Highlights
-
•
Scientific question: Given the waning immune response over time and the emergence of various concerns, the neutralizing capacity of antibodies elicited by inactivated vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants in China remains largely unexplored in the real world.
-
•
Evidence before this study: A third booster dose enhances the immune response against SARS-CoV-2 and its variants. However, the kinetics of the IgG subclass responses remain largely unknown. One study showed that inactivated vaccines predominantly induced IgG1 and IgG3, while IgG2 and IgG4 were nearly undetectable.
-
•
New findings: Despite a booster dose of inactivated vaccine, neutralizing antibody levels against Omicron, particularly the BA.5.2 subvariant, remained low. Notably, the predominant IgG subclass antibodies were IgG1 and IgG2, with a much lower level of IgG4. Repeated administration of inactivated vaccines would lead to a higher conversion of IgG1 to IgG4.
-
•
Significance of the study: First, our real-world seroepidemiological investigation of antibody responses highlights the need for boosting with more effective vaccines or optimization of immunization strategies. Second, the observation of the IgG1/IgG4 class switch alerts us to continue monitoring subclass antibody responses. Further robust clinical investigations should be conducted to provide deeper insight into how this observation contributes to optimizing immunization strategies.
Keywords: Variant of concern (VOC), Inactivated vaccines, Serological study, IgG subclasses, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
Abstract
Inactivated coronavirus disease 2019 (COVID-19) vaccines such as CoronaVac and BBIBP-CorV have been widely used in China. However, more investigation is still needed to understand antibodies' duration and effectiveness against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants in the real world. In this study, 575 participants who had been vaccinated with two or three doses of the inactivated vaccine were recruited. Serum samples were collected and tested for anti-spike IgG and neutralizing antibodies against SARS-CoV-2 (original strain, Dela, and Omicron). Unsurprisingly, a third dose of the vaccine significantly enhanced antibody responses against SARS-CoV-2 and its variants. However, despite a booster dose, the neutralizing antibody levels against Omicron, particularly the BA.5.2 subvariant, remained low. There was no sex bias, but an age bias was observed. Notably, the predominant IgG subclass antibodies were IgG1 and IgG2, with a much lower level of IgG4. After the booster shot, the ratio of IgG4 to IgG1 significantly increased. The observation of IgG1 to the IgG4 class switch after repeated inactivated vaccinations underscores the importance of continuous monitoring of subclass antibody responses. Further clinical investigations are required to understand the implications of this class switch for optimizing immunization strategies.
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to unprecedented global healthcare and economic burdens [1], [2]. Vaccines have been indispensable in preventing infection and severe and critical illnesses and reducing mortality [3], [4], [5], [6]. As of February 2024, more than 13.59 billion vaccine doses were administered globally [7]. However, the emergence of variants of concern (VOCs) with increased transmission and an immune escape capability, such as Delta and Omicron, has driven waves of infections, which present enormous challenges to vaccination strategies [8].
Multiple vaccines have been approved for global use, including inactivated adenovirus-vectored mRNA and protein subunit vaccines [9]. Inactivated vaccines are the primary vaccine type used in China. In December 2020, China initiated primary immunization with two doses of inactivated vaccines at intervals of 3–8 weeks and then implemented a booster program with a third dose of inactivated vaccine in October 2021. A booster program with a fourth dose of an inactivated protein subunit, or adenoviral vector-based vaccine, was also initiated in December 2022 [10]. However, most Chinese people have been vaccinated with only three doses of inactivated vaccines [11]. Despite an ongoing booster program, the neutralizing capacity of the antibodies elicited by inactivated vaccines against SARS-CoV-2 variants in China remains largely unexplored in the real world.
IgG antibodies comprise four subclasses: IgG1, IgG2, IgG3, and IgG4 [12]. IgG1 and IgG3 mediate inflammatory responses and promote cellular immune responses against pathogens. In contrast, IgG2 and IgG4 are involved in noninflammatory functions [12]. Recent studies have found that the repeated administration of mRNA-based SARS-CoV-2 vaccines induced an increase in IgG4 antibody levels, along with a reduced capacity to mediate antibody-dependent cellular phagocytosis (ADCP) and complement deposition (ADCD) [13]. Reduced ADCP and ADCD functions may affect the ability of the immune system to clear viral infections or infected cells [14]. To date, limited research has been conducted on IgG subclasses following vaccination with inactivated vaccines. One previous study found that IgG1 and IgG3 were the most abundant serum antibodies, whereas IgG2 and IgG4 were almost undetectable at any time [15].
Here, we have conducted a serum epidemiological study to comprehensively evaluate the duration of anti-spike IgG antibodies, precisely the subclass antibody responses, and assessed their neutralizing capacity against SARS-CoV-2 variants in healthy individuals immunized with inactivated vaccines in China.
2. Materials and methods
2.1. Study design and participants
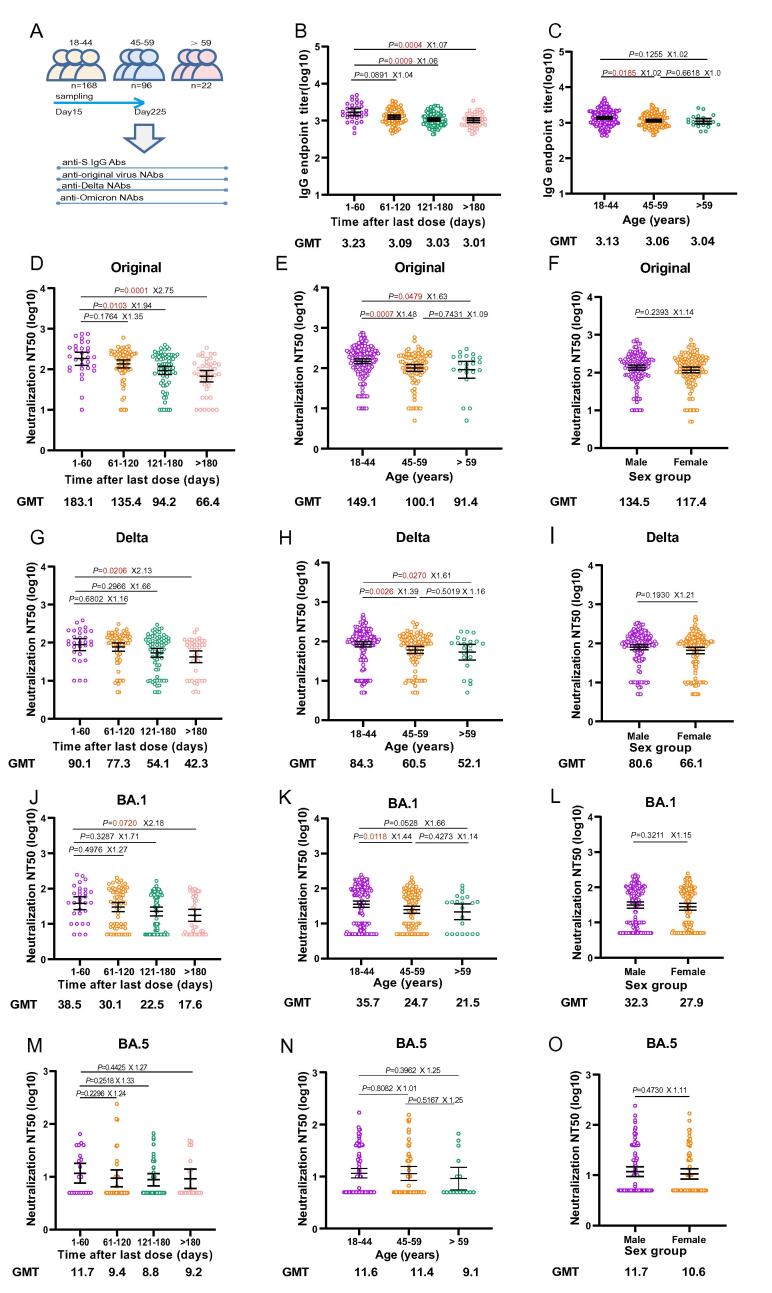
All participants were recruited from the Affiliated Nantong Hospital 3 of the Nantong University Outpatient Department (Nantong, China). Individuals with a history of SARS-CoV-2 infection [based on a surveillance polymerase chain reaction (PCR) test] were excluded. In total, 575 serum samples were collected from vaccinated individuals after receiving the second or third dose of the inactivated vaccines. Two hundred eighty-nine people received two doses of inactivated vaccines, and their serum samples were collected in January 2022. The number of days since the second vaccination ranged from 123 days to 293 days. Two hundred eighty-six participants received three doses of inactivated vaccines, and their serum samples were collected in June 2022—the days since the third vaccination ranged from 15 to 225 days. Fig. 1 shows a schematic outline of the workflow of this study.
Fig. 1.
Schematic diagram outlining the workflow of this study. Abbreviations: ys represents years old; d represents days.
All participants included in the study had no underlying diseases or known history of exposure to or infection with SARS-CoV-2. The serum was separated from peripheral blood in serum-gel tubes via centrifugation, aliquoted, and stored at −80 ℃ before use.
2.2. Cell lines
HEK 293T and Huh7 cells were used for pseudovirus production and titration assays. Both cell lines were maintained in Dulbecco’s Modified Eagle Medium (DMEM, Gibco) with 10 % fetal bovine serum (FBS., Gibco), 100 U/mL penicillin (Gibco) and 100 μg /mL streptomycin (Gibco).
2.3. Vaccines
The participants mainly received two types of inactivated SARS-CoV-2 vaccines approved by the China Food and Drug Administration (CFDA): CoronaVac (Sinovac Biotech) and BBIBP-CorV (Beijing Institute of Biological Products). These two inactivated vaccines have shown promising efficacy and genetic stability in clinical trials and are China’s most popular SARS-CoV-2 vaccines [16], [17].
2.4. Detection of IgG antibody by enzyme-linked immunosorbent assay (ELISA)
In order to examine the levels of IgG antibodies and subclasses in human serum samples, ELISA was performed as described previously [18]. Briefly, SARS-CoV-2 spike (S) protein (S protein from wild type strain was used for Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6 and Supplementary Fig 1; S protein from BA.5.2 was used for Supplementary Fig. 2) was diluted in coating buffer (pH 9.6, 0.05 mol/L Na2CO3-NaHCO3) and then applied to 96-well ELISA plates (Corning) at 100 ng/well overnight at 4 ℃. The pre-coated plates were then blocked with 5 % nonfat milk in phosphate-buffered saline (PBS) containing 0.05 % Tween-20 (PBS-T) for 2 h at 37 ℃ and washed three times with washing buffer (PBS-T). Serum samples were tested at a starting dilution 1:100 and serially diluted two-fold. After 2 h incubation with the diluted serum samples, plates were washed three times with PBS-T and then incubated with five horseradish peroxidase (HRP)-conjugated anti-human IgG antibodies for 1 h at 37 ℃. The following antibody dilutions were used in the experiment: total IgG (Abcam, 1:100,000), IgG1 (SinoBiological, 1:50,000), IgG2 (Abcam, 1:4,000), IgG3 (Invitrogen, 1:3,000) and IgG4 (Abnova, 1:6,000). Plates were washed thrice with PBS-T and developed with a tetramethylbenzidine (TMB, Life Technologies) substrate. After 15 min of incubation in the dark, the reaction was stopped using 2 mol/L H2SO4. A microplate reader (BioTek) was used to read the absorbance at 450 nm with a correction wavelength of 630 nm. Twenty pre-pandemic serum samples collected before January 2019 were tested for IgG antibodies and IgG subclass antibodies to establish the cut-off value for calculating the endpoint titer. The cut-off value was determined using the following equation:
Fig. 2.
Antibody responses after receiving two doses of inactivated vaccines. A) Schematic illustration of the study design. A total of 289 participants were enrolled in this group. Serum samples were evaluated by ELISA for SARS-CoV-2 S protein-specific IgG antibodies in different time post-vaccination groups (B) and in different age groups (C). The 50 % serum neutralization titers (NT50) against SARS-CoV-2 pseudoviruses bearing spike proteins from the original virus, the Delta variant, and the Omicron variant BA.1 or BA.5.2 were calculated by nonlinear regression and compared between time post-vaccination groups (D, G, J, and M), age groups (E, H, K, and N), and sex groups (F, I, L, and O). Black horizontal bars in B - O indicate the mean values with 95 % CIs of each group. Statistical significance was determined by the Mann-Whitney U test and Kruskal-Wallis test. P values and fold changes of GMTs are labeled on each graph. Abbreviations: anti-S, anti-spike; GMT, geometric mean titer; ELISA, enzyme-linked immunosorbent assay; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; CI, confidence interval.
Fig. 3.
Antibody responses after receiving three doses of inactivated vaccine. A) Schematic illustration of the study design. A total of 286 participants were involved in this group. (B) and (C) Serum samples were tested by ELISA for SARS-CoV-2 S protein IgG antibodies at different time points post-vaccination groups (B) and in different age groups (C). The 50 % serum neutralization titers (NT50) against SARS-CoV-2 pseudoviruses bearing spike proteins from the original virus, the Delta variant, or the Omicron variant were calculated by nonlinear regression and compared between different time (D, G, J, and M), age groups (E, H, K, and N), and sex groups (F, I, L, and O). Black horizontal bars in B - O indicate the mean values with 95 % CIs of each group. Statistical significance was determined by the Mann-Whitney U test and Kruskal-Wallis test. P values and fold changes of GMTs are labeled on each graph. Abbreviations: anti-S, anti-spike; GMT, geometric mean titer; ELISA, enzyme-linked immunosorbent assay; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; CI, confidence interval.
Fig. 4.
Comparison of antibody responses between two-dose and three-dose groups. A) SARS-CoV-2 S protein IgG antibody titers were compared between the two-dose group and the three-dose group. B) and C) Correlation analysis between binding antibody titers and neutralizing antibody titers was performed for both the two-dose group (B) and the three-dose group (C). The 50 % serum neutralization titers (NT50) against SARS-CoV-2 pseudoviruses bearing spike proteins from the original virus, the Delta variant, the Omicron variant BA.1 (D), and the BA.5.2 (E) were compared between the two-dose group and three-dose group. The fold changes of GMTs between SARS-CoV-2 and its variants are labeled. Black horizontal bars in (A, D, and E) indicate the means with 95 % CIs of each group. Statistical significance was determined by Mann-Whitney U test for (A). Correlation analyses in B and C were calculated by Pearson’s correlation test. For D and E, a nonparametric test was performed with SPSS 26.0 software because the variables did not follow a normal distribution. A two-tailed test and a P-value < 0.05 were considered statistically significant. Abbreviations: anti-S, anti-spike; GMT, geometric mean titer; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; CI, confidence interval.
Fig. 5.
IgG subclass responses after inactivated vaccine administration. Serum samples were evaluated by ELISA for IgG1, IgG2, and IgG4 antibodies in different time post-vaccination groups in both two-dose group (A) and three-dose group (B). All data were shown as mean values with 95% CIs of each group. Statistical significance was determined by the Mann-Whitney U test and Kruskal-Wallis test. P values and fold changes of GMTs are labeled on each graph. Abbreviations: GMT, geometric mean titer; ELISA, enzyme-linked immunosorbent assay; CI, confidence interval.
Fig. 6.
Ratio of IgG4/IgG1 over time following inactivated vaccines. Endpoint titer ratios of IgG4/IgG1 were compared between different time (A), age group (B) and gender group (C) after the second dose of inactivated vaccines. The comparison of IgG4/IgG1 endpoint titer ratios between different time (D), age group (E), and sex group (F) after the third dose of inactivated vaccine were also performed. G) - I) Endpoint titer ratios of IgG4/IgG1 were compared between the two-dose and the three-dose groups. Statistical significance was determined by the Mann-Whitney U test and Kruskal-Wallis test. Notes: ns represents non-significance (P > 0.05); *, P < 0.05; **, P < 0.01.
is the mean of the optical density (OD) value; SD is the standard deviation; n is the number of independent controls, t is the (1-α)th percentile of the one-tailed t-distribution with v = n-1 degrees of freedom [19]. Based on the results from 20 pre-pandemic serum samples, the cut-off values for total IgG, IgG1, IgG2, and IgG4 were determined to be 0.12, 0.26, 0.26, and 0.18, respectively. In order to calculate the endpoint titer of the binding antibodies, a linear graph was generated by plotting the log10 of serum dilutions versus the corrected OD values (OD450-OD630) within the linear portion of the curve (y = kx + b, r2 > 0.95). The endpoint titer was determined as the log10 of the serum dilution at the point where the curve crossed the defined cut-off value.
2.5. Pseudovirus production and titration
To assess the neutralizing capacity of the serum samples against SARS-CoV-2 and its VOCs, SARS-CoV-2 spike pseudotyped viruses were generated as described previously [20]. Briefly, the codon-optimized gene encoding the spike protein of the SARS-CoV-2 original strain (NC_045512), delta variant (B.1.617.2), or Omicron variant (B.1.1.529 or BA.5.2) with a C-terminal 19-amino acid deletion was synthesized and cloned into the pCAGGS vector. HEK 293T cells were transfected with 1 μg of pCAGGS-spike and 15 μg of pNL4–3. luc. RE using polyethyleneimine (2 μL per 1 μg of plasmids) (Polysciences). Cell culture supernatants were collected at 48 h post-transfection. The 50 % tissue culture infectious dose (TCID50) protocol was used to determine viral titers. Pseudoviruses were serially diluted and added to 1 × 104 Huh7 cells in 96-well plates. The medium was replaced with fresh growth medium 12 h post-infection, and the cells were cultured for an additional 48 h. Luciferase activity was examined using a Steady-Glo Luciferase Assay System (Promega) and measured using a microplate reader (BioTek). Relative luminescence units (RLU) 10-fold were considered positive above the mean background value.
2.6. Pseudovirus neutralization assay
To determine the neutralizing capacity of the sera against SARS-CoV-2 and its VOCs, a pseudovirus neutralization assay was performed as previously described [20]. Briefly, the serially diluted serum samples (two-fold) were incubated with 200 TCID50 of pseudoviruses for 1 h at 37 °C and were then applied to 96-well plates that were pre-seeded with Huh7 cells. Cells were incubated with the mixture at 37 °C for 12 h and then replaced with fresh growth medium. After 48 h, the luciferase activity of the cells was measured as described above. Nonlinear regression was used to calculate 50 % neutralization titers (NT50) against the pseudoviruses (GraphPad Prism 8.0). The NT50 was defined as the half-maximal inhibitory dilution of serum normalized to that of the infection control (virus without serum samples). To define a negative NT50, we performed a two-fold serial dilution of pre-pandemic sera (starting from 1:10) in duplicate; none demonstrated neutralizing activity against the original SARS-CoV-2 and its variants. Therefore, we defined half of the initial dilution, 1:5, as the negative NT50.
2.7. Statistical analysis
All statistical analyses were performed using GraphPad Prism 8.0 unless specified otherwise. A t-test or one-way ANOVA with Tukey’s multiple comparison test was performed for statistical comparisons between groups. Correlations between binding and neutralizing antibody titers were determined using Pearson’s correlation tests. A non-parametric test was performed using the SPSS 26.0 software when the variables did not follow a normal distribution. A two-sided test and P value < 0.05 were considered statistically significant.
3. Results
3.1. Baseline characteristics of the participants
A total of 575 serum samples were obtained from all participants and categorized into two groups based on the doses of vaccines they received: a two-dose group and a three-dose group. Subgroup analyses were conducted within each group based on age, sex, and number of days post-vaccination (Table 1). The participants in the two-dose group ranged from 18 to 76 years old (ys), with a median age of 46 ys, and 67.5 % were male. In the three-dose group, the age ranged from 18 to 74 ys, with a median age of 39 ys, and 53.8 % of patients were male.
Table 1.
Demographic characteristics of study participants.
| Characteristics | Two-dose group (N = 289) |
Three-dose group (N = 286) |
|
|---|---|---|---|
| Sex, number (%) | |||
| Male | 195 (67.5) | 154 (53.8) | |
| Female | 94 (32.5) | 132 (46.2) | |
| Age, years† | 46 (32–55) | 39 (26–52) | |
| Age groups (years), number (%) | |||
| 18–44 | 136 (47.1) | 168 (58.7) | |
| 45–59 | 127 (43.9) | 96 (33.6) | |
| > 59 | 26 (9.0) | 22 (7.7) | |
| Days after vaccination (days), number (%) * | |||
| 1–60 | 0 | 30 (14.1) | |
| 61–120 | 0 | 68 (31.9) | |
| 121–180 | 129 (59.0) | 73 (34.3) | |
| > 180 | 90 (41.0) | 42 (19.7) | |
Note: * represents the number of participants for the two-dose and three-dose groups, which are 219 and 213, respectively; † represents median (interquartile range).
Based on the time post-vaccination, each group was divided into four subgroups: 1–60, 61–120, 121–180, and > 180 days. Of the 575 participants, 432 provided detailed information on the vaccination date (219 in the two-dose group and 213 in the three-dose group). In the two-dose group, 0 (0.0 %), 0 (0.0 %), 129 (59.0 %), and 90 (41.0 %) were at 1–60, 61–120, 121–180 and > 180 days, respectively. In the three-dose group, 30 (14.1 %), 68 (31.9 %), 73 (34.3 %), and 42 (19.7 %) were at 1–60, 61–120, 121–180, and > 180 days, respectively.
3.2. Antibody responses after receiving two doses of inactivated vaccines
First, we evaluated anti-spike IgG antibody levels using ELISA and viral neutralizing antibodies (NAbs) using a pseudovirus neutralization assay (Fig. 2A). In the two-dose group, the geometric mean titers (GMTs) of the anti-spike IgG antibodies were 2.73 (95 % confidence interval (CI) 2.68–2.78) between 121–180 days post the second dose of vaccine, and the GMTs decreased to 2.66 (95 % CI 2.60–2.71) after 180 days (Fig. 2B). Furthermore, a significant reduction in IgG antibodies was observed in senior participants. The GMTs were 2.74 (95 % CI 2.69–2.79), 2.69 (95 % CI 2.64–2.73), and 2.53 (95 % CI 2.40–2.66) for the 18–44, 45–59, and > 59 ys groups, respectively (Fig. 2C).
We further measured the neutralizing titers using a pseudovirus-neutralizing assay. The GMTs of the NAbs against the original virus between 121–180 days was 29.1 (95 % CI 22.2–37.4) and declined to 21.5 (95 % CI 17.6–26.7) after 180 days (Fig. 2D). For the Delta variant, the GMTs NAbs titer was 18.1 (95 % CI 15.5–20.7) in the 121–180 days group and declined to 13.4 (95 % CI 10.9–16.1) in the > 180 days group (Fig. 2G). While for the Omicron BA.1 subvariant, the GMTs was 8.9 (95 % CI 7.1–9.4) between 121–180 days and dramatically declined to 6.0 (95 % CI 5.6–6.7) in the > 180 days group, with a majority of the samples virtually undetectable (Fig. 2J).
Age subgroup analysis revealed that the GMTs NAbs titer in the 18–44 ys group was 33.8 (95 % CI 29.4–38.6), 19.9 (95 % CI 17.2–22.5), and 7.5 (95 % CI 6.8–8.8) for the original, Delta, and Omicron BA.1, respectively. In the 45–59 ys group, the GMTs were 23.9 (95 % CI 20.4–28.0), 15.4 (95 % CI 13.1–17.7), and 6.9 (95 % CI 6.3–7.3), respectively. While in the senior group (>59 ys), the GMTs dropped to 19.1 (95 % CI 13.3–28.1), 12.7 (95 % CI 9.3–17.4), and 6.0 (95 % CI 5.2–6.9), respectively (Fig. 2E, H, and K).
In addition, we evaluated NAbs against BA.5.2, one of the dominant sub-variants during the Omicron wave, at the end of 2022 and early 2023 in China. Unfortunately, 345 serum samples were finished. The remaining 230 serum samples exhibited a comparable distribution of age, sex, and days post-infection with the initial 575 samples (Supplementary Table 1). The binding antibody titers specific to the Omicron BA.5.2 spike were much lower than those to the original spike. They showed significant differences between age groups (Supplementary Fig. 2), which is unsurprising given the over 30 point mutations identified in BA.5.2 spike protein compared to the original spike [21]. The GMTs of NAbs against BA.5.2 remain low among days post-vaccination and age groups (8.8 [95 % CI 6.5–11.8], and 7.5 [95 % CI 6.0–9.3] for 121–180 days and > 180 days groups, respectively; 8.6 [95 % CI 6.9–10.7], 8.0 [95 % CI 6.1–10.5], 6.0 [95 % CI 4.0–9.2] for 18–44, 45–59, and > 59 ys groups, respectively). No significant differences were observed between the subgroups (P > 0.05) (Fig. 2M and N).
We did not observe statistically significant sex differences in antibody responses. In females, the GMTs of NAbs were 24.8 (95 % CI 20.5–30.5), 14.8 (95 % CI 12.5–17.8), 6.9 (95 % CI 6.2–7.6) and 7.6 (95 % CI 5.8–10.1), against Original, Delta, Omicron BA.1 and BA.5.2, respectively. In males, the GMTs of NAbs were 28.5 (95 % CI 25.4–32.4), 18.1(95 % CI 16.3–20.3), 7.1 (95 % CI 6.6–7.5), and 8.4 (95 % CI 6.9–10.3), respectively (Fig. 2F, I, L and O).
3.3. Antibody responses after receiving three doses of inactivated vaccines
In the three-dose group, the time since the third vaccination ranged from 15 to 225 days (Fig. 3A). Our data showed that the antibody titers gradually decreased over time (Fig. 3B). The GMTs were 3.23 (95 % CI 3.13–3.32), 3.09 (95 % CI 3.04–3.14), 3.03 (95 % 2.99–3.07), and 3.01 (95 % CI 2.95–3.07) in the 1–60, 61–120, 121–180, and > 180 days subgroups, respectively. Next, we compared the GMTs between the age groups. The GMTs were 3.13 (95 %CI 3.10–3.17), 3.06 (95 % CI 3.02–3.09), and 3.04 (95 % CI 2.96–3.11) in the 18–44, 45–59, and > 59 ys groups, respectively. A significant difference was observed between the 18–44 and 45–59 ys groups (P < 0.05) (Fig. 3C).
The GMTs of the NAbs against the original virus were 183.1 (95 %CI 127.3–266.4), 135.4 (95 % CI 107.4–170.5), 94.2 (95 % CI 74.4–119.7), and 66.4 (95 %CI 48.5–93.1) in the 1–60, 61–120, 121–180 and > 180 days groups, respectively (Fig. 3D). NAbs showed a significant reduction in 45–59 and > 59 ys groups compared to the 18–44 ys group, with GMTs at 100.1 (95 % CI 83.2–123.2), 91.4 (95 % CI 56.4–149.4), and 149.1 (95 % CI 129.3–172.1), respectively (Fig. 3E).
The GMTs of NAbs against the Delta variant were 90.1 (95 % CI 61.5–128.6), 77.3 (95 % CI 60.1–99.4), 54.1 (95 % CI 41.3–70.9), and 42.3 (95 % CI 28.9–60.5) in the 1–60, 61–120, 121–180 and > 180 days groups, respectively (Fig. 3G). A significant reduction of NAbs in the 45–59 and > 59 ys groups was observed compared to the 18–44 ys group, with the GMTs measured at 60.5 (95 % CI 49.3–74.3), 52.1 (95 % CI 33.6–83.1), and 84.3 (95 % CI 72.1–100.0), respectively (Fig. 3H).
The GMTs of NAbs against the Omicron BA.1 variant were 38.5 (95 % CI 25.3–59.4), 30.1 (95 % CI 22.4–41.2), 22.5 (95 % CI 16.9–30.1) and 17.6 (95 % CI 11.5–26.3) in 1–60, 61–120, 121–180, and > 180 days groups, respectively (Fig. 3J). Regarding the age subgroup analysis, the GMTs in the 18–44 ys group were 35.7 (95 % CI 28.3–43.4), which was higher compared to the 45–59 ys group (24.7, 95 % CI 19.4–31.8) and the > 59 years group (21.5,95 % CI 13.1–37.1) (Fig. 3K).
We observed a significant reduction in NAbs against Omicron BA.5.2. The GMTs were 11.7 (95 % CI 7.6–18.0), 9.4 (95 % CI 6.4–13.6), 8.8 (95 % CI 6.7–11.5), and 9.2 (95 % CI 6.1–13.9) in the 1–60, 61–120, 121–180, and > 180 days groups, respectively (Fig. 3M). Regarding the age subgroup, the GMTs were 11.6 (95 % CI 9.3–14.2), 11.4 (95 % CI 8.3–15.5), 9.1 (95 % CI 5.6–15.1) in the 18–44, 45–59, >59 ys groups, respectively (Fig. 3N). The NAbs against BA.5.2 remain low among groups. No significant differences were observed between subgroups (P > 0.05).
Similar to the two-dose group, no sex bias was observed in the NAbs elicited by the three doses of inactivated vaccines (Fig. 3F, I, L, and O).
3.4. Comparison of antibody responses between two-dose and three-dose groups
Next, we compared the antibody levels between the two- and three-dose groups, aiming to contribute to the knowledge regarding the immunogenicity and protective efficacy of a booster dose of the vaccine.
First, we compared IgG titers between the two- and three-dose groups. A booster shot significantly increased the GMTs of IgG antibodies, from 2.70 (95 % CI 2.67–2.73) in the two-dose group to 3.10 (95 % CI 3.07–3.12) in the three-dose group (Fig. 4A). Correlation analysis revealed a marked positive correlation between binding antibody titers and NAb titers against the original virus in both groups (Fig. 4B and C).
Next, we compared NAbs against SARS-CoV-2 variants. In the two-dose group, the NAbs against Delta and Omicron BA.1 significantly dropped compared to the original virus, with the GMT measured at 16.9 (95 % CI 15.3–19.0), 6.6 (95 % CI 5.9–7.2), and 27.6 (95 % CI 24.4–30.5), respectively. Compared to the original virus, NAb titers decreased by 1.63-fold for the delta variant and 4.18-fold for the Omicron BA.1.
While in the three-dose group, the GMTs of NAbs against the original virus, Delta variant, and Omicron BA.1 were 127.1 (95 % CI 112.5–142.1), 73.1 (95 % CI 65.2–82.8) and 30.3 (95 % CI 25.7–34.7), respectively. Compared to the original virus, NAb titers decreased by 1.74-fold for the Delta variant and 4.19-fold for the Omicron variant, respectively. Furthermore, we compared the two groups and found that the NAb titers against all three SARS-CoV-2 strains were significantly elevated after a third dose of the inactive vaccine (4.6-fold for original, 4.32-fold for Delta, and 4.59-fold for Omicron BA.1), further emphasizing the importance of a third booster dose of the vaccine in enhancing the immune response (Fig. 4D).
Due to the varying number of participants analyzed for Omicron BA.5.2 compared to other variants, we opted to compare the NAbs against BA.5.2 between the two- and three-dose groups instead of comparing them against different variants (Fig. 4E). Our data revealed that the neutralizing antibodies against BA.5.2 persist at a low level even after a third booster dose (only a 1.41-fold increase), unlike the Original, Delta, and BA.1 strains, where we observed a 4.6-, 4.32–, and 5.49- fold increase after a third booster dose, respectively (Fig. 4D and E).
3.5. IgG subclass responses after receiving inactivated vaccines
Next, we evaluated IgG subclass responses. Surprisingly, unlike previous reports [15], our study revealed a substantial amount of IgG2 and IgG4 antibodies, whereas IgG3 antibody responses were almost undetectable (data not shown). In the two-dose group, the GMTs were 2.33 (95 % CI 2.16–2.27) and 2.30 (95 % CI 2.25–2.34) for IgG1, 2.06 (95 % CI 2.01–2.11) and 2.02 (95 % CI 1.97–2.07) for IgG2, 1.84 (95 % CI 1.80–1.89) and 1.90 (95 % CI 1.83–1.96) for IgG4 in the 121–180 days and > 180 days groups, respectively (Fig. 5A).
While in the three-dose group, the GMTs was 2.57 (95 % CI 2.48–2.65), 2.50 (95 % CI 2.46–2.54), 2.40 (95 % CI 2.35–2.44), and 2.37 (95 %CI 2.31–2.43) for IgG1; 2.32 (95 % CI 2.22–2.43), 2.23 (95 % CI 2.17–2.29), 2.18 (95 % CI 2.12–2.23), and 2.13 (95 % CI 2.05–2.20) for IgG2; and 2.18 (95 % CI 2.08–2.27), 2.14 (95 % CI 2.08–2.21), 2.08 (95 % CI 2.01–2.15), and 2.18 (95 % CI 2.10–2.26) for IgG4 in the 1–60, 61–120, 121–180, and > 180 days subgroups, respectively (Fig. 5B).
To investigate whether the administration of inactivated vaccines changes IgG subclass responses, we assessed the ratio of IgG4/IgG1 over time following either two or three doses of inactivated vaccines. After administering two doses of the vaccines, the IgG4/IgG1 ratio significantly increased, indicating the conversion of IgG1 to IgG4 over time (Fig. 6A), suggesting that the immune response shifted towards the IgG4 subclass response after six months. Interestingly, no significant changes in the IgG4/IgG1 ratio were observed over time following the administration of the three doses of the inactivated vaccines (Fig. 6D). Furthermore, we analyzed the IgG4/IgG1 ratio across age and sex subgroups and found no significant differences (Fig. 6B, C, E, and F).
Notably, when comparing the IgG4/IgG1 ratio between the two- and three-dose groups, we found that there was an increase in the IgG4/IgG1 ratio after three doses of vaccines, suggesting that repeated administration of inactivated vaccines may lead to a more pronounced conversion of IgG1 to IgG4 (Fig. 6G, H, and I).
4. Discussion
With the waning immune response over time and the emergence of VOCs with enhanced immune evasion abilities [5], [22], [23], [24], [25], it is crucial to gather more data on vaccine immunogenicity in real-world studies. In this study, our data demonstrated that the antibody immune responses elicited by two doses of the inactivated vaccine dramatically decreased six months post-immunization. Especially for the Omicron variants, two doses of inactivated vaccines failed to maintain an effective immune response after six months, as evidenced by neutralizing antibody titers near the lower detection limit. In comparison, a third booster dose of the inactivated vaccine was found to significantly increase antibody immune responses against the original, Delta, and Omicron BA.1. Consistent with previous studies [5], [26], [27], [28], [29], these findings highlight the importance of a third booster dose in increasing the immune response against SARS-CoV-2 and its VOCs. However, by comparing the neutralizing antibody titers against the original virus, Delta, and Omicron, particularly one of the dominant subvariant of BA.5.2 during the Omicron wave at the end of 2022 and early 2023 in China, our results demonstrated that both two and three doses of inactivated vaccines resulted in shallow levels of neutralizing antibodies against Omicron, particularly for the BA.5.2 subvariant, which is not unexpected given the enhanced immune escape of Omicron [30], [31]. More effective vaccine types and optimization of vaccine strategies should be investigated.
Although it is well known that antibody immune responses wane over time post-vaccination, the waning rate might be differentially affected by factors such as age, sex, serostatus, and specific comorbidities [17], [32], [33], [34], [35], [36], [37]. Understanding how these factors influence the immune response will help determine the optimal vaccination strategies, especially in specific individuals. In this study, we have investigated whether antibody responses are affected by intrinsic host factors such as age and sex. Our results showed no sex bias, but there were age differences in the immune responses. In both the two- and three-dose groups, the antibody responses were more vital in the younger subgroup (18–59 years) than in the older subgroup (>59 years). Neutralizing antibody titers, especially against Omicron, declined substantially six months after two doses of inactivated vaccines among older participants. Vaccination reduces breakthrough and symptomatic cases of SARS-CoV-2 infection in vulnerable individuals [17], [35], [37], [38]. A phase 4 clinical trial showed that, in elderly individuals primed with two doses of CoronaVac, heterologous immunization with an adenoviral vaccine (Ad5-nCOV) induced potent antibodies against wild-type SARS-CoV-2 and its variants, which could be an alternative regimen for enhancing protection in elderly individuals [39].
The subclass of antibody responses elicited by the different SARS-CoV-2 vaccination platforms and their effects on vaccine efficacy have not been thoroughly investigated. The observation of a class switch towards noninflammatory IgG4 antibodies following repeated mRNA-based SARS-CoV-2 vaccination has attracted considerable attention [13], [40]. However, limited research has been conducted on IgG subclass responses to inactivated SARS-CoV-2 vaccination. To the best of our knowledge, only one study has reported that the predominant IgG subclasses are IgG1 and IgG3, with IgG2 and IgG4 being nearly undetectable [15]. Interestingly, our results suggest that the predominant IgG subclasses were IgG1 and IgG2, with a lower IgG4 antibody response, whereas IgG3 was found to be almost undetectable. The observed differences in IgG3 levels between studies could potentially be attributed to different quantification criteria or the time elapsed after immunization. Compared with other IgG subclasses, IgG3 has a relatively short half-life, which may contribute to its transient presence after immunization [41], [42]. Notably, our results revealed an increased IgG1 to IgG4 conversion after vaccination with the inactivated vaccines. This discrepancy from another study indicated that IgG subclass responses after vaccination need to be carefully studied and monitored.
A class switch from IgG1 to IgG4 is associated with reduced capacity for Fc-mediated ADCP and ADCD, which may limit the control of viral infection [14]. IgG4 antibodies induced by repeated mRNA vaccines may generate immune tolerance, which may potentially result in unintended consequences in susceptible individuals, such as comorbidities and immunocompromised individuals [43], [44]. Studies have shown that lethal COVID-19 cases are associated with high levels of IgG4 antibodies [45]. However, accurately deciphering the effect of increased IgG4 levels on immunity remains challenging. How this IgG1/IgG4 switch contributes to the antibody-neutralizing capacity in response to breakthrough infection and its implications for disease outcomes remain largely unexplored.
Nonetheless, our finding of a class switch from IgG1 to IgG4 in inactivated vaccines alerts us to monitor subclass antibody responses closely, which may also contribute to the choice of future booster immunizations against SARS-CoV-2; for example, opting for an adenoviral vector-based vaccine or protein vaccine, not only because of the benefits of heterologous immunization strategies, but may also avoid the induction of IgG4 antibody responses if it is harmful [13], [46]. However, further clinical investigations of IgG subclasses should be conducted in order to draw definitive conclusions.
The present study had several limitations. First, it focused solely on IgG antibodies. Mucosal IgA, considered the critical first line of immune defense, has drawn considerable attention. Studies have shown that higher levels of mucosal IgA are associated with better protection against breakthrough infections than serum IgG [47], [48], [49]. It would be valuable to include IgA or IgM analyses to obtain a comprehensive understanding of the humoral immune responses elicited by inactivated vaccines. Second, two different vaccines (BBIBP-CorV and CoronaVac) were used in this study. While these were combined in the data analysis owing to the absence of observed differences (Supplementary Fig 1), which aligns with another previous study [27], it might be worthwhile to perform separate analyses for a more nuanced understanding of the results. Third, a repeated cross-sectional or longitudinal study should be conducted in the future in order to explore dynamic changes in antibody responses. Finally, follow-up infection information should be collected to assess the effectiveness of two or three doses of vaccines against breakthrough infections.
Currently, several types of vaccines are available for SARS-CoV-2, including mRNA-based, protein subunit-, viral-based, and inactivated vaccines [9]. In China, the last three vaccine platforms are currently available. Previous studies have shown that individuals who received Ad5-vectored or protein-subunit vaccines had higher NAbs against Omicron subvariants than those who received inactivated vaccines [39], [50], [51]. Although the inactivated vaccine was less effective against Omicron, it remained robust in reducing the risk of asymptomatic or mild Delta or Omicron variants of COVID-19 progressing to pneumonia and COVID-related I.C.U. admission [6], [52], [53], [54]. A recently published study found that inactivated vaccines significantly reduced the risk of death from COVID-19 in China compared to other vaccines in a retrospective cohort study involving 1,352 patients between November 2022 and February 2023 [11]. Because different vaccine platforms are used, it is essential to explore the long-term effects of these vaccines. Continued research will contribute to our understanding of the protection provided by different vaccine platforms and inform future vaccination strategies.
In summary, our findings have highlighted the significance of booster vaccine doses in enhancing immune responses, particularly in older individuals. The notable increase in the conversion of IgG1 to IgG4 prompted us to continue monitoring the immune responses after vaccination.
Ethics statement
This study was approved by the Ethics Committee of the Affiliated Nantong Hospital 3 of Nantong University (approval number EL2020006). Written informed consent was obtained from each participant prior to their participation. All procedures were performed under the principles of the Declaration of Helsinki.
Acknowledgements
This study was supported by the Key Project of the Natural Science Foundation of Tianjin, China (No. 20JCZDJC00090). The funders played no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Author contributions
Ziyu Liu: Data curation, Formal analysis, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. Liyan Cai: Data curation, Investigation, Validation, Writing – review & editing. Man Xing: Formal analysis, Investigation, Methodology, Resources, Writing – review & editing. Nan Qiao: Investigation, Methodology, Resources, Validation, Writing – review & editing. Jiaojiao Liu: Investigation, Methodology, Resources, Validation, Writing – review & editing. Xuejun Li: Formal analysis, Software, Writing – review & editing. Chiyu Zhang: Data curation, Investigation, Validation, Writing – review & editing. Naijun Tang: Methodology, Writing – review & editing. Zhelong Xu: Formal analysis, Investigation, Writing – review & editing. Yingying Guo: Conceptualization, Formal analysis, Supervision, Writing – original draft. Renfei Lu: Conceptualization, Project administration, Supervision, Writing – review & editing. Dongming Zhou: Conceptualization, Formal analysis, Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Footnotes
Restrictions apply to the availability of these data, so they are not publicly available. However, data are available from the corresponding author upon reasonable request and with the permission of the institution. Supplementary data to this article can be found online at https://doi.org/10.1016/j.bsheal.2024.04.001.
Contributor Information
Yingying Guo, Email: yingyingguo0228@tmu.edu.cn.
Renfei Lu, Email: rainman78@163.com.
Dongming Zhou, Email: zhoudongming@tmu.edu.cn.
Supplementary data
The following are the Supplementary data to this article:
References
- 1.Fink G., Tediosi F., Felder S. Burden of COVID-19 restrictions: national, regional and global estimates. EClinicalMedicine. 2022;45:101305,. doi: 10.1016/j.eclinm.2022.101305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richards F., Kodjamanova P., Chen X., Li N., Atanasov P., Bennetts L., Patterson B.J., Yektashenas B., Mesa-Frias M., Tronczynski K., et al. Economic burden of COVID-19: A systematic review. Clinicoecon. Outcomes Res. 2022;14:293–307. doi: 10.2147/CEOR.S338225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Perez Marc G., Moreira E.D., Zerbini C., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H., Hua Q., Nani Xu N., Zhang X., Chen B., Ma X., Hu J., Chen Z., Yu P., Lei H., et al. Vol. 12. eLife; 2023. p. e84056. (Evaluation of antibody kinetics and durability in healthy individuals vaccinated with inactivated COVID-19 vaccine (CoronaVac): A cross-sectional and cohort study in Zhejiang, China). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilder-Smith A., Mulholland K. Effectiveness of an inactivated SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;385(10):946–948. doi: 10.1056/NEJMe2111165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO, COVID-19 vaccines | WHO COVID-19 dashboard 2024. https://data.who.int/dashboards/covid19/vaccines, 2024 (accessed 6 Febrary 2024).
- 8.Carabelli A.M., Peacock T.P., Thorne L.G., Harvey W.T., Hughes J., Consortium C.-G.U., Peacock S.J., Barclay W.S., de Silva T.I., Towers G.J., et al. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol. 2023;21(3):162–177. doi: 10.1038/s41579-022-00841-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y., Ye Q. Safety and efficacy of the common vaccines against COVID-19. Vaccines (basel) 2022;10(4):513. doi: 10.3390/vaccines10040513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The State Council of the People's Republic of China, Authoritative releases. https://www.gov.cn/xinwen/gwylflkjz218/index.htm, 2022 (accessed 6 February 2024).
- 11.Yao W., Chen Y., Huang Q., Luo W., Chen Y., Xie C. SARS-CoV-2 vaccines in China could reduce COVID-19-related respiratory syndromes and deaths: A retrospective cohort study. Vaccine X. 2024;16 doi: 10.1016/j.jvacx.2024.100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vidarsson G., Dekkers G., Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front. Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irrgang P., Gerling J., Kocher K., Lapuente D., Steininger P., Habenicht K., Wytopil M., Beileke S., Schafer S., Zhong J., et al. Class switch toward noninflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Sci. Immunol. 2023;8(79):eade2798. doi: 10.1126/sciimmunol.ade2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang A., Stacey H.D., D'Agostino M.R., Tugg Y., Marzok A., Miller M.S. Beyond neutralization: Fc-dependent antibody effector functions in SARS-CoV-2 infection. Nat. Rev. Immunol. 2023;23(6):381–396. doi: 10.1038/s41577-022-00813-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W., Zhang L., Li J., Bai S., Wang Y., Zhang B., Zheng Q., Chen M., Zhao W., Wu J. The kinetics of IgG subclasses and contributions to neutralizing activity against SARS-CoV-2 wild-type strain and variants in healthy adults immunized with inactivated vaccine. Immunology. 2022;167(2):221–232. doi: 10.1111/imm.13531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H., Zhang Y., Huang B., Deng W., Quan Y., Wang W., Xu W., Zhao Y., Li N., Zhang J., et al. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020;182:713–721.e9. doi: 10.1016/j.cell.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng G., Wu Q., Pan H., Li M., Yang J., Wang L., Wu Z., Jiang D., Deng X., Chu K., et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: Interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect. Dis. 2022;22(4):483–495. doi: 10.1016/S1473-3099(21)00681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M., Liu J., Lu R., Zhang Y., Du M., Xing M., Wu Z., Kong X., Zhu Y., Zhou X., et al. Longitudinal immune profiling reveals dominant epitopes mediating long-term humoral immunity in COVID-19-convalescent individuals. J. Allergy Clin. Immunol. 2022;149(4):1225–1241. doi: 10.1016/j.jaci.2022.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frey A., Di Canzio J., Zurakowski D. A statistically defined endpoint titer determination method for immunoassays. J. Immunol. Methods. 1998;221(1–2):35–41. doi: 10.1016/s0022-1759(98)00170-7. [DOI] [PubMed] [Google Scholar]
- 20.Liu J., Xu K., Xing M., Zhuo Y., Guo J., Du M., Wang Q., An Y., Li J., Gao P., et al. Heterologous prime-boost immunizations with chimpanzee adenoviral vectors elicit potent and protective immunity against SARS-CoV-2 infection. Cell Discov. 2021;7(1):123. doi: 10.1038/s41421-021-00360-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tegally H., Moir M., Everatt J., Giovanetti M., Scheepers C., Wilkinson E., Subramoney K., Makatini Z., Moyo S., Amoako D.G., et al. Emergence of SARS-CoV-2 Omicron lineages BA.4 and BA.5 in South Africa. Nat. Med. 2022;28(9):1785–1790. doi: 10.1038/s41591-022-01911-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., Doolman R., Asraf K., Mendelson E., Ziv A., et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N. Engl. J. Med. 2021;385(24):e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng Q., Zhou R., Wang Y., Zhao M., Liu N., Li S., Huang H., Yang D., Au K.K., Wang H., et al. Waning immune responses against SARS-CoV-2 variants of concern among vaccinees in Hong Kong. EBioMedicine. 2022;77 doi: 10.1016/j.ebiom.2022.103904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caniels T.G., Bontjer I., van der Straten K., Poniman M., Burger J.A., Appelman B., Lavell A.H.A., Oomen M., Godeke G.J., Valle C., et al. Emerging SARS-CoV-2 variants of concern evade humoral immune responses from infection and vaccination. Sci. Adv. 2021;7(36):eabj5365. doi: 10.1126/sciadv.abj5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilboa M., Regev-Yochay G., Mandelboim M., Indenbaum V., Asraf K., Fluss R., Amit S., Mendelson E., Doolman R., Afek A., et al. Durability of immune response after COVID-19 booster vaccination and association with COVID-19 Omicron infection. JAMA Netw. Open. 2022;5(9) doi: 10.1001/jamanetworkopen.2022.31778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan Y., Xu J., Ma B., Chen G., Wang Z., Wang S., Jing N., Zhang J., Wang B., Yan W., et al. Characteristics of humoral and cellular responses to coronavirus disease 2019 (COVID-19) inactivated vaccine in central China: a prospective, multicenter, longitudinal study. Front. Immunol. 2023;14:1107866. doi: 10.3389/fimmu.2023.1107866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuo F., Abolhassani H., Du L., Piralla A., Bertoglio F., de Campos-Mata L., Wan H., Schubert M., Cassaniti I., Wang Y., et al. Heterologous immunization with inactivated vaccine followed by mRNA-booster elicits strong immunity against SARS-CoV-2 Omicron variant. Nat. Commun. 2022;13(1):2670. doi: 10.1038/s41467-022-30340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yue L., Zhou J., Zhou Y., Yang X., Xie T., Yang M., Zhao H., Zhao Y., Yang T., Li H., et al. Antibody response elicited by a third boost dose of inactivated SARS-CoV-2 vaccine can neutralize SARS-CoV-2 variants of concern. Emerg Microbes Infect. 2021;10(1):2125–2127. doi: 10.1080/22221751.2021.1996210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji R., Zhang J., Liang D., Quan H., Wu Y., Peng A., Li W., Lu S., Zhang X., Ke C., et al. Potent antibody response elicited by a third booster dose of inactivated COVID-19 vaccine in healthy subjects. Viral Immunol. 2023;36(9):593–599. doi: 10.1089/vim.2023.0072. [DOI] [PubMed] [Google Scholar]
- 30.Willett B.J., Grove J., MacLean O.A., Wilkie C., De Lorenzo G., Furnon W., Cantoni D., Scott S., Logan N., Ashraf S., et al. SARS-CoV-2 Omicron is an immune escape variant with an altered cell entry pathway. Nat. Microbiol. 2022;7(8):1161–1179. doi: 10.1038/s41564-022-01143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X., Wu S., Wu B., Yang Q., Chen A., Li Y., Zhang Y., Pan T., Zhang H., He X. SARS-CoV-2 Omicron strain exhibits potent capabilities for immune evasion and viral entrance. Signal Transduct. Target. Ther. 2021;6(1):430. doi: 10.1038/s41392-021-00852-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gebhard C., Regitz-Zagrosek V., Neuhauser H.K., Morgan R., Klein S.L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol. Sex Differ. 2020;11(1):29. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bai F., Tomasoni D., Falcinella C., Barbanotti D., Castoldi R., Mule G., Augello M., Mondatore D., Allegrini M., Cona A., et al. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin. Microbiol. Infect. 2022;28(4):611.e9–611.e16. doi: 10.1016/j.cmi.2021.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng Y., Wu P., Lu W., Liu K., Ma K., Huang L., Cai J., Zhang H., Qin Y., Sun H., et al. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: A retrospective study of 168 severe patients. PLoS Pathog. 2020;16(4) doi: 10.1371/journal.ppat.1008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muecksch F., Wise H., Templeton K., Batchelor B., Squires M., McCance K., Jarvis L., Malloy K., Furrie E., Richardson C., et al. Longitudinal variation in SARS-CoV-2 antibody levels and emergence of viral variants: A serological analysis. Lancet Microbe. 2022;3(7):e493–e502. doi: 10.1016/S2666-5247(22)00090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawasuji H., Morinaga Y., Tani H., Saga Y., Kaneda M., Murai Y., Ueno A., Miyajima Y., Fukui Y., Nagaoka K., et al. Age-dependent reduction in neutralization against Alpha and Beta variants of BNT162b2 SARS-CoV-2 vaccine-induced immunity. Microbiol. Spectr. 2021;9(3) doi: 10.1128/Spectrum.00561-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collier D.A., Ferreira I., Kotagiri P., Datir R.P., Lim E.Y., Touizer E., Meng B., Abdullahi A., Elmer A. CITIID-NIHR BioResource COVID-19 Collaboration, et al., Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596(7872):417–422. doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butt A.A., Yan P., Shaikh O.S., Mayr F.B. Outcomes among patients with breakthrough SARS-CoV-2 infection after vaccination in a high-risk national population. EClinicalMedicine. 2021;40 doi: 10.1016/j.eclinm.2021.101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin P.F., Guo X.L., Gou J.B., Hou L.H., Song Z.Z., Zhu T., Pan H.X., Zhu J.H., Shi F.J., Du P., et al. Immunogenicity and safety of heterologous immunisation with Ad5-nCOV in healthy adults aged 60 years and older primed with an inactivated SARS-CoV-2 vaccine (CoronaVac): A phase 4, randomised, observer-blind, non-inferiority trial. Lancet Reg. Health West Pac. 2023;38 doi: 10.1016/j.lanwpc.2023.100829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pillai S. Is it bad, is it good, or is IgG4 just misunderstood? Sci. Immunol. 2023;8(81):eadg7327. doi: 10.1126/sciimmunol.adg7327. [DOI] [PubMed] [Google Scholar]
- 41.Kapur R., Einarsdottir H.K., Vidarsson G. IgG-effector functions: “the good, the bad and the ugly”. Immunol. Lett. 2014;160(2):139–144. doi: 10.1016/j.imlet.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 42.Stapleton N.M., Andersen J.T., Stemerding A.M., Bjarnarson S.P., Verheul R.C., Gerritsen J., Zhao Y., Kleijer M., Sandlie I., de Haas M., et al. Competition for FcRn-mediated transport gives rise to short half-life of human IgG3 and offers therapeutic potential. Nat. Commun. 2011;2:599. doi: 10.1038/ncomms1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao F.X., Wu R.X., Shen M.Y., Huang J.J., Li T.T., Hu C., Luo F.Y., Song S.Y., Mu S., Hao Y.N., et al. Extended SARS-CoV-2 RBD booster vaccination induces humoral and cellular immune tolerance in mice. iScience. 2022;25(12) doi: 10.1016/j.isci.2022.105479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uversky V.N., Redwan E.M., Makis W., Rubio-Casillas A. IgG4 antibodies induced by repeated vaccination may generate immune tolerance to the SARS-CoV-2 spike protein. Vaccines (basel) 2023;11(5):991. doi: 10.3390/vaccines11050991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moura A.D., da Costa H.H.M., Correa V.A., de S.L.A.K., Lindoso J.A.L., De Gaspari E., Hong M.A., Cunha-Junior J.P., Prudencio C.R. Assessment of avidity related to IgG subclasses in SARS-CoV-2 Brazilian infected patients. Sci. Rep. 2021;11(1):17642. doi: 10.1038/s41598-021-95045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalkeri R., Zhu M., Cloney-Clark S., Plested J.S., Parekh A., Gorinson D., Cai R., Mahato S., Ramanathan P., Aurelia L.C., et al. Altered IgG4 antibody response to repeated mRNA versus protein COVID vaccines [Preprint] medRxiv. 2024 doi: 10.1101/2024.01.17.24301374. [DOI] [PubMed] [Google Scholar]
- 47.Havervall S., Marking U., Svensson J., Greilert-Norin N., Bacchus P., Nilsson P., Hober S., Gordon M., Blom K., Klingstrom J., et al. Anti-spike mucosal IgA protection against SARS-CoV-2 Omicron infection. N. Engl. J. Med. 2022;387(14):1333–1336. doi: 10.1056/NEJMc2209651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuo F., Marcotte H., Hammarstrom L., Pan-Hammarstrom Q. Mucosal IgA against SARS-CoV-2 Omicron infection. N. Engl. J. Med. 2022;387(21):e55. doi: 10.1056/NEJMc2213153. [DOI] [PubMed] [Google Scholar]
- 49.Zuo F., Cao Y., Sun R., Yisimayi A., Du L., Bertoglio F., Schubert M., Guerra C., Cavalli A., Hust M., et al. Neutralisation activity of mucosal IgA against XBB sublineages and BA.2.86. Lancet Infect. Dis. 2024;24(1):e7–e9. doi: 10.1016/S1473-3099(23)00732-6. [DOI] [PubMed] [Google Scholar]
- 50.Li Y., Qiao S., Dong L., Zhang R., Li R., Qin S., Yu D., Liu X., Li Y., Ma Y., et al. Antibody response assessment of immediate breakthrough infections after zero-COVID policy adjustment in China. Lancet Reg. Health West Pac. 2023;40 doi: 10.1016/j.lanwpc.2023.100945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li J.X., Hou L.H., Gou J.B., Yin Z.D., Wu S.P., Wang F.Z., Zhang Z., Peng Z.H., Zhu T., Shen H.B., et al. Safety, immunogenicity and protection of heterologous boost with an aerosolised Ad5-nCoV after two-dose inactivated COVID-19 vaccines in adults: A multicentre, open-label phase 3 trial. Lancet Infect. Dis. 2023;23(10):1143–1152. doi: 10.1016/S1473-3099(23)00350-X. [DOI] [PubMed] [Google Scholar]
- 52.Wu D., Zhang Y., Tang L., Wang F., Ye Y., Ma C., Zheng H., Yu W., Cao L., Song Y., et al. Effectiveness of inactivated COVID-19 vaccines against symptomatic, pneumonia, and severe disease caused by the Delta variant: real world study and evidence - China, 2021. China CDC Wkly. 2022;4(4):57–65. doi: 10.46234/ccdcw2022.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu H., Li H., You H., Zhang P., Li N., Jiang N., Cao Y., Qin L., Qin G., Qu H., et al. Effectiveness of inactivated COVID-19 vaccines against mild disease, pneumonia, and severe disease among persons infected with SARS-CoV-2 Omicron variant: real-world study in Jilin Province, China. Emerg. Microbes Infect. 2023;12(1):2149935. doi: 10.1080/22221751.2022.2149935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu Z., Tao B., Li Z., Song Y., Yi C., Li J., Zhu M., Yi Y., Huang P., Wang J. Effectiveness of inactivated COVID-19 vaccines against severe illness in B.1.617.2 (Delta) variant-infected patients in Jiangsu, China. Int. J. Infect. Dis. 2022;116:204–209. doi: 10.1016/j.ijid.2022.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.