Highlights
-
•
Scientific question: An effective technique of genus-level identification for human enterovirus (HEV) is highly needed.
-
•
Evidence before this study: The gold-standard method for HEV, quantitative realtime reverse transcription polymerase chain reaction (qRT-PCR), still has shortfalls in diagnostic sensitivity and timeliness. In our previous study, RAP (recombinase-aided PCR), with a wide potential application in the detection of DNA/RNA pathogens, is more rapid and sensitive than the conventional qPCR.
-
•
New findings: We established a one-step real-time reverse-transcription RAP (RT-RAP) to detect HEV rapidly. Furthermore, RT-RAP in this study was evaluated by 15 HEV types with high sensitivity and universality.
-
•
Significance of the study: The established RT-RAP provides a reliable and sensitive method to rapidly identify HEV. Moreover, optimization of RT-RAP in this study further improved the RT-RAP’s practicality.
Keywords: Human enteroviruses, Nucleic acid detection, RT-RAP assay
Abstract
Human enteroviruses (HEVs) include many different types that cause a wide range of diseases, and an effective method of genus-level identification has therefore significant clinical implications. However, quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR), the gold-standard method, still has shortfalls in diagnostic sensitivity and timeliness. Here we established a one-step real-time reverse-transcription recombinase-aided PCR assay (RT-RAP) to detect HEV fragment within an hour. The RT-RAP assay showed a detection limit of 5 copies/μL using recombinant plasmids and was extensively verified using 15 HEV strains. Among 15 types of HEV (species A-C), the sensitivity of RT-RAP was approximately 2–8 folds lower than that of the qRT-PCR in 9 types, and no-cross reaction with other viruses was observed. RT-RAP was further applied to analyze CSF and fecal specimens; the clinical performance demonstrated that the RT-RAP and the commercial qRT-PCR kit provided consistent results. These results indicated that RT-RAP assay may be a promising approach for rapid and sensitive detection of HEV.
1. Introduction
Enteroviruses (EVs) are a widespread class of picornaviruses. Human enterovirus (HEV) can be categorized into four species: EV-A to D, including over 100 serotypes [1], [2], [3], [4]. While most humans show mild symptoms after infection, HEVs are associated with some severe diseases like meningitis and paralysis, which may be life-threatening [5], [6], [7]. Over the last decade, there has been an increasing number of sporadic cases of polio-like illness with central or peripheral paralysis caused by different HEVs globally [8]. Given the health and economic burden, rapid and sensitive identification of HEV is highly in demand.
It should be noted that nucleic acid detection has been widely used for disease diagnostics in clinical laboratories. Routine practice can identify HEV within 3 h, such as quantitative real-time reverse transcription-PCR (qRT-PCR). However, the detection capacity of the qRT-PCR assay is not satisfying in detecting samples with lower viral loads, like cerebrospinal fluid (CSF) [9], [10]. As a result, the pathogen detection rate is underestimated, and the number of confirmed pathogen diagnoses is inadequate [10]. To date, many PCR-based molecular assays for HEV have been developed such as two-step nested RT-PCR [11] and single-tube real-time nested RT-PCR (RTN RT-PCR) [9], [12]. These methods have problems such as easy contamination or being time-consuming, which restrict their widespread application.
Recombinase-aided amplification (RAA) is an emerging isothermal amplification method and has been widely used to detect microbial pathogens [13], [14], [15], [16]. Within 5–20 min, the target gene can be amplified several million times [17]. Real-time detection of the RAA products can be achieved by adding exonuclease III (Exo) and Exo-probe (46–52 bp) [18]. However, RAA does not fit for the detection of HEV with high sequence variability because the long RAA probes are difficult to design to meet the specificity requirements, and the introduction of degenerated bases in the probe can limit the RAA sensitivity and result in non-specific amplification. In our previous work, we reported a novel nested PCR approach, named RAP (recombinase-aided PCR), by integrating the merits of RAA and qPCR, which is superior to the conventional qPCR and avoids the design of the Exo-RAA probe [13]. In this research, we attempted to develop a one-step RT-RAP assay for the detection of HEV. Furthermore, the usage of docosane further improved the RT-RAP’s practicality in this study.
2. Material and methods
2.1. HEV RNA and clinical samples
Fifteen types of HEV RNA were obtained from the National Laboratory for Poliomyelitis, National Institute for Viral Disease Control and Prevention of the Chinese Center for Disease Control and Prevention, including EV-A71, coxsackievirus group A serotypes (CVA1, 4, 5, 10, 11, 12, 16), coxsackievirus group B serotypes (CVB1, 2, 4, 5), echoviruses (E1, 11), and poliovirus type 1 (PV1).
A total of 139 CSF specimens were collected from patients with suspected neurological infections visiting Hebei Children’s Hospital (Shijiazhuang, Hebei, China) and Hebei General Hospital (Shijiazhuang, Hebei, China) between June 2022–November 2022. In addition, 42 nucleic acid samples from stool specimens, 23 of which tested positive for CVA6 by qRT-PCR, were obtained from the Yantai Center for Disease Control and Prevention. This research was authorized by the Institutional Review Boards of the National Institute for Viral Disease Control and Prevention, Center for Disease Control and Prevention of China (approved number: LL2022001). The patients were made aware of the study’s goal.
2.2. Nucleic acid extraction
CSF specimens were first thoroughly shaken, then centrifuged at 5,000g for 5 min, and 200 μL supernatant was subjected to total nucleic acid extraction using EX-DNA/RNA Virus Kits 4.0 (Tianlong, Suzhou, China). All nucleic acid samples for this study were either tested immediately or stored at −80 °C until used.
2.3. Primers and probe design
The primers and probe were designed based on the regions of 5′UTR. A multiple sequence alignment of HEVs 5′UTR, submitted to the GenBank database from 2017 to 2021, was performed with the Vector NTI 11.5.1 software to locate the conserved region of HEVs. According to the design requirement, the RAA primers as the RAP outer primers were designed using Oligo7, and the specificity of primers and amplicons was checked by applying BLASTN on the GenBank nucleotide collection (nr/nt) from the National Center for Biotechnology Information (NCBI) database. The RAP inner primers were adopted from a previous study [19]. Primers and probe were synthesized and purified by Shanghai Bioengineering (Shanghai, China) and sequences are shown in Table 1. Additional information on primers can be found in the supplemental information.
Table 1.
Primers and probes designed for the real-time reverse-transcription recombinase-aided PCR (RT-RAP) assay.
| Primers and probes | Sequence 5′–3′ | Primer length (bp) | Reference |
|---|---|---|---|
| Outer-F | CTCCGGCCCCTGAATGCGGCTAATCCYAAC | 30 | This study |
| Outer-R | GATGGCCAATCCAATAGCTATATGGYAACA | 30 | This study |
| Inner-F | GCCCCTGAATGCGGC | 15 | [19] |
| Inner-R | RATTGTCACCATAAGCAGC | 19 | Adapted from [19] |
| Probe | FAM-CGGAACCGACTACTTTGGGTGWCCGT-BHQ | 26 | Adapted from [19] |
Notes: FAM, 6-carboxyfluorescein; BHQ, black hole quencher.
2.4. RT-RAP assay
RT-RAP assay involves two amplification stages in a single tube and we use a thermally removable barrier to separate one tube into two compartments. In the lower compartment, the 40 μL qPCR contained 2.5 μL of forward primer, 2 μL of reverse primer, 0.5 μL of probe, 12.5 μL of qPCR buffer (Entrans qPCR Probe Set V2, ABclonal, Wuhan, China), 2.2 μL of MgCl2, 0.6 μL of dNTP mix, and 2.5 units of DNA polymerase. In the upper compartment, the 10 μL RAA reaction volume contained 2 μL of eluted RNA, 14 mM of magnesium ion (Mg2+), 400 nM of each RAA primer, and RAA reaction buffer (Qitian, Jiangsu, China). A total of 30 μL of docosane (98%, Aladdin) was inserted between the two compartments.
The RT-RAP assay was conducted in Archimed X6 (RocGene Technology Limited, China) and the final procedure was as follows. Two microliter samples were assayed in duplicate reactions in a total volume of 50 μL; above the solid docosane barrier, the 10 μL RT-RAA reaction was initially performed at 42 °C for 20 min and then followed by an initial denaturation at 95 °C for 5 min to deactivate the recombinase and activate DNA polymerase; simultaneously, the barrier melted floating to the top; then the products were dropped into the qPCR mixture and amplified for 20 cycles (15 s at 95 °C and 1 min at 59 °C). Positive and negative controls were included in each analytical round.
2.5. Sensitivity and specificity of the RT-RAP assay
For sensitivity analysis, 10-fold dilutions of a recombinant plasmid from 5 to 5 × 104 copies/μL were used, containing the target fragment at 5′UTR cloned into the vector pUC57 (Tsingke Biotechnology Co., Ltd). Determination of the specificity was performed with nucleic acids of several other RNA and DNA viruses characterized in our laboratory previously. RNA viruses included influenza A, respiratory syncytial virus (RSV), rotavirus, and norovirus genogroups II. DNA viruses were also tested including human adenovirus (ADV) 3 and 7 and Epstein-Barr virus (EBV).
Meanwhile, 15 strains of HEV were also tested to confirm the universality performance of the RT-RAP assay by comparing the results of RT-RAP and qRT-PCR. The qRT-PCR assay of HEV was performed as the previous study described [19].
2.6. Clinical evaluation and HEV typing
A total of 181 nucleic acid samples (139 from CSF and 42 from stool) were tested using RT-RAP and commercial qRT-PCR kits in parallel for comparison. Commercial qRT-PCR kits for HEV were obtained from DaAn Gene Corporation (Guangzhou, China). Moreover, positive CSF samples were subjected to nested RT-PCR in an ArtGene (TM) A300 device (LongGene) using ABScript III One Step RT-qPCR Probe Kit (ABclonal) described previously [11]. The HEV types were confirmed by sending the inner product of nested RT-PCR to Sangon Biotech for Sanger sequencing. The resulting sequences were aligned with HEVs genomes in the NCBI database, and the similarity comparison was obtained by BLAST analysis.
3. Results
3.1. Sensitivity and specificity of the RT-RAP assay
To determine the sensitivity, we performed this RT-RAP assay on serial dilutions of recombinant plasmids containing the EV-A71 5′UTR fragment. The sensitivity of the RT-RAP assay was 5 copies/μL, equivalent to 10 copies per reaction (Fig. 1).
Fig. 1.
Sensitivity test of the real-time reverse-transcription recombinase-aided PCR (RT-RAP) assay using recombinant plasmids. Notes: A panel of serially diluted recombinant plasmid from 5 to 5 × 104 copies/μL and negative control were used to determine the detection limit of the RT-RAP assay.
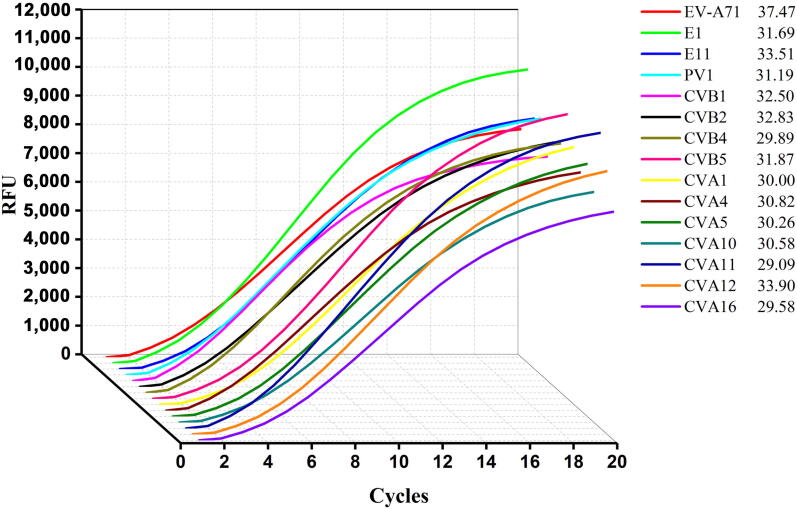
The assay was also validated on a panel of 15 different HEVs belonging to species A–C (6 HEV-A, 6 HEV-B, and 3 HEV-C) to determine the specificity. Amplification of viral genome from these strains showed that all serotypes gave positive signals (Fig. 2). Additionally, no amplification was observed with DNAs (or RNAs) extracted from a panel of 7 other viruses, resulting in an analytic specificity of 100% (Fig. 3).
Fig. 2.
Real-time reverse-transcription recombinase-aided PCR (RT-RAP) assay amplification curves and quantitative real-time reverse transcription PCR (qRT-PCR) assay CT values of 15 HEV.
Fig. 3.
Specificity test of the real-time reverse-transcription recombinase-aided PCR (RT-RAP) assay using 7 pathogens related to human enteroviruses (HEVs). Notes: The RT-RAP assay was positive for EV-A71 and negative for respiratory syncytial virus (RSV), human adenovirus (ADV3 and 7), norovirus genogroups II, rotavirus, Epstein-Barr virus (EBV), and the negative control.
3.2. Universality performance comparison of RT-RAP assay with qRT-PCR
We next compared the universality performance of the RT-RAP and qRT-PCR on 15 HEV types. The nucleic acids in Fig. 2, with Ct (cycle threshold) values ranging from 29.09 to 37.47 by the qRT-PCR assay, were serially two-fold diluted making 7 samples for each type, respectively. Notably, 6 types had the same tested results in two methods among the 15 strains, whereas 1–3 more samples were detected only in the RT-RAP experiments of the other 9 types but not in qRT-PCR. These results show that the RT-RAP assay’s sensitivity was equal to the qRT-PCR assay in 6 types (E1, CVA5, CVA10, CVA11, CVA12, and CVA16) and approximately 2–8 folds lower than that of the qRT-PCR in the other 9 types (EV-A71, E11, PV1, CVB1, CVB2, CVB4, CVB5, CVA1, and CVA4) (Table 2).
Table 2.
Comparison of real-time reverse-transcription recombinase-aided PCR (RT-RAP) assay with quantitative real-time reverse transcription PCR (qRT-PCR) assay performed on 15 diverse human enteroviruses (HEVs).
| Serotypes | Name of Strains | RT-RAP | qRT-PCR |
|
|---|---|---|---|---|
| n/N | n/N | Ct-value | ||
| EV-A71 | EV-A71-FY | 3/7 | 2/7 | 37.18–37.47 |
| CVA4 | GS2008-0266 | 5/7 | 4/7 | 30.82–37.21 |
| CVA5 | HeB2009-018 | 5/7 | 5/7 | 30.26–36.07 |
| CVA10 | HeB2009-035 | 6/7 | 6/7 | 30.58–38.47 |
| CVA12 | HeN2009-817 T | 5/7 | 5/7 | 33.90–38.21 |
| CVA16 | 521–017 T | 7/7 | 7/7 | 29.58–37.22 |
| E1 | XZ2005-T48 | 7/7 | 7/7 | 31.69–36.96 |
| E11 | SD2003-478–2 | 5/7 | 4/7 | 33.51–36.9 |
| CVB1 | SZ2008-1011 | 7/7 | 4/7 | 32.21–35.96 |
| CVB2 | SD2007-518–02 | 7/7 | 5/7 | 32.83–37.26 |
| CVB4 | NM2007-0174 | 7/7 | 6/7 | 29.76–36.65 |
| CVB5 | SD2002-307 | 6/7 | 5/7 | 31.87–38.68 |
| PV1 | CHN15115/Xinjiang/CHN/2011 | 6/7 | 5/7 | 31.19–38.62 |
| CVA1 | HT-THLH02F/XJ/CHN/2011 | 7/7 | 6/7 | 30.0–38.01 |
| CVA11 | XJ2011-653129–17 | 6/7 | 6/7 | 29.09–36.04 |
Notes: n, the number of positive samples detected; N, the total number of samples used in the actual operation. For the nine HEV strains in bold, RT-RAP achieved a higher sensitivity compared with qRT-PCR.
3.3. Clinical performance of RT-RAP assay
Among 139 nucleic acid samples from CSF specimens, three had a positive RT-RAP result, consistent with a diagnosis of the commercial qRT-PCR kit (Ct 33.92–37.27). For these 3 positive samples, the purified inner products of nested RT-PCR were subjected to sequencing to identify the types of HEV, and 2 different genotypes have been determined: CVB1 and CVB2. The remaining 42 samples from stool were detected by commercial qRT-PCR kit, of which 23 were positive for CVA6 (Ct 20.27–28.75), and were also detected by RT-RAP assay (Table 3).
Table 3.
Clinical performance of real-time reverse-transcription recombinase-aided PCR (RT-RAP) assay and comparison with commercial quantitative real-time reverse transcription PCR (qRT-PCR).
| Sample types | RT-RAP assay | qRT-PCR |
Agreement | |
|---|---|---|---|---|
| Positive | Negative | |||
| CSF samples | Positive | 3 | 0 | 100% |
| Negative | 0 | 136 | ||
| Stool samples | Positive | 23 | 0 | 100% |
| Negative | 0 | 19 | ||
4. Discussion
HEVs are known to cause a wide spectrum of diseases [7], [20], some of which, such as aseptic meningitis and encephalitis, can result in high mortality [21], [22]. Although many commercial qRT-PCR kits for HEV have been routinely applied in well-equipped laboratories, the detection of certain types of samples (e.g., CSF) may face bigger challenges due to the low virus load [23]. Traditional two-step nested PCR has higher sensitivity but the test samples are prone to contamination. The RTN RT-PCR for HEV developed in our laboratory significantly reduced the risk but consumed more time than qRT-PCR [9].
In our previous study, the RAP method for respiratory viruses (ADV3 and ADV7) was highly sensitive and efficient. Also, the potential of the RT-RAP in detecting samples with low viral load has been raised. With these in mind, we established a novel and sensitive RT-RAP assay for detecting HEV rapidly. As reported above, the RT-RAP in this study has been extensively validated with 15 HEV strains (species A–C), and the sensitivities of this assay for 9 out of 15 were 2–8 folds more sensitive than the qRT-PCR assay. Further, when the test was applied to 181 clinical specimens, the RT-RAP and the commercial qRT-PCR kit provided consistent results. Three positive samples were sequenced to determine the HEV genotype, and sequencing results verified that these samples are all true positives.
To our knowledge, this is the first study to develop the RT-RAP for detecting HEV infection. The same principle of RAP described in our previous research was used in this study, but it is worth mentioning that we added the RAA solution on the surface of the docosane instead of the lid of the tubes, avoiding the risk of droplets falling off during the RAA reaction. Indeed, several studies of isothermal amplification techniques to detect HEV have already been reported, such as recombinase polymerase amplification (RPA) [24] and loop-mediated isothermal amplification (LAMP) [25], [26]. Compared to Yang’s research [24], our assay integrates reverse transcription with RAP in a single tube, which can prevent contamination and simplify operation. In addition, a distinct advantage to our RT-RAP assay over LAMP is that the design of RAP primers is much simpler. Owing to the efficient RAA reaction, we finally set the number of RAP cycles to 20, which allows the whole RT-RAP to run completely within 1 h.
Nonetheless, our assay has several limitations. First, the current RT-RAP assay only achieved a higher sensitivity for 9 of 15 strains compared with qRT-PCR. One reason for this outcome may be that the reverse RAA primer as RT primer is not conducive to the RT step. This is similar to a recent study that shows the RT efficiency of the RPA reverse primer was 6-fold less efficient than the qPCR reverse primer [27]. For this reason, we expect that further optimization of RAA primers and RAA systems will be feasible to overcome this issue. Second, despite intensive efforts, we collected a small number of clinical samples. Future work with more samples of different types from various regions will be needed to further evaluate the performance of RT-RAP.
Our study demonstrated the merits of RAP in the detection of low viral load samples in a single tube format. To offer the potential for clinical translation, we intend to incorporate the RT-RAP strategy into microfluidic chips wherever possible. Overall, the established one-step RT-RAP assay is a promising approach for detecting HEV in a timely and sensitive manner.
Acknowledgments
Acknowledgements
This work was supported by the National Key R&D Program of China (2021YFC2301102), National Natural Science Foundation of China (82202593), and the Central Guidance on Local Science and Technology Development Fund of Hebei Province (216Z7713G).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Author contributions
Xiuli Sun: Investigation, Validation, Writing – original draft. Huanhuan Lu: Validation. Yanqing Tie: Resources. Mengchuan Zhao: Resources. Ruiqing Zhang: Methodology, Writing – review & editing. Zhenlu Sun: Resources. Guohao Fan: Methodology. Fengyu Li: Validation. Fengyu Tian: Validation. Yaxin Hu: Validation, Mengyi Zhang: Validation. Xinxin Shen: Conceptualization, Supervision. Xuejun Ma: Conceptualization, Writing – review & editing, Project Administration. Zhishan Feng: Conceptualization, Supervision.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bsheal.2023.03.002.
Contributor Information
Xinxin Shen, Email: x616815@126.com.
Xuejun Ma, Email: maxj@ivdc.chinacdc.cn.
Zhishan Feng, Email: 15131129999@139.com.
Supplementary data
The following are the Supplementary data to this article:
References
- 1.Esposito S., Principi N. Hand, foot and mouth disease: current knowledge on clinical manifestations, epidemiology, aetiology and prevention. Eur. J. Clin. Microbiol. Infect. Dis. 2018;37(3):391–398. doi: 10.1007/s10096-018-3206-x. [DOI] [PubMed] [Google Scholar]
- 2.Jain S., Patel B., Bhatt G.C. Enteroviral encephalitis in children: clinical features, pathophysiology, and treatment advances. Pathog. Glob. Health. 2014;108(5):216–222. doi: 10.1179/2047773214Y.0000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J., Ye X., Zhang X.Y., Zhu Z., Zhang X., Xu Z., Ding Z., Zou G., Liu Q., Kong L., et al. Coxsackievirus A10 atomic structure facilitating the discovery of a broad-spectrum inhibitor against human enteroviruses. Cell Discov. 2019;5:4. doi: 10.1038/s41421-018-0073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han Z., Zhang Y., Huang K., Cui H., Hong M., Tang H., Song Y., Yang Q., Zhu S., Yan D., Xu W. Genetic characterization and molecular epidemiological analysis of novel enterovirus EV-B80 in China. Emerg. Microbes Infect. 2018;7(1):1–12. doi: 10.1038/s41426-018-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown D.M., Zhang Y., Scheuermann R.H. Epidemiology and sequence-based evolutionary analysis of circulating non-polio enteroviruses. Microorganisms. 2020;8(12):1856. doi: 10.3390/microorganisms8121856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Crom S.C., Rossen J.W., van Furth A.M., Obihara C.C. Enterovirus and parechovirus infection in children: a brief overview. Eur. J. Pediatr. 2016;175(8):1023–1029. doi: 10.1007/s00431-016-2725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brouwer L., Moreni G., Wolthers K.C., Pajkrt D. World-Wide Prevalence and Genotype Distribution of Enteroviruses. Viruses. 2021;13(3):434. doi: 10.3390/v13030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer T.K., Simmonds P., Harvala H. The importance of enterovirus surveillance in a post-polio world. Lancet Infect. Dis. 2022;22(1):e35–e40. doi: 10.1016/s1473-3099(20)30852-5. [DOI] [PubMed] [Google Scholar]
- 9.Shen X., Qiu F., Zhao H., Yang M., Hong L., Xu S., Zhou S., Li G., Feng Z., Ma X. A novel and highly sensitive real-time nested RT-PCR assay in a single closed tube for detection of enterovirus. Diagn. Microbiol. Infect. Dis. 2018;90(3):181–185. doi: 10.1016/j.diagmicrobio.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Zhu X., Liu P., Lu L., Zhong H., Xu M., Jia R., Su L., Cao L., Sun Y., Guo M., Sun J., Xu J. Development of a multiplex droplet digital PCR assay for detection of enterovirus, parechovirus, herpes simplex virus 1 and 2 simultaneously for diagnosis of viral CNS infections. Virol. J. 2022;19(1):70. doi: 10.1186/s12985-022-01798-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge S., Yan Q., He S., Zhuang S., Niu J., Xia N. Specific primer amplification of the VP1 region directed by 5' UTR sequence analysis: enterovirus testing and identification in clinical samples from hand-foot-and-mouth disease patients. J. Virol. Methods. 2013;193(2):463–469. doi: 10.1016/j.jviromet.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Niu P., Qi S., Yu B., Zhang C., Wang J., Li Q., Ma X. Development of a highly sensitive real-time nested RT-PCR assay in a single closed tube for detection of enterovirus 71 in hand, foot, and mouth disease. Arch. Virol. 2016;161(11):3003–3010. doi: 10.1007/s00705-016-2985-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan G., Zhang R., He X., Tian F., Nie M., Shen X., Ma X. RAP: A Novel Approach to the rapid and highly sensitive detection of respiratory viruses. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.766411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen X., Qiu F., Shen L., Yan T., Zhao M., Qi J., Chen C., Zhao L., Wang L., Feng Z., Ma X. A rapid and sensitive recombinase aided amplification assay to detect hepatitis B virus without DNA extraction. BMC Infect. Dis. 2019;19(1):229. doi: 10.1186/s12879-019-3814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu T., Ge Y., Zhao K., Zhu X., Chen Y., Wu B., Zhu F., Zhu B., Cui L. A reverse-transcription recombinase-aided amplification assay for the rapid detection of N gene of severe acute respiratory syndrome coronavirus 2(SARS-CoV-2) Virology. 2020;549:1–4. doi: 10.3390/ijms232315269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma X. Isothermal amplification technology for diagnosis of COVID-19: Current status and future prospects. Zoonoses. 2022;2(1):123388. doi: 10.15212/zoonoses-2021-0022. [DOI] [Google Scholar]
- 17.Nie M., Zhang R., Zhao M., Tan H., Hu Y., Fan G., Li J., He A., Tian F., Li F., et al. Development of a duplex recombinase-aided amplification assay for direct detection of Mycoplasma pneumoniae and Chlamydia trachomatis in clinical samples. J. Microbiol. Methods. 2022;198 doi: 10.1016/j.mimet.2022.106504. [DOI] [PubMed] [Google Scholar]
- 18.Munawar M.A. Critical insight into recombinase polymerase amplification technology. Expert Rev. Mol. Diagn. 2022;22(7):725–737. doi: 10.1080/14737159.2022.2109964. [DOI] [PubMed] [Google Scholar]
- 19.Mohamed N., Elfaitouri A., Fohlman J., Friman G., Blomberg J. A sensitive and quantitative single-tube real-time reverse transcriptase-PCR for detection of enteroviral RNA. J. Clin. Virol. 2004;30(2):150–156. doi: 10.1016/j.jcv.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Tapparel C., Siegrist F., Petty T.J., Kaiser L. Picornavirus and enterovirus diversity with associated human diseases. Infect. Genet. Evol. 2013;14:282–293. doi: 10.1016/j.meegid.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Chen B.S., Lee H.C., Lee K.M., Gong Y.N., Shih S.R. Enterovirus and encephalitis. Front. Microbiol. 2020;11:261. doi: 10.3389/fmicb.2020.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang H.I., Shih S.R. Neurotropic enterovirus infections in the central nervous system. Viruses. 2015;7(11):6051–6066. doi: 10.3390/v7112920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lafolie J., Labbé A., L’Honneur A.S., Madhi F., Pereira B., Decobert M., Adam M.N., Gouraud F., Faibis F., Arditty F., et al. Assessment of blood enterovirus PCR testing in paediatric populations with fever without source, sepsis-like disease, or suspected meningitis: a prospective, multicentre, observational cohort study. Lancet Infect. Dis. 2018;18(12):1385–1396. doi: 10.1016/s1473-3099(18)30479-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X., Xie J., Hu S., Zhan W., Duan L., Chen K., Zhang C., Yin A., Luo M. Rapid and visual detection of enterovirus using recombinase polymerase amplification combined with lateral flow strips. Sens. Actuators B. 2020;311 doi: 10.1016/j.snb.2020.127903. [DOI] [Google Scholar]
- 25.Daskou M., Dimitriou T.G., Alexopoulou D.S., Tsakogiannis D., Amoutzias G.D., Mossialos D., Kyriakopoulou Z., Markoulatos P. WarmStart colorimetric RT-LAMP for the rapid, sensitive and specific detection of Enteroviruses A-D targeting the 5'UTR region. J. Appl. Microbiol. 2021;130(1):292–301. doi: 10.1111/jam.14770. [DOI] [PubMed] [Google Scholar]
- 26.Arita M., Ling H., Yan D., Nishimura Y., Yoshida H., Wakita T., Shimizu H. Development of a reverse transcription-loop-mediated isothermal amplification (RT-LAMP) system for a highly sensitive detection of enterovirus in the stool samples of acute flaccid paralysis cases. BMC Infect. Dis. 2009;9:208. doi: 10.1186/1471-2334-9-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu S., Tong X., Han Y., Zhang K., Zhang Y., Chen Q., Duan J., Lei X., Huang M., Qiu Y., et al. Fast and sensitive detection of SARS-CoV-2 RNA using suboptimal protospacer adjacent motifs for Cas12a. Nat. Biomed. Eng. 2022;6(3):286–297. doi: 10.1038/s41551-022-00861-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.