein, we describe our preliminary efforts of introducing secondary amino groups via the Pd/NBE catalysis and primary amino groups via subsequent dealkylation (Scheme 1d).
Why has a decade elapsed without addressing the limitation of the amino group scope in the Pd/NBE-catalyzed ortho amination reaction? This is because simply replacing the benzoyloxy dialkylamines with the corresponding mono N-alkylamine reagents under the identical reaction conditions led to no desired ortho amination product (Scheme 2). For example, when N-(n-butyl)-O-benzoylhydroxylamine (R = n-butyl) was used as the amination reagent, a complex mixture of unidentifiable compounds was obtained with no target product detected under the standard conditions reported for the ortho amination/ipso alkenylation[12a] or alkynylation[12d]. While the use of N-(tert-butyl)-O-benzoylhydroxylamine (R = t-butyl) also yielded no desired products, some side products (i-iv) could nevertheless be identified (Scheme 2, for details, see the Supporting Information), which provides some insights about the potential issues using mono N-alkylamine reagents. The main concern is associated with the acidic N–hydrogen for the primary amine-derived electrophiles. For example, after their reaction with ANP, deprotonation of the aniline moiety can trigger the reductive elimination to give the indoline side product (iii).[16] In addition, nitrene is known to be generated from benzoyloxy mono N-alkylamines upon reacting with a transition metal,[17] which can lead to decomposition of the amine electrophile and/or undesired pathways, including ortho arylation (for side product i), reductive elimination of ANP (for side product ii) and ipso amination (for side product iv). Moreover, phosphines are known to reduce related NH-OBz species to form iminophosphoranes,[18] which is another pathway for decomposition of the amine electrophiles. Hence, these competing reactions underscore the challenges of introducing secondary or primary amino groups via the Pd/NBE-catalyzed ortho amination.
Scheme 2.

Challenges of ortho amination with mono N-alkylamine-derived electrophiles. E, electrophile.
To realize the desired ortho amination for preparing secondary anilines, the key is to avoid decomposition of the mono N-alkylamine reagents and to avoid NBE-mediated reductive elimination. We hypothesized that the use of an electron-deficient less-nucleophilic phosphine ligand could diminish unwanted reduction of the amine electrophile and the use of C2-substituted NBE should minimize NBE-mediated reductive elimination.[19] To test this hypothesis, 2-iodoanisole (1a) was employed as the model substrate and the ortho amination/ipso Heck coupling reaction was tested using N-(tert-butyl)-O-benzoylhydroxylamine (2a) as the electrophile. Indeed, after examining a range of phosphine ligands and structurally modified NBEs (smNBEs),[20] the electron-poor tris[3,5-bis(trifluoromethyl)phenyl]phosphine (L1) and C2-substituted NBE (N1)[19] were found to be the optimal ligand/NBE combination, which ultimately delivered the desired secondary aniline product (4a) in 79% yield (Table 1, entry 1). Under the standard conditions, the previous side products (i-iv) were effectively inhibited, and the current side reaction is the direct ipso Heck. A number of control experiments were conducted to understand the role of the reactants. First, unsurprisingly, in the absence of Pd(OAc)2, ligand or NBE, no desired product was observed (entries 2, 5, and 7). Other palladium pre-catalysts, such as PdCl2 and Pd(COD)Cl2, were somewhat less efficient (entries 3 and 4). Given the importance of phosphine ligands to the Pd/NBE catalysis as first demonstrated by Lautens,[21] monodentate phosphine ligands with varying electronic properties were tested (entry 6). While simple PPh3 gave almost no desired product with obvious formation of four-membered side product (ii), the use of the more electron deficient P(4-CF3C6H4)3 gave much improved yield, likely through inhibiting the iminophosphorane formation. The best result was obtained with tris[3,5-bis(trifluoromethyl)phenyl]phosphine (L1); in contrast, P(C6F5)3 gave no reactivity, probably because it is too electron-deficient to promote oxidative addition of the aryl iodide substrate. The electronic effect of the benzoate leaving group on the electrophile was also assessed. While the electro-rich variant (2b) gave a comparable result, the more electro-deficient 2c, i.e. the one with a better leaving group, led to considerably lower yield, possibly owing to the decreased stability of 2c under the reaction conditions. On the other hand, the NBE effect is also evident (entry 8). Except C2-substituted NBEs (N1, isopropyl ester-derived N3[22] and amide-derived N4[23]), other smNBEs and simple NBE gave very low yield of the desired products and accompanied with some reductive elimination side products (ii and iii). THF proves to be the optimal solvent, and the use of less polar toluene or more polar acetonitrile was not effective (entries 9 and 10). Finally, replacing Cs2CO3 with K2CO3 led to significantly lower yield; however, the use of CsOAc afforded comparable efficiency (entries 11 and 12).
Table 1.
Control experiments.

| |||
|---|---|---|---|
|
| |||
| Entry[a] | Variation from the “standard conditions” | Yield of 4a (%)[b] | Yield of 4a’ (%)[b] |
|
| |||
| 1 | none | 79 | 12 |
| 2 | No Pd | 0 | 0 |
| 3 | PdCl2 instead of Pd(OAc)2 | 56 | 16 |
| 4 | Pd(COD)Cl2 instead of Pd(OAc)2 | 52 | 20 |
| 5 | No L1 | 0 | 0 |
| 6 | other ligands instead of L1 | listed above | |
| 7 | No N1 | 0 | <5 |
| 8 | other norbornenes instead of N1 | listed above | |
| 9 | toluene instead of THF | 16 | 15 |
| 10 | CH3CN instead of THF | trace | trace |
| 11 | K2CO3 instead of Cs2CO3 | 5 | 28 |
| 12 | CsOAc instead of Cs2CO3 | 62 | 16 |
Reaction conditions: 1 (0.10 mmol), 2 (0.2 mmol), 3 (0.11 mmol), Pd(OAc)2 (0.01 mmol), L1 (0.025 mmol), N1 (0.10 mmol), Cs2CO3 (0.25 mmol), THF (1.0 mL), 100 °C, 12 h.
Yield was determined by 1H NMR using dibromomethane as the internal standard. COD, 1,5-cyclooctadiene.
With the optimized reaction conditions in hand, the scope of the ortho amination/ipso Heck reaction was explored (Table 2). Regarding the aryl iodide substrates,[24] those featuring diverse electronic properties, encompassing electron-donating or electron-withdrawing groups (4b–4t), all furnished desired products in good yield. This method exhibited excellent tolerance toward various functional groups (FGs), including fluoro (4b), bromo (4c, 4g and 4j), chloro (4k), ester (4h and 4p), silyl ether (4i), secondary aniline (4l), tertiary amine (4m), free alcohol (4n), amide (4o), cyclopropane (4p), epoxide (4q), and terminal olefin (4r). In addition, aryl iodides with diverse fused structures, such as 2,3-dihydrobenzofuran (4t), naphthalene (4u), and dibenzofuran (4v), also afforded the desired products in decent yield. Moreover, pyridine-based substrates (4w, 4x and 4y) also worked well. Ph-Davephos[15,25] worked better for the more electron-rich substrate (4s). Furthermore, the method was amenable to complex substrates derived from natural products and pharmaceuticals (4z, 4aa and 4ab), indicating potential for late-stage modifications. Simple para-substituted aryl iodides gave a di-ortho amination product (4ac) under the current conditions, albeit in lower yield.
Table 2.
Reaction scope of the ortho amination/ipso Heck.[a]

|
Reaction conditions: 1 (0.20 mmol), 2 (0.40 mmol), 3 (0.22 mmol), Pd(OAc)2 (0.02 mmol), L1 (0.05 mmol), N1 (0.20 mmol), Cs2CO3 (0.5 mmol), THF (2.0 mL), 100 °C, 12 h. Isolated yield.
Ph-Davephos was used instead of L1.
4-Iodoanisole 1ac (0.20 mmol) was used as the substrate, and 0.8 mmol of 2a was used.
0.4 mmol of N1 was used. TBS, tert-butyldimethylsilyl; Boc, tert-butyloxycarbonyl.
Besides tert-butyl acrylate, a variety of Michael acceptors, including methyl acrylate (4ad), 2-(trimethylsilyl)ethyl acrylate (4ae) and acrylamides (4af and 4ag), were found to be effective for the ipso alkenylation. The scope of the amine electrophile was also evaluated using various N-benzoyloxy mono-alkyl amines (4ah-4aq). While at this stage only N-tertiary alkylamine-based reagents afforded good yield (vide infra, Scheme 3), a variety of interesting scaffolds derived from carbocycles (4al), heterocycles (4am-4ao), and [2.2.2] bicycles (4ap), along with meaningful FGs (4ah-4ak), can be tolerated. In addition, the ortho amination followed by intramolecular ipso Heck (4aq) can also be realized with the alkene-tethered amine-electrophile. Note that it is not possible to introduce the N-tertiary alkylamino group by the hypothetical two-step procedure: ortho amination with the N-benzyl-N-tertiary alkylamine reagent, followed by benzyl deprotection (Eq. 1), likely due to the steric hindrance of the electrophile.
Scheme 3.
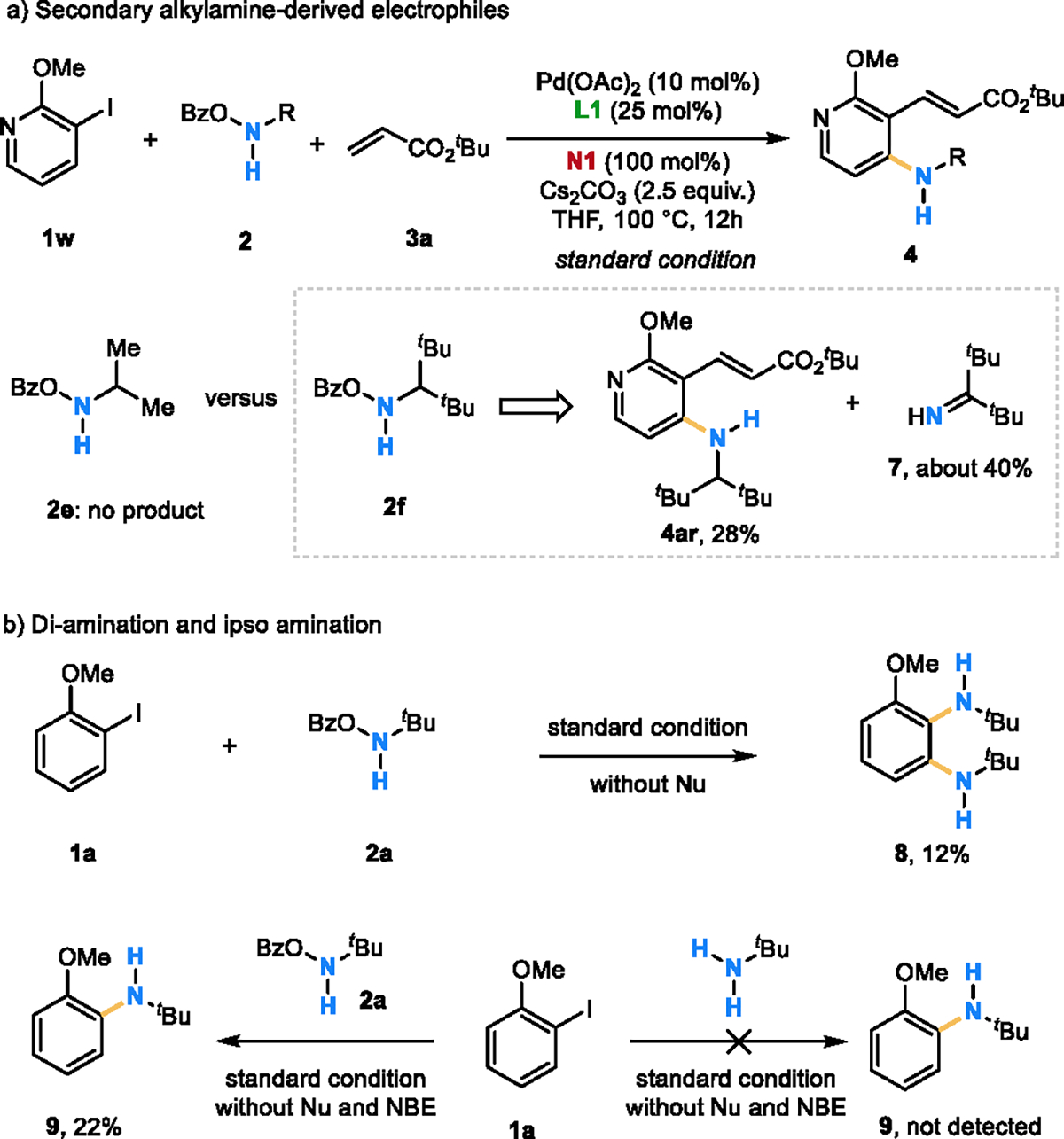
Control experiments. a) Secondary alkyl amine-derived electrophiles can also be used; b) Di-amination and ipso amination have been obtained with the amine electrophile.
 |
Apart from the Heck quench, alkynylation also proved to be feasible for the ipso functionalization (Table 3).[26] Simple (triisopropylsilyl)acetylene (5a)[27] was found to be the most effective coupling partner for this reaction, whereas other reagents, such as phenylacetylene, 2-methyl-4-phenyl-3-butyn-2-ol, and phenylpropiolic acid, were not effective.
Table 3.
Ortho amination with ipso alkyne termination.[a]

|
Reaction conditions: 1 (0.20 mmol), 2 (0.40 mmol), 3 (0.22 mmol), Pd(OAc)2 (0.02 mmol), L1 (0.05 mmol), N1 (0.40 mmol), Cs2CO3 (0.5 mmol), THF (2.0 mL), 100 °C, 12 h. Isolated yield. TIPS, triisopropylsilyl.
A series of additional control experiments were carried out to gain insight into the reactivity of the amine electrophile. As shown in Scheme 3a, while the isopropylamine-derived reagent (2e) failed to give any desired ortho amination product, the use of a bulkier secondary alkylamine (2f) afforded the desired product 4ar in 28% yield, accompanied with forming imine side-product 7 in about 40% yield (based on the initial amount of 2f). The reactivity difference between α-tertiary and secondary alkylamine-derived electrophiles and the steric effect observed with different secondary alkylamine-derived electrophiles could be possibly explained by the relative stability of the amine electrophiles and/or aminopalladium intermediates under the reaction conditions. The formation of imine 7 indicates an E2 elimination or a Pd-mediated β-H elimination pathway with secondary alkylamine-derived reagents. Interestingly, in the absence of the nucleophile, 1,2-di-amination product 8 was isolated in 12% yield under the standard condition (Scheme 3b). This is a surprising result because intermolecular ipso amination has not been successful by directly using amines as the nucleophile.[10d,28] In addition, our further control experiments show that the Pd-catalyzed ipso amination cannot take place with the free amine under the standard condition (in the absence of nucleophile and NBE).[29] In contrast, replacement of the free amine with the electrophilic 2a led to the formation of the ipso amination product in 22%. Although the exact source of the reductant in the latter reaction remains to be uncovered, these unusual outcomes suggest that the observed ipso amination may not go through the normal Buchwald–Hartwig pathway. This, on the other hand, implies a Pd-catalyzed nitrene-mediated ipso amination,[30] as it is known that nitrene can be generated from this type of N–O reagents.[17] Altogether, these experiments suggest that β- and/or α-elimination of N-mono alkylamine reagents can compete with the desired ortho amination pathway.
One benefit of introducing the t-butylamino group by this method is that the t-butyl group can be easily removed under acidic conditions in high yield (Scheme 4a).[31] As a result, this method offers a convenient approach to access primary anilines, which can be further transformed to other important moieties, such as amides, diarylamines, heterocycles, etc. For example, the primary amine generated from the ortho amination product can cyclize with the enoate group to afford a 2-hydroxyquinoline product (11) upon treatment with hydrochloric acid (Scheme 4b). This transformation can be operated in either two steps or a one-pot manner, which involves t-butyl group removal, alkene isomerization and lactam bond formation. Notably, the previous route to access 2-hydroxyquinoline 11 requires five steps from aniline 12;[32] here our method offers a single-step approach to prepare this important heterocycle directly from the commercially available aryl iodide (1b). Finally, a unique application of the ortho amination/ipso alkynylation is the rapid preparation of aza-indole products from the corresponding simple aryl iodides (Scheme 4c). The triisopropylsilyl (TIPS) group of the reaction intermediate can be removed in situ by adding tetrabutylammonium fluoride (TBAF), and the resulting terminal alkyne in compound 13 can undergo a gold (III)-catalyzed annulation[33] to deliver 5-aza-indole 14.
Scheme 4.

Synthetic applications. a) Introducing primary amines via N-deprotection; b) Efficient preparation of functionalized 2-hydroxyquinoline; c) Synthesis of 5-aza-indole. TFA, trifluoroacetic acid.
In summary, beyond forming tertiary anilines, we have developed the first method to directly prepare secondary amines via the Pd/NBE catalysis. Diverse aryl substrates and N-tertiary-alkyl amine-derived electrophiles are identified as suitable coupling partners, leading to versatile synthetic applications, such as primary amino group installation and concise heterocycle formations. While it remains challenging to introduce other types of amino groups at this stage, they can potentially be accessed via the primary amine intermediate. In addition, important mechanistic insights have been obtained, and preliminary success to install a bulky secondary alkyl amino group has been achieved. Efforts on new reagent/catalyst design to further expand the scope of the nitrogen-containing moieties that can be introduced, as well as deeper mechanistic understanding of these transformations, are ongoing.
Supplementary Material
Acknowledgements
The research was supported by the National Institute of General Medical Sciences (R01GM124414). We thank Professor Qiu Wang (Duke University) for inspiring discussions. We also thank S. Anferov and A. Filatov (University of Chicago) for the X-ray crystallography.
Footnotes
Supporting Information
The authors have cited additional references within the Supporting Information.[34–57]
References
- [1].a) Ricci A, Amino Group Chemistry: from Synthesis to the Life Sciences, John Wiley & Sons, 2008; [Google Scholar]; b) McGrath NA, Brichacek M, Njardarson JT, A Graphical Journey of Innovative Organic Architectures That Have Improved Our Lives, J. Chem. Educ. 2010, 87, 1348–1349. [Google Scholar]
- [2].Rohrbach S, Smith AJ, Pang JH, Poole DL, Tuttle T, Chiba S, Murphy JA, Concerted Nucleophilic Aromatic Substitution Reactions, Angew. Chem., Int. Ed. 2019, 58, 16368–16388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].a) Hartwig JF, Negishi E, Handbook of Organopalladium Chemistry for Organic Synthesis, Wiley-Interscience, New York, 2002; [Google Scholar]; b) Jiang L, Buchwald SL, Metal-Catalyzed Cross-Coupling Reactions, 2nd, completely rev. and enl. ed., Wiley-VCH, Weinheim, 2004; [Google Scholar]; c) Hartwig JF, Evolution of a Fourth Generation Catalyst for the Amination and Thioetherification of Aryl Halides, Acc. Chem. Res. 2008, 41, 1534–1544; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Aubin Y, Fischmeister C, Thomas CM, Renaud JL, Direct Amination of Aryl Halides with Ammonia, Chem. Soc. Rev. 2010, 39, 4130–4145; [DOI] [PubMed] [Google Scholar]; e) Maiti D, Fors BP, Henderson JL, Nakamura Y, Buchwald SL, Palladium-Catalyzed Coupling of Functionalized Primary and Secondary Amines with Aryl and Heteroaryl Halides: Two Ligands Suffice in Most Cases, Chem. Sci. 2011, 2, 2428–2428; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Surry DS, Buchwald SL, Dialkylbiaryl Phosphines in Pd-Catalyzed Amination: a User’s Guide, Chem. Sci. 2011, 2, 27–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].a) Monnier F, Taillefer M, Copper-Catalyzed C(aryl)-N Bond Formation, Amination and Formation of Sp2 C-N Bonds 2013, 46, 173–204; [Google Scholar]; b) Echeverry-Gonzalez CA, Kouznetsov VV, in Copper in N-Heterocyclic Chemistry, Elsevier, 2021, pp. 399–430. [Google Scholar]
- [5].a) Chan DMT, Monaco KL, Wang RP, Winters MP, New N- and O-Arylations with Phenylboronic Acids and Cupric Acetate, Tetrahedron Lett. 1998, 39, 2933–2936; [Google Scholar]; b) Evans DA, Katz JL, West TR, Synthesis of Diaryl Ethers through the Copper-Promoted Arylation of Phenols with Arylboronic Acids. An Expedient Synthesis of Thyroxine, Tetrahedron Lett. 1998, 39, 2937–2940; [Google Scholar]; c) Lam PYS, Clark CG, Saubern S, Adams J, Winters MP, Chan DMT, Combs A, New Aryl/Heteroaryl C-N Bond Cross-Coupling Reactions via Arylboronic Acid Cupric Acetate Arylation, Tetrahedron Lett. 1998, 39, 2941–2944. [Google Scholar]
- [6].a) Berman AM, Johnson JS, Copper-Catalyzed Electrophilic Amination of Diorganozinc Reagents, J. Am. Chem. Soc. 2004, 126, 5680–5681; [DOI] [PubMed] [Google Scholar]; b) Hendrick CE, Wang Q, Emerging Developments Using Nitrogen-Heteroatom Bonds as Amination Reagents in the Synthesis of Aminoarenes, J. Org. Chem. 2017, 82, 839–847. [DOI] [PubMed] [Google Scholar]
- [7].a) Louillat ML, Patureau FW, Oxidative C-H Amination Reactions, Chem. Soc. Rev. 2014, 43, 901–910; [DOI] [PubMed] [Google Scholar]; b) Park Y, Kim Y, Chang S, Transition Metal-Catalyzed C-H Amination: Scope, Mechanism, and Applications, Chem. Rev. 2017, 117, 9247–9301. [DOI] [PubMed] [Google Scholar]
- [8].a) For selected reviews, see: Jiao J, Murakami K, Itami K, Catalytic Methods for Aromatic C-H Amination: An Ideal Strategy for Nitrogen-Based Functional Molecules, ACS Catal. 2016, 6, 610–633; [Google Scholar]; b) Kim H, Chang S, Transition-Metal-Mediated Direct C-H Amination of Hydrocarbons with Amine Reactants: The Most Desirable but Challenging C-N Bond-Formation Approach, ACS Catal. 2016, 6, 2341–2351. [Google Scholar]
- [9].a) Dong Z, Dong G, Ortho vs ipso: Site-Selective Pd and Norbornene-Catalyzed Arene C-H Amination Using Aryl Halides, J. Am. Chem. Soc. 2013, 135, 18350–18353; [DOI] [PubMed] [Google Scholar]; b) Dong Z, Lu G, Wang JC, Liu P, Dong G, Modular ipso/ortho Difunctionalization of Aryl Bromides via Palladium/Norbornene Cooperative Catalysis, J. Am. Chem. Soc. 2018, 140, 8551–8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].a) For the seminal work, see: Catellani M, Frignani F, Rangoni A, A Complex Catalytic Cycle Leading to a Regioselective Synthesis of o,o’-Disubstituted Vinylarenes, Angew. Chem., Int. Ed. 1997, 36, 119–122; For selected reviews, see: [Google Scholar]; b) Ye JT, Lautens M, Palladium-Catalysed Norbornene-Mediated C-H Functionalization of Arenes, Nat. Chem. 2015, 7, 863–870; [DOI] [PubMed] [Google Scholar]; c) Della Ca N, Fontana M, Motti E, Catellani M, Pd/Norbornene: A Winning Combination for Selective Aromatic Functionalization via C-H Bond Activation, Acc. Chem. Res. 2016, 49, 1389–1400; [DOI] [PubMed] [Google Scholar]; d) Wang JC, Dong G, Palladium/Norbornene Cooperative Catalysis, Chem. Rev. 2019, 119, 7478–7528; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Marchese AD, Mirabi B, Lautens M, Recent Developments of Palladium- and Rhodium-Catalyzed β-Carbon Elimination Strategies, Synthesis 2023, 55, 2285–2303. [Google Scholar]
- [11].For a recent review, see: Dong X, Liu Q, Dong YH, Liu H, Transition-Metal-Catalyzed Electrophilic Amination: Application of O-Benzoylhydroxylamines in the Construction of the C-N Bond, Chem. - Eur. J. 2017, 23, 2481–2511. [DOI] [PubMed] [Google Scholar]
- [12].a) For selected examples of ortho amination, see: Chen ZY, Ye CQ, Zhu H, Zeng XP, Yuan JJ, Palladium/Norbornene-Mediated Tandem C-H Amination/C-I Alkenylation Reaction of Aryl Iodides with Secondary Cyclic O-Benzoyl Hydroxylamines and Activated Terminal Olefins, Chem. - Eur. J. 2014, 20, 4237–4241; [DOI] [PubMed] [Google Scholar]; b) Ye CQ, Zhu H, Chen ZY, Synthesis of Biaryl Tertiary Amines through Pd/Norbornene Joint Catalysis in a Remote C-H Amination/Suzuki Coupling Reaction, J. Org. Chem. 2014, 79, 8900–8905; [DOI] [PubMed] [Google Scholar]; c) Zhou PX, Ye YY, Ma JW, Zheng L, Tang Q, Qiu YF, Song B, Qiu ZH, Xu PF, Liang YM, Palladium-Catalyzed/Norbornene-Mediated ortho-Amination/N-Tosylhydrazone Insertion Reaction: An Approach to the Synthesis of ortho-Aminated Vinylarenes, J. Org. Chem. 2014, 79, 6627–6633; [DOI] [PubMed] [Google Scholar]; d) Pan SF, Ma X, Zhong DN, Chen WZ, Liu MC, Wu HY, Palladium-Catalyzed One-Pot Consecutive Amination and Sonogashira Coupling for Selective Synthesis of 2-Alkynylanilines, Adv. Synth. Catal. 2015, 357, 3052–3056; [Google Scholar]; e) Shi H, Babinski DJ, Ritter T, Modular C-H Functionalization Cascade of Aryl Iodides, J. Am. Chem. Soc. 2015, 137, 3775–3778; [DOI] [PubMed] [Google Scholar]; f) Sun FG, Gu ZH, Decarboxylative Alkynyl Termination of Palladium-Catalyzed Catellani Reaction: A Facile Synthesis of α-Alkynyl Anilines via C-H Amination and Alkynylation, Org. Lett. 2015, 17, 2222–2225; [DOI] [PubMed] [Google Scholar]; g) Luo B, Gao JM, Lautens M, Palladium-Catalyzed Norbornene-Mediated Tandem Amination/Cyanation Reaction: A Method for the Synthesis of ortho Aminated Benzonitriles, Org. Lett. 2016, 18, 4166–4169; [DOI] [PubMed] [Google Scholar]; h) Wang J, Gu ZH, Synthesis of 2-(1-Alkoxyvinyl)anilines by Palladium/Norbornene-Catalyzed Amination Followed by Termination with Vinyl Ethers, Adv. Synth. Catal. 2016, 358, 2990–2995; [Google Scholar]; i) Fan LX, Liu JJ, Bai L, Wang YY, Luan XJ, Rapid Assembly of Diversely Functionalized Spiroindenes by a Three-Component Palladium-Catalyzed C-H Amination/Phenol Dearomatization Domino Reaction, Angew. Chem., Int. Ed. 2017, 56, 14257–14261; [DOI] [PubMed] [Google Scholar]; j) Fu WC, Zheng B, Zhao QY, Chan WTK, Kwong FY, Cascade Amination and Acetone Monoarylation with Aryl Iodides by Palladium/Norbornene Cooperative Catalysis, Org. Lett. 2017, 19, 4335–4338; [DOI] [PubMed] [Google Scholar]; k) Whyte A, Olson ME, Lautens M, Palladium-Catalyzed, Norbornene-Mediated, ortho-Amination ipso-Amidation: Sequential C-N Bond Formation, Org. Lett. 2018, 20, 345–348; [DOI] [PubMed] [Google Scholar]; l) Zhang BS, Li YK, An Y, Zhang Z, Liu C, Wang XG, Liang YM, Carboxylate Ligand-Exchanged Amination/C(sp3)-H Arylation Reaction via Pd/Norbornene Cooperative Catalysis, ACS Catal. 2018, 8, 11827–11833; [Google Scholar]; m) Gao QW, Liu ZS, Hua Y, Li LS, Cheng HG, Cong HJ, Zhou QH, A Palladium/Norbornene Cooperative Catalysis to Access N-Containing Bridged Scaffolds, Chem. Commun. 2019, 55, 8816–8819; [DOI] [PubMed] [Google Scholar]; n) An Y, Zhang BS, Zhang Z, Liu C, Gou XY, Ding YN, Liang YM, A Carboxylate-Assisted Amination/Unactivated C(sp2)-H Arylation Reaction via a Palladium/Norbornene Cooperative Catalysis, Chem. Commun. 2020, 56, 5933–5936; [DOI] [PubMed] [Google Scholar]; o) Zhang BS, Li YK, Gou XY, Zhang Z, An Y, Wang XG, Liang YM, DMAP and PivOH-Promoted Amination/Allenization Reaction, Chem. Commun. 2020, 56, 9202–9205; [DOI] [PubMed] [Google Scholar]; p) Zhang Z, Zhang BS, Li KL, An Y, Liu C, Gou XY, Liang YM, Palladium-Catalyzed Amination/Dearomatization Reaction of Indoles and Benzofurans, J. Org. Chem. 2020, 85, 7817–7839; [DOI] [PubMed] [Google Scholar]; q) Wang CT, Li M, Ding YN, Wei WX, Zhang Z, Gou XY, Jiao RQ, Wen YT, Liang YM, Alkylation-Terminated Catellani Reactions by Cyclobutanol C-C Cleavage, Org. Lett. 2021, 23, 786–791; [DOI] [PubMed] [Google Scholar]; r) Bao ZC, Wu CQ, Wang JB , Palladium-Catalyzed Catellani Reaction with 1,1-Bis[(pinacolato)boryl]methane as the Nucleophilic Component, Eur. J. Org. Chem. 2023, 26; [Google Scholar]; s) Du XY, Yang XZ, Wang H, Li XG, Wang MR, Li X, Tao Y, Yang YX, Tan XQ, Ren F, Zhou PX, Liang YM, Homoallyl Alcohol as an Allylation Reagent for Termination of the Catellani-Lautens Reaction via Retro-Allylation, Org. Chem. Front. 2023, 10, 898–904; [Google Scholar]; t) Zhang BS, Zhao SY, Li SX, Jia WY, Yang YX, Wang YM, Gou XY, Liang YM, Wang XC, Quan ZJ, Synthesis of C4-Aminated Carbazoles and Their Derivatives via Pd/NBE Chemistry, J. Org. Chem. 2023, 88, 1786–1795. [DOI] [PubMed] [Google Scholar]
- [13].a) Li J, Yang Y, Liu YX, Liu Q, Zhang LZ, Li XJ, Dong YH, Liu H, Palladium/Norbornene Catalyzed ortho Amination/Cyclization of Aryl Iodide: Process to 3-Methyl-indole Derivates and Controllable Reductive Elimination against the Second Amination, Org. Lett. 2021, 23, 2988–2993; [DOI] [PubMed] [Google Scholar]; b) Rago AJ, Dong G, Synthesis of C3,C4-Disubstituted Indoles via the Palladium/Norbornene-Catalyzed ortho-Amination/ipso-Heck Cyclization, Org. Lett. 2021, 23, 3755–3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liu X, Wang J, Dong G, Modular Entry to Functionalized Tetrahydrobenzo[b]azepines via the Palladium/Norbornene Cooperative Catalysis Enabled by a C7-Modified Norbornene, J. Am. Chem. Soc. 2021, 143, 9991–10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wu Z, Xu XL, Wang J, Dong G, Carbonyl 1,2-Transposition Through Triflate-Mediated α-Amination, Science 2021, 374, 734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].a) Wu FF, Wang HH, Chen WZ, Synthesis and Characterization of Palladium Amido Complexes Containing Pincer CNO Ligands through Nitrene Insertion, Appl. Organomet. Chem. 2019, 33; [Google Scholar]; b) Ghasemi M, Jafarpour F, Habibi A, Palladium/Norbornene Chemistry in the Synthesis of Polycyclic Indolines with Simple Nitrogen Sources, Synthesis 2020, 52, 2092–2098; [Google Scholar]; c) Jafarpour F, Jalalimanesh N, Teimouri M, Shamsianpour M, Palladium/Norbornene Chemistry: an Unexpected Route to Methanocarbazole Derivatives via Three Csp3-Csp2/Csp3-N/Csp2-N Bond Formations in a Single Synthetic Sequence, Chem. Commun. 2015, 51, 225–228. [DOI] [PubMed] [Google Scholar]
- [17].Noda H, Asada Y, Shibasaki M, O-Benzoylhydroxylamines as Alkyl Nitrene Precursors: Synthesis of Saturated N-Heterocycles from Primary Amines, Org. Lett. 2020, 22, 8769–8773. [DOI] [PubMed] [Google Scholar]
- [18].Lin S, Lin B, Zhang ZT, Chen JH, Luo YS, Xia YZ, Construction of N-Acyliminophosphoranes via Iron(II)-Catalyzed Imidization of Phosphines with N-Acyloxyamides, Org. Lett. 2022, 24, 3302–3306. [DOI] [PubMed] [Google Scholar]
- [19].Shen PX, Wang XC, Wang P, Zhu RY, Yu JQ, Ligand-Enabled Meta-C-H Alkylation and Arylation Using a Modified Norbornene, J. Am. Chem. Soc. 2015, 137, 11574–11577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li R, Dong G, Structurally Modified Norbornenes: A Key Factor to Modulate Reaction Selectivity in the Palladium/Norbornene Cooperative Catalysis, J. Am. Chem. Soc. 2020, 142, 17859–17875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lautens M, Piguel S, A New Route to Fused Aromatic Compounds by Using a Palladium-catalyzed Alkylation - Alkenylation Sequence, Angew. Chem., Int. Ed. 2000, 39, 1045–1046. [DOI] [PubMed] [Google Scholar]
- [22].Li R, Dong G, Direct Annulation between Aryl Iodides and Epoxides through Palladium/Norbornene Cooperative Catalysis, Angew. Chem., Int. Ed. 2018, 57, 1697–1701. [DOI] [PubMed] [Google Scholar]
- [23].Wang J, Dong Z, Yang C, Dong G, Modular and Regioselective Synthesis of All-Carbon Tetrasubstituted Olefins Enabled by an Alkenyl Catellani Reaction, Nat. Chem. 2019, 11, 1106–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Attempts to use aryl bromides were unfruitful at this stage, leading to mainly recovery of the substrate.
- [25].Ph-Davephos is a pre-ligand in this system, which is converted to a phosphafluorene ligand. For our recent study, see: Wang JC, Zhou Y, Xu XL, Liu P, Dong GB, Entry to 1,2,3,4-Tetrasubstituted Arenes through Addressing the “Meta Constraint” in the Palladium/Norbornene Catalysis, J. Am. Chem. Soc. 2020, 142, 3050–3059. Ph-Davephos gave lower yield than L1 for model substrate 1a (58%, NMR yield) and was far less efficient for electron-deficient substrates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Other ipso termination, such as hydrogen or Suzuki quenches, remains challenging at this stage, leading to ortho, ipso diamination products, see Scheme 3.
- [27].a) Motti E, Rossetti M, Bocelli G, Catellani M, Palladium Catalyzed Multicomponent Reactions in Ordered Sequence: New Syntheses of o, o’-Dialkylsubstituted Diarylacetylenes and Diarylalkylidenehexahydromethanofluorenes, J. Organomet. Chem. 2004, 689, 3741–3749; [Google Scholar]; b) Lei CH, Jin XJ, Zhou JR, Palladium-Catalyzed Alkynylation and Concomitant Ortho Alkylation of Aryl Iodides, ACS Catal. 2016, 6, 1635–1639; [Google Scholar]; c) Lv WW, Chen YH, Wen S, Ba D, Cheng GL, Modular and Stereoselective Synthesis of C-Aryl Glycosides via Catellani Reaction, J. Am. Chem. Soc. 2020, 142, 14864–14870. [DOI] [PubMed] [Google Scholar]
- [28].Ye J, Lautens M, in Remote C-H Bond Functionalizations: Methods and Strategies in Organic Synthesis, 2021, pp. 56–114. [Google Scholar]
- [29].To further check this result, the use of a heavier amine was attempted under the same conditions, which also did not yield any ipso amination product.
- [30].Efforts to trap the nitrene species by adding different alkenes were unfruitful at this stage.
- [31].Deposition number 2303994 (for 10), 2308602 (for 4as) and 2303993 (for 11) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- [32].Shimizu H, Kawano Y, Ishikawa S, Uematsu Y, Shinohara T, Itotani M, Haraguchi Y, Takemura I, Kaneshige A, Nakai Y, Hariguchi N, Hayashi Y, Matsumoto M, (Otsuka Pharmaceutical Co., Ltd.), WO, 2016, p. 443 pages. [Google Scholar]
- [33].Das S, Recent Progress in Gold-Catalyzed Reactions of Alkynes for the Construction of Indole Frameworks, Asian J. Org. Chem. 2023, 12, e202300267. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


