Highlights
-
•
Laboratory diagnosis of non-polio enterovirus infections is important in determining a patient's prognosis and guiding clinical management, for surveillance and for the investigation of outbreaks.
-
•
Laboratory diagnosis of non-polio enteroviruses is mainly based on molecular techniques, but classical virus-isolation techniques are still used in references laboratories.
-
•
An important part of diagnosis and surveillance of enteroviruses infections include viral typing by VP1 gene sequencing and/or VP4-VP2 sequencing using conventional Sanger technique, and more recently, full-genome next-generation sequencing.
Keywords: Nonpolio enteroviruses, Virus isolation, Nucleic acid amplification tests, Sanger sequencing, Next-generation sequencing
Abstract
Infections by nonpolio enteroviruses (EVs) are highly prevalent, particularly among children and neonates, where they may cause substantial morbidity and mortality. Laboratory diagnosis of these viral infections is important in patient prognosis and guidance of clinical management. Although the laboratory diagnosis of nonpolio EVs is mainly based on molecular techniques, classical virus-isolation techniques are still used in reference laboratories. Other techniques, such as antigen detection and serology, are becoming obsolete and rarely used in diagnosis. An important part of diagnosis and surveillance of EV infections is viral typing by VP1 gene sequencing using conventional Sanger technique and more recently, full-genome next-generation sequencing. The latter allows the typing of all EVs, better investigation of EV outbreaks, detection of coinfection, and identification of severity markers in the EV genome.
1. Introduction
Human enteroviruses (HEVs) constitute a large group of viral pathogens that cause diseases, which range from mild respiratory infections to paralysis or severe central nervous system infection [1]. Enteroviruses (EVs) are among the most common viruses infecting humans. They consist of ubiquitous, small (∼30 nm in diameter), non-enveloped, positive-sense, single-stranded RNA viruses belonging to the Picornaviridae family [1]. EVs have been recognized and characterized since the first electron microscopy images of the poliovirus, which were captured in 1952 by Reagan et al [2]. Coxsackievirus was the first human EV discovered after polioviruses, and it was named after the first geographical site of its isolation in New York. The name echovirus (enteric, cytopathogenic, human, and orphan virus) was selected for these viruses, which are associated with various clinical manifestations, such as gastroenteritis, meningitis, and respiratory illness [3].
The classification based on specific clinical manifestations becomes difficult with every newly discovered EV. Therefore, since 1974, novel EVs with serological properties have been numbered by their order of identification and more recently, by their molecular type [4].
Over a hundred types of EVs exist, and most of them are classified into four species based on molecular and biological characteristics: “enterovirus A (EV-A), enterovirus B (EV-B), enterovirus C (EV-C), and enterovirus D (EV-D)” (Table 1) [5].
Table 1.
Human enterovirus (EV) species and main types (adapted from Pons-Salort et al [5]).
| Human enterovirus species | Types |
|---|---|
| Enterovirus A | Coxsackievirus A2-A8, A10, A12, A14, A16 Enterovirus A71, A76, A89-92, A114, A119, A120, A121 |
| Enterovirus B | Coxsackievirus A9, B1-6 Echovirus 1–9, 11–21, 24–27, 29–33 Enterovirus B69, B73-75, B77-88, B93, B97, B98, B100, B101, B106, B107, B110-113 |
| Enterovirus C | Coxsackievirus A1, A11, A13, A17, A19-A22, A24 Poliovirus 1–3 Enterovirus C95, C96, C99, C102, C104, C105, C109, C113, C116-118 |
| Enterovirus D | Enterovirus D68, D70, D94, D111, D120 |
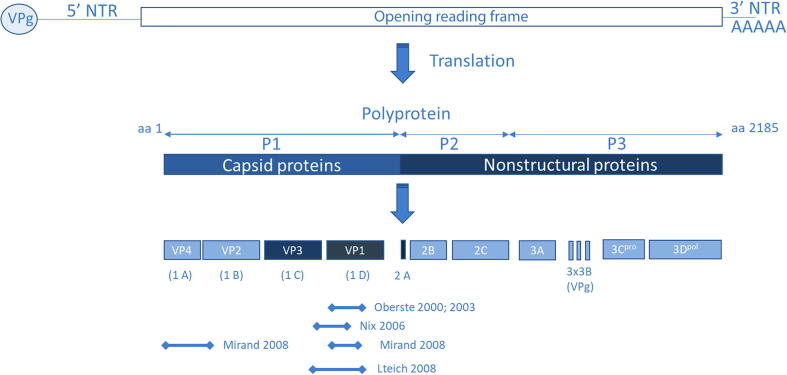
A single open reading frame encodes approximately 2,185 amino acids (range: 2,138–2,214) flanked by 5′- and 3′-non-translated regions (5′NTR and 3′NTR) of approximately 750 and 1,000 nucleotides, respectively (Fig. 1). The 5′NTR harbors secondary RNA structural elements needed for replication, and the 3′NTR is needed for replication control [6], [7]. All EVs have a similar genomic organization (7.2–8.5 Kb). The coding region is subdivided into the following three regions (from 5′ to 3′): the precursor 1 (P1) region that encodes structural capsid proteins (VP4, VP2, VP3, and VP1); the P2 and P3 regions that encode non-structural proteins, such as the RNA-dependent RNA polymerase, proteases, and other necessary proteins, needed for intracellular replication [1].
Fig. 1.
Organization of Enterovirus genome. Top: schematic of the viral RNA genome, with the genome linked protein VPg at the 5′ end, the 5′ non-translated region, the protein coding region, the 3′ non-translated and the poly(A) tail. Bottom: Processing pattern of Enterovirus protein: capsid proteins (VP1 – VP4), non-structural proteins 2A, 2B, and 3A, and conserved non-structural proteins polymerase 3Dpol, protease 3Cpro, helicase 2C. Amino acid sequence variation across the enterovirus genome are deduced from polyprotein amino acid sequences alignment from reference strains of human enteroviruses (darker boxes: more conservative; lighter boxes: more variable). The binding regions for the most commonly used primers are marked below (adapted from Racaniello et al. [1]).
EV infections are prevalent worldwide, although rates of infections vary by location and are seasonal. Individuals living in regions with temperate climates experience considerably higher rates of EV infections in the summer and fall months (from June to October) [8]. Meanwhile, transmission occurs throughout the year in tropical and subtropical areas [9]. Although EV infections occur in all age groups, the highest rates of infection and diseases are observed in infants and young children [8].
Human EV infections are usually acquired directly or indirectly by contact with the virus, which is shed in feces or the upper respiratory tract [6]. The incubation period for EV infections may vary based on the clinical syndrome but generally lasts for 7–14 days (range: 3–35 days) [10]. Reinfection with the same type of EV can occur despite previous immunity [10].
Most EV infections observed in clinical practice are asymptomatic/benign or result in mild symptoms, such as uncomplicated Hand, Foot, and mouth disease (HFMD), herpangina, and pleurodynia [6]. The most common symptomatic manifestation of EV infection is an acute, non-focal febrile illness that mainly affects infants aged < 1 year [11], [12], [13]. The same viruses causing these common syndromes have been incriminated in severe and life-threatening infections, such as aseptic meningitis, encephalitis, acute myocarditis, myositis, neonatal sepsis, and acute flaccid paralysis [14], [15]. Nonpolio EV infections have also been implicated in cases of Guillain–Barre syndrome, acute transverse myelitis, and cerebellar ataxia [16], [17], [18].
Protective immunity against EV infection is type specific [10]. Although humoral- and cell-mediated immune response mechanisms occur following EV infection, a type-specific antibody is the most important factor in limiting diseases and eradicating the virus [6]. Frequent recombination and mutations in EVs are the primary mechanisms for their high rate of evolution. This condition has contributed to the rapid response and adaptation of EVs to new environmental challenges. The molecular epidemiology of EVs is well studied compared with other enteric viruses because they are easy to isolate in cell culture [7]. Recent advances in molecular biology have led to improved diagnostics of EV and typing of EV strains [19], [20], [21], [22]. This review covers the currently available techniques and advances in the diagnosis and typing of EV. Rhinovirus species and “non-human” EV species E–J are outside the scope of this review.
2. Laboratory diagnosis on collected clinical samples
Diagnosis of EV infection can be complicated and challenging. Such difficulty is due to the biology and epidemiology of EV infections and limitations of current diagnostic techniques [6]. Diagnosis of a specific EV infection requires the detection of the virus itself, specific antibodies, or viral genome in patient samples [10].
Given the myriad of clinical manifestations of EV infections, confirmation of diagnosis may be necessary to reduce hospitalization, antibiotic use, and additional expensive diagnostic tests, which are often performed to exclude or treat other conditions [23].
In addition to their improved sensitivity and quantitative results, molecular tests can be used to identify specific EV types. Sequencing of the EV VP1 capsid coding region correlates well with the neutralization serotyping and represents an ideal target for EV detection and identification tests [24]. Specific EVs, notably EV-A71 and EV-D68, have been associated with outbreaks, and they occasionally result in significant morbidity and mortality in Asia, Europe, and Northern America [25], [26], [27], [28]. EV-A71 has been involved in severe neurological manifestations, ranging from aseptic meningitis to acute paralysis and brainstem encephalitis associated with systemic features, such as severe pulmonary edema and shock [25]. Meanwhile, EV-D68 has been implicated mainly in severe respiratory illnesses, including severe asthma and bronchiolitis, in children [26], [27].
2.1. Clinical samples collection
Samples should be collected immediately after the onset of symptoms. The most common clinical samples include stool, cerebrospinal fluid (CSF), nasopharyngeal aspirate/swab, vesicular fluid, bronchoalveolar lavage, blood, conjunctival swab, biopsy specimen, and urine [28].
Multiple samples are collected from different sites in case of a suspected EV infection.[28]. As severe infection has been described in children, EVs can be detected in the plasma, stool, respiratory secretions, CSF, and biopsies [8]. Further, viral loads are usually higher in stool and respiratory specimens compared with those in CSF [29]. Therefore, these samples are recommended for EV identification [28].
2.2. Direct methods for primary diagnosis
2.2.1. Viral antigen detection tests
Immunohistochemistry detection has been described primarily not only for the diagnosis of myocardial infections but also for other tissues, such as the spleen, lung, kidney, intestine, bone marrow, and pancreas [30], [31], [32]. Detecting viruses in tissues can be important for understanding viral pathogenesis. Most of these detection tests locate the conserved region of the VP1 peptide [30], [31], [32]. Direct detection of EV antigens in stool samples by enzyme immune-assay (EIA) is as sensitive as traditional cell culture but only achieves 58% sensitivity compared with reverse-transcription polymerase chain reaction (RT-PCR) [33]. Available commercialized tests include either immunofluorescence tests (IMAGENTM Enterovirus, Thermo-fisher) or rapid POCT chromatographic immunoassays (CDIA Enterovirus Antigen Test, Creative Diagnostics). The main problem with these assays is their failure to type EVs; thus, they are rarely used in common practice [10].
2.2.2. Nucleic acid amplification tests (NAATs)
The application of molecular biology techniques has significantly changed approaches to EV diagnostics. The most common use of PCR for EV diagnostics is the direct detection of EV genome in cell cultures, clinical specimens, and biopsy tissues [24]. In contrast to antigen detection and viral culture, NAATs have been applied successfully in the diagnosis of EV infections. These tests are extremely sensitive, and they attain 100 % specificity in the absence of laboratory cross-contamination. RT-PCR techniques for EV detection in clinical samples are more sensitive than cell culture [34], [35], [36]. These assays are mainly based on the detection of viral RNA, which targets the highly conserved 5′NTR region in EVs [36], [37], [38], [39]. Different primers targeting this region have been used with various sequences and product sizes. Given the genetic conservation of this region, sequencing prevents the accurate identification of EV type [40] and should not be used for typing [28].
Correct sample preparation prior to nucleic acid extraction is primordial, especially for fecal specimens. To avoid PCR inhibitors, medical experts should disaggregate fecal samples in normal saline and clarify them by centrifugation [28]. The addition of an internal control during extraction is important to identify the presence of these inhibitory compounds (blood and dietary supplement) [41].
Several commercial real-time PCR kits are available for EV detection in CSF (Xpert EV, Cepheid; NucliSENS EasyQ Enterovirus, BioMérieux) and respiratory specimens (FilmArray Respiratory Panel EV/HRVb, BioFire; xTAG Respiratory Viral Panel EV/HRV; Luminex). However, more data are needed to confirm the sensitivity of commercial 5′NTR-based assays for the possible detection of all EV strains [10], [39], [42].
As with all PCR-based techniques, the sensitivity of RNA amplification from biological specimens is extremely variable and depends on the nature of the specimen. Some specimens have modest sensitivity (e.g., CSF), and others exhibit excellent sensitivity (e.g., stool) [6]. Extensive studies of the capsid region of EV isolates have led to the design of primers that selectively target single types. This progress was accomplished using inosine in the primer synthesis despite the high rate of analogy in nucleotide substitutions [19], [24].
PCR is useful for the diagnosis of pediatric EV meningitis, non-specific fever illnesses, and neonatal infections. In related studies, PCR testing of CSF, serum, and throat specimens from children with acute illnesses showed > 90% sensitivity and high specificity; meanwhile, urine testing showed less sensitivity [10]. Patients suffering from aseptic meningitis, encephalitis, or myelitis have detectable virus in stool and respiratory secretions for periods longer than those for CSF, where virus remains undetectable on occasions. EV RNA can be detected in CSF by PCR in the majority of meningitis cases but inconsistently in encephalitis. EV excretion in throat and stool samples is prolonged, but detection is not a confirmation of infection [28]. Any detection in stool or throat samples should be carefully interpreted because it does not apply causation and may simply reflect prolonged excretion or carrier status [43]. Droplet digital PCR is a useful tool for the identification and detection of EV 71 [44] and quantification of EVs in untreated sewage waters [45].
2.3. Viral isolation and identification
The isolation of EV in cell culture has historically been the primary method for diagnosis mainly because most EV strains grow readily [10]. Traditional methods for EV detection and characterization rely on time-consuming and labor-intensive procedures of viral isolation in cell culture and neutralization by reference antisera [6].
The viral culture procedure involves the inoculation of appropriate specimens onto susceptible cell lines. The World Health Organization (WHO) suggested L20B, RD, and former HEp-2 cell lines for poliovirus isolation in a global poliovirus laboratory network. At least three different cell lines are often used for EV culture; these cell lines include monkey kidney cells, A549 (human lung carcinoma), buffalo green monkey kidney (BGMK), human embryonic lung fibroblasts, epithelial, and rhabdomyosarcoma cell lines [6], [15], [46]. Transgenic and co-cultured cell lines can increase the sensitivity of EV recovery and eliminate the need for multiple shell vials. BGMK cells transfected with human decay-accelerating factor have been co-cultured with Caco-2 cells (human colon-adenocarcinoma cell line) [47]. These mixed cell cultures showed higher sensitivity for recovery of EV from clinical samples than single cell lines [47], [48].
In routine diagnostic testing, EV growth is detected by its cytopathic effects, which include visible rounding, shrinking, nuclear pyknosis, refractility, and cell degeneration [6]. The cytopathic effect is confirmed as that of a specific EV by neutralization with type-specific antisera, immunofluorescence with type-specific monoclonal antibodies, or polymerase chain reaction (PCR) coupled with sequencing [6].
In general, virus-isolation techniques possess high sensitivity and specificity. The highest yield in neonatal EV infections can be obtained using viral culture specimens, such as those from the rectum or stool (91%–93% positive), CSF (62%–83% positive), and nasopharynx or throat (52%–67% positive) [49]. Serum and urine cultures provide lower yields (24%–74%) [10].
Viral isolation faces significant limitations. First, this process requires expertise and is relatively expensive. Second, no single optimal cell line can be used to grow all EVs. Most EVs can be isolated in cell cultures of mammalian cell lines; however, some EVs exhibit poor growth [50]. Most coxsackie A viruses, except CVA9 and CVA16, grow poorly in cultures [51]. Third, the sensitivity of culture is further limited by neutralization of antibodies in diagnostic specimens and inadequate specimen collection or handling. Lastly, growth in culture can be slow, lasting for 3–8 days [6].
However, cell culture is still applied in many applications and can be used to confirm new variants of EV, detect EV coinfection (more than one EV strain), type EVs by neutralization, and assess the efficacy of antiseptic antiviral agents.
2.4. EV typing
2.4.1. Sequencing by Sanger technique
Single detection of an EV during an outbreak, even in a patient with typical clinical manifestations, does not ensure an epidemiological link with an outbreak without molecular typing [7]. EVs are among the most rapidly changing viruses [52]. A goal in virus identification is determining the viral genome sequence. Originally, EV typing was based on sero-neutralization. However, nowadays, the sequencing part of the VP1 capsid protein gene has been the pillar for EV typing and is widely used worldwide [53]. This step is performed directly by PCR in conjunction with nucleic acid sequencing of a full (about 900 nucleotides) or partial VP1 genome region (a minimum of 350 nucleotide sequences are required) and is recommended by WHO [42]. The type of an unknown isolate is inferred by comparing the VP1 sequence with a nucleotide sequence database, e.g., GenBank and RIVM [6], [54]. The VP1 gene encodes an important type-specific neutralization epitope, and therefore, its sequence correlates very well with the classical type of classification [55], [56].
VP1 nucleotide identity of > 75% (amino acid sequence>85%) between an EV isolate and a prototype strain may be used to establish the isolate type [6], [19]. The best-match nucleotide sequence identity of < 70% may indicate a distinct new type. Any sequence identity between 70% and 75% requires further characterization before final identification [6]. This approach reduces the time required to type EV strains and can be used to type difficult or untypable isolates using sero-neutralization. This method also helps in the rapid determination of the epidemiological relationship of viruses isolated during an outbreak [6]. The complete VP1 sequence (∼900 nucleotides) is used for formal type identification and required for the characterization of new types [28].
Given a good reference dataset, the VP1 genome region is commonly used for phylogenetic analysis. The VP2 genome region is also applied for typing and phylogenetic analysis, although less frequently [7]. Assays targeting other genomic regions (mainly capsid proteins VP4/VP2) can be used if VP1 sequencing fails; some of these tests display increased sensitivity [6], [28], [56], [57], [58], [59], [60]. However, VP4/VP2-based tests can limit the comparability of data between laboratories because of a small database [28]. In addition, the VP4 region may include a recombination hotspot at the 5′NTR-VP4 junction, and recombination events within the VP4 domain may compromise phylogenetic analysis [7]. Other genome regions are less suitable for phylogenetic analysis due to ubiquitous recombination outside the VP2/VP1 region [61] and are used to highlight the circulation of the recombinant forms of EVs [62].
According to the WHO EV surveillance guidelines, Sanger sequencing technique for EV typing is based on a semi-nested PCR (snPCR) amplification of the VP1 region using specific primers [53] (Supplementary Material 1). Subsequently, the Nix et al.'s protocol was improved by conducting snPCR separately for all EV, EV-A, EV-B, EV-C, and EV-D [59], [62], [63]. These techniques include other sets of primers [59], [62] or their combination [63], [64] (Table 2).
Table 2.
Primers used for amplification and sequencing of VP1 (in pairs, where first primer is forward).
| Primer | Use fora | Specificityb | Sequence (5ˊ-3ˊ)c | Reference |
|---|---|---|---|---|
| AN32 | RT | All EV | GTY TGC CA | Nix et al., 2006 [19] |
| AN 33 | RT | All EV | GAY TGC CA | |
| AN 34 | RT | All EV | CCR TCR TA | |
| AN 35 | RT | All EV | RCT YTG CCA | |
| 224 | 1st round | All EV | GCI ATG YTI GGI ACI CAY RT | Nix et al., 2006 [19] |
| 222 | 1st round | All EV | C ICC IGG IGG IAY RWA CAT | Oberste et al., 2003 [89] |
| HEVBS1695 | 1st round, 2nd round | EV-A | CTTGTGCTTTGTGTCGGCRTGYAAYGAYTTYTCWG | Mirand et al., 2016 [59] |
| EV2C | 1st round | EV-A | CAATACGGCATTTGGACTTGAACTGTATG | |
| EV-OS | 1st round | All EV | TTA AAA CAG CCT GTG GGT TG | Leitch et al., 2009 [62] |
| EV-OAS | 1st round | All EV | ATT GTC ACC ATA AGC AGC CA | |
| EV-A-OS | 1st round, 2nd round, SR | EV-A | CCN TGG ATH AGY AAC CAN CAY T |
|
| EV-A-OAS | 1st round | EV-A | GGR TAN CCR TCR TAR AAC CAY TG |
|
| EVB-OS | 1st round | EV-B | GGY TAY ATN CAN TGY TGG TAY CAR AC |
|
| EV-B-OAS | 1st round | EV-B | GGT GCT CAC TAG GAG GTC YCT RTT RTA RTC YTC CCA |
|
| AN89 | 2nd round, SR | All EV | CCA GCA CTG ACA GCA GYN GAR AYN GG | Nix et al., 2006 [19] |
| AN88 | 2nd round, SR | All EV | TAC TGG ACC ACC TGG NGG NAY RWA CAT | |
| HEVBR132 | 2nd round | EV-A | GGTGCTCACTAGGAGGTCYCTRTTRTARTCYTCCCA | Mirand et al., 2016 [59] |
| 5NCS663 | 2nd round, SR | EV-B | GCGGAACCGACTACTTTGGGTGTCCGTGTTTC | |
| HEVR436 | 2nd round, SR | EV-B | CCCATGTCAGTCAGCGCATCIGGIARITTCCAIYACCAICC | |
| EV-A-IAS | 2nd round, SR | EV-A | GAN GGR TTN GTN GKN GTY TGC CA |
Leitch et al., 2009 [62] |
| EV-B-IS | 2nd round, SR | EV-B | CTT GTG CTT TGT GTC GGC RTG YAA YGA YTT YTC WG |
|
| EV-B-IAS | 2nd round, SR | EV-B | TCY TCC CAC ACR CAV TTY TGC CAR TC |
|
| EV-IS | 2nd round, SR | All EV | GGN AYY YTW GTR CGC CTG TTT T |
|
| EV-IAS | 2nd round, SR | All EV | CAC CCA AAG TAG TCG GTT CCG C |
|
| 189 | 2nd round, SR | EV-A | CAR GCI GCI GAR ACI GGN GC | Oberste et al., 2000 [90] |
| 187 | 2nd round, SR | EV-B | ACI GCI GYI GAR ACI GGN CA | |
| 188 | 2nd round, SR | EV-C&D | CAR GCI GCI GAR ACI GGN | |
| AN232 | SR | All EV | CCAGCACTGACAGCA | Nix et al., 2006 [19] |
| AN233 | SR | All EV | TACTGGACCACCTGG | |
| P1S1695S | SR | EV-A | CTTGTGCTTTGTGTCGGC | Mirand et al., 2016 [59] |
| P1R132S | SR | EV-A | GGTGCTCACTAGGAGGTC |
a) Abbreviations: RT, reverse transcription; 1st round, first round of nested polymerase chain reaction (PCR); 2nd round, second round of nested PCR; SR, sequencing reaction.
b) Abbreviations: EV-A, enterovirus A; EV-B, enterovirus B, EV-C, enterovirus C, EV-D, enterovirus D.
c) Sequences are written using the IUPAC nucleotide ambiguity codes, where I = deoxyinosine; N = G, A, T or C; Y = C or T; W = A or T; R = A or G.
Despite its sensitivity, when Nix et al.'s protocol is applied directly to clinical samples, non-typed EV isolates can subsequently be detected after the samples are subjected to cell culture using a susceptible and permissive cell line [63]. This approach was used by Faleye et al. to improve protocol performance. Consequently, these authors added species-specific primers in the second round PCR and used an RD cell line additionally; they noticed the following results. First, Nix et al.'s protocol, although extremely sensitive for detecting EV genomes, will often mask the presence of more than one EV isolate per sample. Second, even when a clinical sample is negative for EVs by pan-EV VP1 screen-based primers, the designed species-specific screen (using the first-round product as a template) still types EVs successfully at times. Finally, non-detected EV types in native clinical samples will emerge after cell culture. Thus, the pan-EV VP1 screen (Nix et al.'s protocol) is fallible, and virus concentration significantly influences the detectability of virions present in samples.
However, a unique set of primers is hardly sufficient for the amplification of all EV types, and the use of degenerate inosine primers as the ones in Nix's protocol may carry non-specific amplification [19], [55].
2.4.2. Next-generation sequencing (NGS)
Conventional Sanger sequencing has been the gold standard of the analysis of pathogens for over three decades. Sequencing of the whole EV genome (∼7,800 nucleotides) by the Sanger method is time consuming, difficult to scale-up, and a laborious procedure because the use of multiplex samples is not possible. However, with the expansion of NGS techniques, the demand for low-cost high-throughput sequencing has increased. NGS has high sensitivity and specificity, and a great sequencing depth as a single nucleotide is sequenced for at least 50 times. The PCR cycle threshold value of 30 is an assay limit required for NGS, and samples with low viral loads are not usually recommended [65].
NGS has been successfully applied in poliovirus typing and identification [66]. This success led to the use of NGS techniques in EV typing. Viral RNA is obtained either from purification and extraction from cell culture supernatant [67] or direct isolation from clinical samples [68]. Different strategies for reverse transcription of viral RNA into cDNA fragments, which can be subsequently sequenced by high-throughput techniques, have been reported. Some strategies are based on random amplification using non-specific primers [69], [70]. These strategies generally lead to the generation of DNA libraries, which mainly consist of non-viral sequences, thus decreasing the number of relevant reads obtained by sequencing. Alternate strategies are based on primers that specifically target conserved genomic regions of the viral RNA to be sequenced [71] or the whole-genome sequencing approach, which directly targets native RNA [72]. Such strategies have been used for EV-A71 [73], [74] and EV-C species [71]. These specific primers are usually degenerated and target either the full EV genome [68], [71], [72], [75], [76] or parts of the viral genome as the VP1 gene [77], [78].
After quality assessment of the prepared library, sequencing is performed on the NGS platform. To date, three different platforms are used for EV typing: MinION sequencing (Oxford Nanopore technologies), MiSeq sequencing (Illumina), and ion torrent sequencing (Thermo-Fisher). Sequence assembly is performed by mapping on a reference sequence or de novo assembly to obtain a full or partial EV genome [71].
The minimum batch size for NGS is eight samples, with typical sets of 24–96 samples [66], [75]. The processing time from extraction to nearly complete sequencing for a typical set of 24–96 specimens ranges from 3 days [66] to 4–5 days [65], [68], [75]. The total reagent costs for NGS technology per sample range from 70 USD to 120 USD and vary significantly when samples are multiplexed in one run [65], [67]. In addition, the number of reads for each sample varies from 0.3 million reads to 1.5 million reads [67]. The total cost per run, together with hardware-associated costs combined with bioinformatic challenges, may remain as major obstacles preventing Illumina-based sequencing.
NGS has become a useful tool for sub-typing, particularly in outbreak investigations [79] or in situations when the infectious agent is difficult to identify [80]. Other studies reported the successful application of NGS-based assays to typing of the HFMD-causing strain EV-A71 [57], [58], [65], [68] and severe respiratory disease-causing strain EV-D68 [81].
NGS of EV has several advantages over single-gene analysis.
-
i.
Detection of recombination events
NGS techniques showed good correlation with classic Sanger techniques for the analysis of serologically untypable EV [67]. This approach has been used to detect 23 genome variants, which are potential genetic recombinants [67].
-
ii.
Capability to identify mutations and virulence markers across the genome
Huang et al. conducted a retrospective study of the genomic diversity of EV-D68 strains collected during an outbreak in the late summer and fall of 2014. Their analysis showed that most EV-D68 strains (26 out of 29) differed significantly from prior ones. Two functional mutations in the protease cleavage sites may have been responsible for the increased rate of viral replication and transmission [81]. Routine sequencing of longer genome fragments or the complete genome is required to obtain a better image. These genetic determinants can be located anywhere in the genome but not in the highly exploited VP1 genome region.
-
iii.
Identification of potential new biomarkers throughout the genome
In a study by Grädel et al., direct RNA sequencing using nanopore technology for fast whole-genome sequencing of viruses from clinical samples showed a good correlation with cDNA Illumina MiSeq Sequencing. This technology correctly identifies EV genotypes from stool samples and provides rich meta-transcriptomics information [72].
-
iv.
More sensitive and specific tracking of viral evolution and further identification and investigation of EV outbreaks and coinfection:
An Australian study by Stelzer-Braid et al. investigated and sequenced the near full-length genome of 23 EV strains identified during a known outbreak of EV-A71 in 2012/2013. The identified EV-A71 strains were remarkably similar to those circulating in Asia during the same period [82]. In addition, Issacs et al. similarly characterized 58 clinical stool samples and discovered coinfection in four clinical samples, with each containing two distinct EV genotypes [83]. Similar results from the work of Chien et al. showed coinfection with two virus genomes in eight sequenced samples [67].
-
v.
Detection of EV circulation in the human population and sewage
Recent studies have described the isolation of EVs in sewage by NGS. This process allowed the monitoring of EV diversity in wastewater and monthly analysis of concentrated samples [77], [84], [85], [86]. A genotype C1-like EV-A71 variant was recovered from wastewater in Arizona, and the strain was suggested to be circulating for more than two years [78]. Such a wastewater-based surveillance will help in outbreak prediction before the onset of cases in the community [78].
3. Indirect methods by serology
As in classical virology, serological diagnosis of EV infections can be made by comparing titers in acute and convalescent-phase serum specimens [6]. This process has a limited clinical value in the routine diagnosis of EV because of the time needed to obtain the samples, large number of existing types, and lack of a common antigen for serological assays. In general, EV serodiagnosis is more suitable for epidemiological studies than clinical diagnosis [6]. A more than fourfold increase in EV-specific antibodies between acute and convalescent samples is suggestive of a recent infection and may be useful in epidemiological investigations, such as studies of outbreaks caused by a specific serotype [10], [28].
Detection of neutralizing antibodies is used to quantify neutralizing antibodies against selected EV types [38]. Several commercial enzyme-linked immunosorbent assay (ELISA) tests are available on the market for EV-specific IgM [6].
ELISA tests have been successfully applied for epidemiological investigators of outbreaks and specific diagnostic use. As with neutralization IgM assays, EIAs possess exceptionally good sensitivity and specificity. In most cases, IgM tests are not serotype specific, and sensitivity is widely variable. A total of 10% to nearly 70% of serum samples showed a heterotypic response caused by other EV infections [87], [88].
4. Conclusion and perspectives
As technology advances, laboratories progress toward the implementation of novel state-of-the-art sequencing techniques to rapidly characterize and identify EVs in clinical specimens. NGS of pooled samples provides advantages as a screening tool to complement standard molecular assays during the surveillance and diagnosis of EV infection. In addition, NGS is a broad-spectrum approach that is not limited to pathogen-specific primers and probes. However, the cost of NGS technology is still an issue, and the provided results are complex and thus require careful pre-analytical phase and sample preparation, quality assessment of DNA libraries, and exhaustive data processing by experienced bioinformaticians to manage the large, generated databases. Global cooperation between experts in EVs is highly desirable to provide sufficient sample coverage and a good reference dataset. However, specific virologic diagnostic skills, including virus-isolation and histopathological investigations, should be continually implemented in reference laboratories to investigate newly emerging, re-emerging, and less-characterized or rare EV strains.
Acknowledgements
This study was funded by the Russian Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing, grant ID 121041500041−1.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Author contributions
Tarek Itani: Conceptualization. Vladislav Chalapa: Visualization. Aleksandr Semenov: Conceptualization, Supervision, Writing – review & editing. Aleksandr Sergeev: Supervision, Writing – review & editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bsheal.2022.12.002.
Supplementary data
The following are the Supplementary data to this article:
References
- 1.Racaniello V.R. Fields Virol. 6th ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2013. Picornaviridae: The viruses and their replication; pp. 453–489. [Google Scholar]
- 2.Reagan R., Brueckner A. Morphological observations by electron microscopy of the Lansing strain of poliomyelitis virus after propagation in the Swiss albino mouse. Tex. Rep. Biol. Med. 1952;10:425–428. [PubMed] [Google Scholar]
- 3.Grist N., Bell E., Assaad F. Enteroviruses in human disease. Prog. Med. Virol. 1978;24:114–157. [PubMed] [Google Scholar]
- 4.Poyry T., Kinnunen L., Hyypia T., Brown B., Horsnell C., Hovi T., Stanway G. Genetic and phylogenetic clustering of enteroviruses. J. Gen. Virol. 1996;77:1699–1717. doi: 10.1099/0022-1317-77-8-1699. [DOI] [PubMed] [Google Scholar]
- 5.Pons-Salort M., Parker E.P.K., Grassly N.C. The epidemiology of non-polio enteroviruses: recent advances and outstanding questions. Curr. Opin. Infect. Dis. 2015;28:479–487. doi: 10.1097/QCO.0000000000000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pallansch M.A., Oberste M.S., Whitton L.J. Fields Virol. 6th ed, Lippincott Williams & Wilkins; Philadelphia, PA: 2013. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses; pp. 490–530. [Google Scholar]
- 7.Lukashev A.N., Vakulenko Y.A., Turbabina N.A., Deviatkin A.A., Drexler J.F. Molecular epidemiology and phylogenetics of human enteroviruses: Is there a forest behind the trees? Rev. Med. Virol. 2018;28 doi: 10.1002/rmv.2002. [DOI] [PubMed] [Google Scholar]
- 8.Khetsuriani N., LaMonte A., Oberste M.S., Pallansch M. Neonatal enterovirus infections reported to the National Enterovirus Surveillance System in the United States, 1983 - 2003. Pediatr. Infect. Dis. J. 2006;25:889–893. doi: 10.1097/01.inf.0000237798.07462.32. [DOI] [PubMed] [Google Scholar]
- 9.Tunkel A.R., Van de Beek D.W., Scheld M. Mand. Douglas Bennetts Princ. Pract. Infect. Dis. Eighth Edition. 2015. Acute Meningitis; pp. 1097–1137. [Google Scholar]
- 10.Dunn J.J. Enteroviruses and Parechoviruses. Microbiol. Spectr. 2016;4:22. doi: 10.1128/microbiospec.DMIH2-0006-2015. [DOI] [PubMed] [Google Scholar]
- 11.Jenista J.A., Powell K.R., Menegus M.A. Epidemiology of neonatal enterovirus infection. J. Pediatr. 1984;104:685–690. doi: 10.1016/S0022-3476(84)80944-0. [DOI] [PubMed] [Google Scholar]
- 12.Rittichier K.R., Bryan P.A., Bassett K.E., Taggart E.W., Enriquez F.R., Hillyard D.R., Byington C.L. Diagnosis and outcomes of enterovirus infections in young infants. Pediatr. Infect. Dis. J. 2005;24:546–550. doi: 10.1097/01.inf.0000164810.60080.ad. [DOI] [PubMed] [Google Scholar]
- 13.Rotbart H.A., Mccracken G.H.J., Whitley R.J., Modlin J.F., Cascino M., Shah S., Blum D. Clinical significance of enteroviruses in serious summer febrile illnesses of children. Pediatr. Infect. Dis. J. 1999;18:869–874. doi: 10.1097/00006454-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Sawyer M.H. Enterovirus infections: Diagnosis and treatment. Semin. Pediatr. Infect. Dis. 2002;13:40–47. doi: 10.1053/spid.2002.29756. [DOI] [PubMed] [Google Scholar]
- 15.Rotbart H. Richman DD Whitley RJ Hayden FG Clin. Virol. 2nd ed. ASM Press; Washington, D.C.: 2002. Enteroviruses; pp. 971–994. [Google Scholar]
- 16.Minami K., Tsuda Y., Maeda H., Yanagawa T., Izumi G., Yoshikawa N. Acute transverse myelitis caused by Coxsackie virus B5 infection. J. Paediatr. Child Health. 2004;40:66–68. doi: 10.1111/j.1440-1754.2004.00295.x. [DOI] [PubMed] [Google Scholar]
- 17.Starakis I., Marangos M., Giali S., Bassaris H. Acute transverse myelitis due to Coxsackie virus. J. Clin. Neurosci. 2005;12:296–298. doi: 10.1016/j.jocn.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi S., Miyamoto A., Oki J. Acute transverse myelitis caused by ECHO virus type 18 infection. Eur. J. Pediatr. 1995;154:378–380. doi: 10.1007/BF02072107. [DOI] [PubMed] [Google Scholar]
- 19.Nix W.A., Oberste M.S., Pallansch M.A. Sensitive, Seminested PCR Amplification of VP1 Sequences for direct identification of all enterovirus serotypes from original clinical specimens. J. Clin. Microbiol. 2006;44:2698–2704. doi: 10.1128/JCM.00542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton M.S., Jackson M.A., Abel D. Clinical utility of polymerase chain reaction testing for enteroviral meningitis. Pediatr. Infect. Dis. J. 1999;18:533–537. doi: 10.1097/00006454-199906000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Read S.J., Kurtz J.B. Laboratory diagnosis of common viral infections of the central nervous system by using a single multiplex pcr screening assay. J. Clin. Microbiol. 1999;37:1352–1355. doi: 10.1128/JCM.37.5.1352-1355.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson C.C., Willis M., Meagher A., Gieseker K.E., Rotbart H., Glode M.P. Impact of rapid polymerase chain reaction results on management of pediatric patients with enteroviral meningitis. Pediatr. Infect. Dis. J. 2002;21:283–286. doi: 10.1097/00006454-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Ramers C. Impact of a diagnostic cerebrospinal fluid enterovirus polymerase chain reaction test on patient management. J. Am. Med. Assoc. 2000;283:2680. doi: 10.1001/jama.283.20.2680. [DOI] [PubMed] [Google Scholar]
- 24.Oberste M.S., Maher K., Kilpatrick D.R., Pallansch M.A. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J. Virol. 1999;73:1941–1948. doi: 10.1128/JVI.73.3.1941-1948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solomon T., Lewthwaite P., Perera D., Cardosa M.J., McMinn P., Ooi M.H. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect. Dis. 2010;10:778–790. doi: 10.1016/S1473-3099(10)70194-8. [DOI] [PubMed] [Google Scholar]
- 26.Schuffenecker I., Mirand A., Josset L., Henquell C., Hecquet D., Pilorgé L., Petitjean-Lecherbonnier J., Manoha C., Legoff J., Deback C., Pillet S., Lepiller Q., Mansuy J.M., Marque-Juillet S., Antona D., Peigue-Lafeuille H., Lina B. Epidemiological and clinical characteristics of patients infected with enterovirus D68, France, July to December 2014. Eurosurveillance. 2016;21:30226. doi: 10.2807/1560-7917.ES.2016.21.19.30226. [DOI] [PubMed] [Google Scholar]
- 27.Midgley C.M., Jackson M.A., Selvarangan R., Turabelidze G., Obringer E., Johnson D., Giles B.L., Patel A., Echols F., Oberste M.S., Nix W.A., Watson J.T., Gerber S.I. Severe respiratory illness associated with enterovirus D68 — Missouri and Illinois, 2014. MMWR Morb. Mortal Wkly. Rep. 2014;63(36):798–799. [PMC free article] [PubMed] [Google Scholar]
- 28.Harvala H., Broberg E., Benschop K., Berginc N., Ladhani S., Susi P., Christiansen C., Wiewel M., Niesters H., Fischer T.K., et al. Recommendations for enterovirus diagnostics and characterisation within and beyond Europe. J. Clin. Virol. 2018;101:11–17. doi: 10.1016/j.jcv.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Verboon-Maciolek M.A., Nijhuis M., van Loon A.M., van Maarssenveen N., van Wieringen H., Pekelharing-Berghuis M.A., Krediet T.G., Gerards L.J., Fleer A., Diepersloot R.J.A., Thijsen S.F.T. Diagnosis of enterovirus infection in the first 2 months of life by real-time polymerase chain reaction. Clin. Infect. Dis. 2003;37:1–6. doi: 10.1086/375222. [DOI] [PubMed] [Google Scholar]
- 30.Cioc A.M., Nuovo G.J. Histologic and in situ viral findings in the myocardium in cases of sudden, unexpected death. Mod. Pathol. 2002;15:914–922. doi: 10.1097/01.MP.0000024291.37651.CD. [DOI] [PubMed] [Google Scholar]
- 31.Li Y., Bourlet T., Andreoletti L., Mosnier J.-F., Peng T., Yang Y., Archard L.C., Pozzetto B., Zhang H. Enteroviral capsid protein VP1 is present in myocardial tissues from some patients with myocarditis or dilated cardiomyopathy. Circulation. 2000;101:231–234. doi: 10.1161/01.CIR.101.3.231. [DOI] [PubMed] [Google Scholar]
- 32.Andréoletti L., Bourlet T., Moukassa D., Rey L., Hot D., Li Y., Lambert V., Gosselin B., Mosnier J., Stankowiak C., Wattré P. Enteroviruses can persist with or without active viral replication in cardiac tissue of patients with end-stage ischemic or dilated cardiomyopathy. J. Infect. Dis. 2000;182:1222–1227. doi: 10.1086/315818. [DOI] [PubMed] [Google Scholar]
- 33.Terletskaia-Ladwig E., Metzger C., Schalasta G., Enders G. A new enzyme immunoassay for the detection of enteroviruses in faecal specimens. J. Med. Virol. 2000;60:439–445. doi: 10.1002/(SICI)1096-9071(200004)60:4<439::AID-JMV12>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 34.Chonmaitree T., Ford C., Sanders C., Lucia H.L. Comparison of cell cultures for rapid isolation of enteroviruses. J. Clin. Microbiol. 1988;26:2576–2580. doi: 10.1128/jcm.26.12.2576-2580.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.M. Beld, R. Minnaar, J. Weel, C. Sol, M. Damen, H. van der Avoort, P. Wertheim-van Dillen, A. van Breda, R. Boom, Highly sensitive assay for detection of enterovirus in clinical specimens by reverse transcription-PCR with an armored RNA internal control, J. Clin. Microbiol. 42 (2004) 3059–3064, 10.1128/JCM.42.7.3059-3064.2004. [DOI] [PMC free article] [PubMed]
- 36.Iturriza-Gómara M., Megson B., Gray J. Molecular detection and characterization of human enteroviruses directly from clinical samples using RT-PCR and DNA sequencing. J. Med. Virol. 2006;78:243–253. doi: 10.1002/jmv.20533. [DOI] [PubMed] [Google Scholar]
- 37.van Doornum G.J.J., Schutten M., Voermans J., Guldemeester G.J.J., Niesters H.G.M. Development and implementation of real-time nucleic acid amplification for the detection of enterovirus infections in comparison to rapid culture of various clinical specimens. J. Med. Virol. 2007;79:1868–1876. doi: 10.1002/jmv.21031. [DOI] [PubMed] [Google Scholar]
- 38.Nijhuis M., van Maarseveen N., Schuurman R., Verkuijlen S., de Vos M., Hendriksen K., van Loon A.M. Rapid and sensitive routine detection of all members of the genus Enterovirus in different clinical specimens by real-time PCR. J. Clin. Microbiol. 2002;40:3666–3670. doi: 10.1128/JCM.40.10.3666-3670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaramillo-Gutierrez G., Benschop K.S.M., Claas E.C.J., de Jong A.S., van Loon A.M., Pas S.D., Pontesilli O., Rossen J.W., Swanink C.M.A., Thijsen S., van der Zanden A.G.M., van der Avoort H.G.A.M., Koopmans M.P.G., Meijer A. September through October 2010 multi-centre study in the Netherlands examining laboratory ability to detect enterovirus 68, an emerging respiratory pathogen. J. Virol. Methods. 2010;190(2013):53–62. doi: 10.1016/j.jviromet.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 40.Thoelen I., Lemey P., Van der Donck I., Beuselinck K., Lindberg A.M., Van Ranst M. Molecular typing and epidemiology of enteroviruses identified from an outbreak of aseptic meningitis in Belgium during the summer of 2000. J. Med. Virol. 2003;70:420–429. doi: 10.1002/jmv.10412. [DOI] [PubMed] [Google Scholar]
- 41.Oikarinen S., Tauriainen S., Viskari H., Simell O., Knip M., Virtanen S., Hyöty H. PCR inhibition in stool samples in relation to age of infants. J. Clin. Virol. 2009;44:211–214. doi: 10.1016/j.jcv.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 42.Piralla A., Daleno C., Scala A., Greenberg D., Usonis V., Principi N., Baldanti F., Esposito S. CAP-PRI Study Group, Genome characterisation of enteroviruses 117 and 118: A new group within human enterovirus species C. PLoS One. 2013;8:e60641. doi: 10.1371/journal.pone.0060641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Q., Fu X., Jiang L., Yang R., Cun J., Zhou X., Zhou Y., Xiang Y., Gu W., Fan J., Li H., Xu W. Prevalence of enteroviruses in healthy populations and excretion of pathogens in patients with hand, foot, and mouth disease in a highly endemic area of southwest China. PLoS One. 2017;13:e0181234. doi: 10.1371/journal.pone.0181234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lui Y.L.E., Tan E.L. Droplet digital PCR as a useful tool for the quantitative detection of Enterovirus 71. J. Virol. Methods. 2014;207:200–203. doi: 10.1016/j.jviromet.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 45.Kiulia N.M., Gonzalez R., Thompson H., Aw T.G., Rose J.B. Quantification and Trends of Rotavirus and Enterovirus in Untreated Sewage Using Reverse Transcription Droplet Digital PCR. Food Environ. Virol. 2021;13:154–169. doi: 10.1007/s12560-020-09455-9. [DOI] [PubMed] [Google Scholar]
- 46.Kok T.W., Pryor T., Payne L. Comparison of rhabdomyosarcoma, buffalo green monkey kidney epithelial, A549 (human lung epithelial) cells and human embryonic lung fibroblasts for isolation of enteroviruses from clinical samples. J. Clin. Virol. 1998;11:61–65. doi: 10.1016/S0928-0197(98)00026-9. [DOI] [PubMed] [Google Scholar]
- 47.Huang Y.T., Yam P., Yan H., Sun Y. Engineered BGMK cells for sensitive and rapid detection of enteroviruses. J. Clin. Microbiol. 2002;40:366–371. doi: 10.1128/JCM.40.2.366-371.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buck G.E., Wiesemann M., Stewart L. Comparison of mixed cell culture containing genetically engineered BGMK and CaCo-2 cells (Super E-Mix) with RT-PCR and conventional cell culture for the diagnosis of enterovirus meningitis. J. Clin. Virol. 2002;25:13–18. doi: 10.1016/S1386-6532(02)00029-X. [DOI] [PubMed] [Google Scholar]
- 49.Abzug M.J. Presentation, diagnosis, and management of enterovirus infections in neonates. Pediatr. Drugs. 2004;6:1–10. doi: 10.2165/00148581-200406010-00001. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt N.J., Ho H.H., Lennette E.H. Propagation and isolation of group A coxsackieviruses in RD cells. J. Clin. Microbiol. 1975;2:183–185. doi: 10.1128/jcm.2.3.183-185.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Modlin J.F., Dolin R. Mand. Douglas Bennetts Princ. Pract. Infect. Dis. Seventh edition. Churchill Livingstone Elsevier; Philadelphia, PA: 2010. Enteroviruses and parechoviruses; pp. 2237–2241. [Google Scholar]
- 52.Sanjuán R. From Molecular Genetics to Phylodynamics: Evolutionary Relevance of Mutation Rates Across Viruses. PLoS Pathog. 2012;8:e1002685. doi: 10.1371/journal.ppat.1002685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.World Health Organization (WHO), Enterovirus surveillance guidelines, Guideline for enterovirus surveillance in support of the Polio eradication initiative. https://apps.who.int/iris/handle/10665/344375, 2015 (accessed 25 August 2022).
- 54.Norder H., Bjerregaard L., Magnius L.O. Homotypic echoviruses share aminoterminal VP1 sequence homology applicable for typing. J. Med. Virol. 2001;63:35–44. doi: 10.1002/1096-9071(200101)63:1<35::AID-JMV1005>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 55.Oberste M.S., Peñaranda S., Rogers S.L., Henderson E., Nix W.A. Comparative evaluation of Taqman real-time PCR and semi-nested VP1 PCR for detection of enteroviruses in clinical specimens. J. Clin. Virol. 2010;49:73–74. doi: 10.1016/j.jcv.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 56.Wollants E., Maes P., Merino M., Bloemen M., Van Ranst M., Vanmechelen B. First genomic characterization of a Belgian Enterovirus C104 using sequence-independent Nanopore sequencing. Infect. Genet. Evol. 2020;81 doi: 10.1016/j.meegid.2020.104267. [DOI] [PubMed] [Google Scholar]
- 57.Nasri D., Bouslama L., Pillet S., Bourlet T., Aouni M., Pozzetto B. Basic rationale, current methods and future directions for molecular typing of human enterovirus, Expert Rev. Mol. Diagn. 2007;7:419–434. doi: 10.1586/14737159.7.4.419. [DOI] [PubMed] [Google Scholar]
- 58.Benschop K.S.M., Rahamat-Langendoen J.C., van der Avoort H.G.A.M., Claas E.C.J., Pas S.D., Schuurman R., Verweij J.J., Wolthers K.C., Niesters H.G.M., Koopmans M.P.G. on behalf of VIRO-TypeNed, VIRO-TypeNed, systematic molecular surveillance of enteroviruses in the Netherlands between 2010 and 2014. Euro. Surveill. 2016;21:29. doi: 10.2807/1560-7917.ES.2016.21.39.30352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mirand A., le Sage F.V., Pereira B., Cohen R., Levy C., Archimbaud C., Peigue-Lafeuille H., Bailly J.L., Henquell C. Ambulatory pediatric surveillance of hand, foot and mouth disease as signal of an outbreak of coxsackievirus A6 infections, France, 2014–2015. Emerg. Infect. Dis. 2016;22:1884–1893. doi: 10.3201/eid2211.160590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ibrahim W., Boukhadra N., Nasri-Zoghlami D., Berthelot P., Omar S., Bourlet T., Pozzetto B., Pillet S. Partial sequencing of the VP2 capsid gene for direct enterovirus genotyping in clinical specimens. Clin. Microbiol. Infect. 2014;20:O558–O565. doi: 10.1111/1469-0691.12520. [DOI] [PubMed] [Google Scholar]
- 61.Lukashev A.N., Shumilina E.Y., Belalov I.S., Ivanova O.E., Eremeeva T.P., Reznik V.I., Trotsenko O.E., Drexler J.F., Drosten C. Recombination strategies and evolutionary dynamics of the Human enterovirus A global gene pool. J. Gen. Virol. 2014;95:868–873. doi: 10.1099/vir.0.060004-0. [DOI] [PubMed] [Google Scholar]
- 62.Leitch E.C.M., Harvala H., Robertson I., Ubillos I., Templeton K., Simmonds P. Direct identification of human enterovirus serotypes in cerebrospinal fluid by amplification and sequencing of the VP1 region. J. Clin. Virol. 2009;44:119–124. doi: 10.1016/j.jcv.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 63.Faleye T., Adewumi M., Adeniji J. Defining the Enterovirus Diversity Landscape of a Fecal Sample: A Methodological Challenge? Viruses. 2016;8:18. doi: 10.3390/v8010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krasota A., Loginovskih N., Ivanova O., Lipskaya G. Direct identification of enteroviruses in cerebrospinal fluid of patients with suspected meningitis by nested PCR amplification. Viruses. 2016;8:10. doi: 10.3390/v8010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nguyen A.T., Tran T.T., Hoang V.M.T., Nghiem N.M., Le N.N.T., Le T.T.M., Phan Q.T., Truong K.H., Le N.N.T., Ho V.L., Do V.C., Ha T.M., Nguyen H.T., Nguyen C.V.V., Thwaites G., van Doorn H.R., Le T.V. Development and evaluation of a non-ribosomal random PCR and next-generation sequencing based assay for detection and sequencing of hand, foot and mouth disease pathogens. Virol. J. 2016;13:125. doi: 10.1186/s12985-016-0580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Montmayeur A.M., Ng T.F.F., Schmidt A., Zhao K., Magaña L., Iber J., Castro C.J., Chen Q., Henderson E., Ramos E., Shaw J., Tatusov R.L., Dybdahl-Sissoko N., Endegue-Zanga M.C., Adeniji J.A., Oberste M.S., Burns C.C. High-throughput next-generation sequencing of polioviruses. J. Clin. Microbiol. 2017;55:606–615. doi: 10.1128/JCM.02121-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chien Y.S., Luo S.T., Tsao K.C., Huang Y.C., Chung W.Y., Liao Y.C., Tan Y., Das S.R., Lee M.S. Genomic analysis of serologically untypable human enteroviruses in Taiwan. J. Biomed. Sci. 2019;26:49. doi: 10.1186/s12929-019-0541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tan L.V., Tuyen N.T.K., Thanh T.T., Ngan T.T., Van H.M.T., Sabanathan S., Van T.T.M., Thanh L.T.M., Thwaites G., van Doorn H.R., et al. A generic assay for whole-genome amplification and deep sequencing of enterovirus A71. J. Virol. Methods. 2015;215–216:30–36. doi: 10.1016/j.jviromet.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berthet N., Reinhardt A.K., Leclercq I., van Ooyen S., Batéjat C., Dickinson P., Stamboliyska R., Old I.G., Kong K.A., Dacheux L., Bourhy H., Kennedy G.C., Korfhage C., Cole S.T., Manuguerra J.-C. Phi29 polymerase based random amplification of viral RNA as an alternative to random RT-PCR. BMC Mol. Biol. 2008;9:77. doi: 10.1186/1471-2199-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Djikeng A., Halpin R., Kuzmickas R., DePasse J., Feldblyum J., Sengamalay N., Afonso C., Zhang X., Anderson N.G., Ghedin E., Spiro D.J. Viral genome sequencing by random priming methods. BMC Genomics. 2008;9:5. doi: 10.1186/1471-2164-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bessaud M., Sadeuh-Mba S.A., Joffret M.-L., Razafindratsimandresy R., Polston P., Volle R., Rakoto-Andrianarivelo M., Blondel B., Njouom R., Delpeyroux F. Whole genome sequencing of enterovirus species C isolates by high-throughput sequencing: Development of generic primers. Front. Microbiol. 2016;7:1294. doi: 10.3389/fmicb.2016.01294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grädel C., Terrazos Miani M.A., Baumann C., Barbani M.T., Neuenschwander S., Leib S.L., Suter-Riniker F., Ramette A. Whole-genome sequencing of human enteroviruses from clinical samples by nanopore direct rna sequencing. Viruses. 2020;12:841. doi: 10.3390/v12080841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wright C.F., Morelli M.J., Thébaud G., Knowles N.J., Herzyk P., Paton D.J., Haydon D.T., King D.P. Beyond the consensus: Dissecting within-host viral population diversity of foot-and-mouth disease virus by using next-generation genome sequencing. J. Virol. 2011;85:2266–2275. doi: 10.1128/JVI.01396-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baronti C., Piorkowski G., Leparc-Goffart I., de Lamballerie X., Dubot-Pérès A. Rapid next-generation sequencing of dengue, EV-A71 and RSV-A viruses. J. Virol. Methods. 2015;226:7–14. doi: 10.1016/j.jviromet.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 75.Tan Y., Hassan F., Schuster J.E., Simenauer A., Selvarangan R., Halpin R.A., Lin X., Fedorova N., Stockwell T.B., Lam T.-T.-Y., Chappell J.D., Hartert T.V., Holmes E.C., Das S.R., Evolution M., Recombination I., of Enterovirus D68 during the, Outbreak in the United States. J. Virol. 2014;90(2016):1997–2007. doi: 10.1128/JVI.02418-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Geoghegan J.L., Tan L.V., Kühnert D., Halpin R.A., Lin X., Simenauer A., Akopov A., Das S.R., Stockwell T.B., Shrivastava S., Van Doorn H.R., et al. Phylodynamics of enterovirus A71-associated hand, foot, and mouth disease in Viet Nam. J. Virol. 2015;89:8871–8879. doi: 10.1128/JVI.00706-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lizasoain A., Mir D., Masachessi G., Farías A., Rodríguez-Osorio N., Victoria M., Nates S., Colina R. Environmental surveillance through next-generation sequencing to unveil the diversity of human enteroviruses beyond the reported clinical cases. Viruses. 2021;13:120. doi: 10.3390/v13010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Faleye T.O.C., Driver E., Bowes D., Adhikari S., Adams D., Varsani A., Halden R.U., Scotch M. Pan-enterovirus amplicon-based high-throughput sequencing detects the complete capsid of a EVA71 genotype C1 variant via wastewater-based epidemiology in Arizona. Viruses. 2021;13:74. doi: 10.3390/v13010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duong V., Mey C., Eloit M., Zhu H., Danet L., Huang Z., Zou G., Tarantola A., Altmeyer R., Buchy P., et al. Molecular epidemiology of human enterovirus 71 at the origin of an epidemic of fatal hand, foot and mouth disease cases in Cambodia. Emerg. Microbes Infect. 2016;5:1–9. doi: 10.1038/emi.2016.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rutvisuttinunt W., Klungthong C., Thaisomboonsuk B., Chinnawirotpisan P., Ajariyakhajorn C., Manasatienkij W., Phonpakobsin T., Lon C., Saunders D., Fernandez S., et al. Retrospective use of next-generation sequencing reveals the presence of Enteroviruses in acute influenza-like illness respiratory samples collected in South/South-East Asia during 2010–2013. J. Clin. Virol. 2017;94:91–99. doi: 10.1016/j.jcv.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang W., Wang G., Zhuge J., Nolan S.M., Dimitrova N., Fallon J.T. Whole-genome sequence analysis reveals the enterovirus D68 Isolates during the United States 2014 outbreak mainly belong to a novel clade. Sci. Rep. 2015;5:15223. doi: 10.1038/srep15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stelzer-Braid S., Wynn M., Chatoor R., Scotch M., Ramachandran V., Teoh H.L., Farrar M.A., Sampaio H., Andrews P.I., Craig M.E., MacIntyre C.R., Varadhan H., Kesson A., Britton P.N., Newcombe J., Rawlinson W.D. Next generation sequencing of human enterovirus strains from an outbreak of enterovirus A71 shows applicability to outbreak investigations. J. Clin. Virol. 2020;122:104216. doi: 10.1016/j.jcv.2019.104216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Isaacs S.R., Kim K.W., Cheng J.X., Bull R.A., Stelzer-Braid S., Luciani F., Rawlinson W.D., Craig M.E. Amplification and next generation sequencing of near full-length human enteroviruses for identification and characterisation from clinical samples. Sci. Rep. 2018;8:11889. doi: 10.1038/s41598-018-30322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Strubbia S., Phan M.V.T., Schaeffer J., Koopmans M., Cotten M., Le Guyader F.S. Characterization of norovirus and other human enteric viruses in sewage and stool samples through next-generation sequencing. Food Environ. Virol. 2019;11:400–409. doi: 10.1007/s12560-019-09402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bisseux M., Debroas D., Mirand A., Archimbaud C., Peigue-Lafeuille H., Bailly J.-L., Henquell C. Monitoring of enterovirus diversity in wastewater by ultra-deep sequencing: An effective complementary tool for clinical enterovirus surveillance. Water Res. 2020;169 doi: 10.1016/j.watres.2019.115246. [DOI] [PubMed] [Google Scholar]
- 86.Tao Z., Chen P., Cui N., Lin X., Ji F., Liu Y., Xiong P., Zhang L., Xu Q., Song Y., Xu A. Detection of enteroviruses in urban sewage by next generation sequencing and its application in environmental surveillance. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138818. [DOI] [PubMed] [Google Scholar]
- 87.Boman J., Nilsson B., Juto P. Serum IgA, IgG, and IgM responses to different enteroviruses as measured by a coxsackie B5-based indirect ELISA. J. Med. Virol. 1992;38:32–35. doi: 10.1002/jmv.1890380108. [DOI] [PubMed] [Google Scholar]
- 88.Swanink C.M., Veenstra L., Poort Y.A., Kaan J.A., Galama J.M. Coxsackievirus B1-based antibody-capture enzyme-linked immunosorbent assay for detection of immunoglobulin G (IgG), IgM, and IgA with broad specificity for enteroviruses. J. Clin. Microbiol. 1993;31:3240–3246. doi: 10.1128/jcm.31.12.3240-3246.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oberste M.S., Nix W.A., Maher K., Pallansch M.A. Improved molecular identification of enteroviruses by RT-PCR and amplicon sequencing. J. Clin. Virol. 2003;26:375–377. doi: 10.1016/S1386-6532(03)00004-0. [DOI] [PubMed] [Google Scholar]
- 90.Oberste M.S., Maher K., Flemister M.R., Marchetti G., Kilpatrick D.R., Pallansch M.A. Comparison of classic and molecular approaches for the identification of untypeable enteroviruses. J. Clin. Microbiol. 2000;38:1170–1174. doi: 10.1128/JCM.38.3.1170-1174.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.