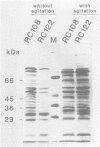
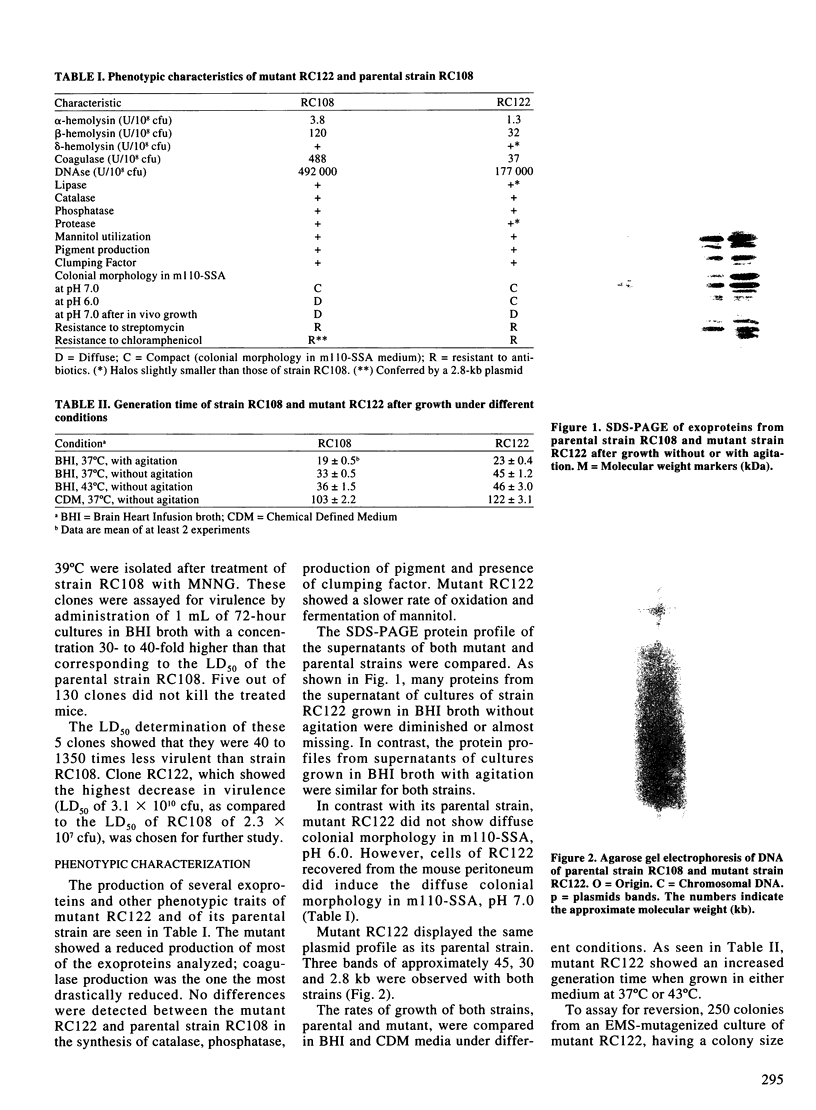
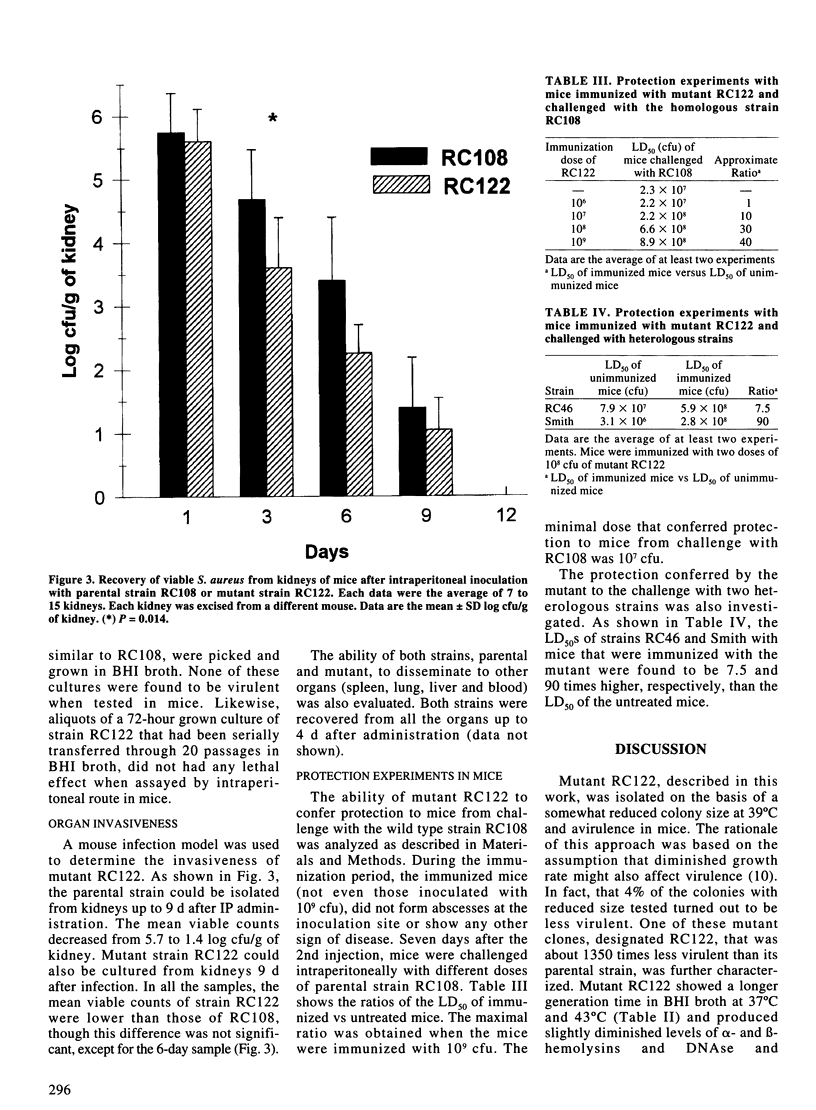
Abstract
An avirulent mutant, designated RC122, was derived from Staphylococcus aureus bovine mastitis strain RC108 after N-methyl-N'-nitro-N-nitrosoguanidine mutagenesis. Mutant RC122, which was isolated on the basis of reduced colony size, showed diminished virulence in mice (LD50 of RC122: 3.1 x 10(10) cfu vs LD50 of RC108: 2.3 x 10(7) cfu). Mutant RC122 grew more slowly than its parental strain and showed decreased production of several exoproteins, such as alpha- and beta-hemolysin, DNAse and coagulase. The production of its capsule was induced only under in vivo growth conditions. Clearance studies performed in the mouse kidney revealed that the kinetics of disappearance of the mutant was similar to that of its parental strain. Protection experiments carried out by intraperitoneal administration in mice showed that mutant RC122 conferred a good degree of protection from challenge with homologous and heterologous strains.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelnour A., Arvidson S., Bremell T., Rydén C., Tarkowski A. The accessory gene regulator (agr) controls Staphylococcus aureus virulence in a murine arthritis model. Infect Immun. 1993 Sep;61(9):3879–3885. doi: 10.1128/iai.61.9.3879-3885.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayles K. W., Iandolo J. J. Genetic and molecular analyses of the gene encoding staphylococcal enterotoxin D. J Bacteriol. 1989 Sep;171(9):4799–4806. doi: 10.1128/jb.171.9.4799-4806.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzolari A., Giraudo J. A., Rampone H., Odierno L., Giraudo A. T., Frigerio C., Bettera S., Raspanti C., Hernández J., Wehbe M. Field trials of a vaccine against bovine mastitis. 2. Evaluation in two commercial dairy herds. J Dairy Sci. 1997 May;80(5):854–858. doi: 10.3168/jds.S0022-0302(97)76007-7. [DOI] [PubMed] [Google Scholar]
- Cheung A. L., Yeaman M. R., Sullam P. M., Witt M. D., Bayer A. S. Role of the sar locus of Staphylococcus aureus in induction of endocarditis in rabbits. Infect Immun. 1994 May;62(5):1719–1725. doi: 10.1128/iai.62.5.1719-1725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkle L. M., Blair L. L., Fortune K. P. Transformation of a plasmid encoding an adhesin of Staphylococcus aureus into a nonadherent staphylococcal strain. J Infect Dis. 1986 Apr;153(4):670–675. doi: 10.1093/infdis/153.4.670. [DOI] [PubMed] [Google Scholar]
- Foster T. J. Potential for vaccination against infections caused by Staphylococcus aureus. Vaccine. 1991 Apr;9(4):221–227. doi: 10.1016/0264-410x(91)90103-d. [DOI] [PubMed] [Google Scholar]
- Giraudo A. T., Rampone H., Calzolari A., Nagel R. Phenotypic characterization and virulence of a sae- agr- mutant of Staphylococcus aureus. Can J Microbiol. 1996 Feb;42(2):120–123. doi: 10.1139/m96-019. [DOI] [PubMed] [Google Scholar]
- Giraudo A. T., Raspanti C. G., Calzolari A., Nagel R. Characterization of a Tn551-mutant of Staphylococcus aureus defective in the production of several exoproteins. Can J Microbiol. 1994 Aug;40(8):677–681. doi: 10.1139/m94-107. [DOI] [PubMed] [Google Scholar]
- Giraudo J. A., Calzolari A., Rampone H., Rampone A., Giraudo A. T., Bogni C., Larriestra A., Nagel R. Field trials of a vaccine against bovine mastitis. 1. Evaluation in heifers. J Dairy Sci. 1997 May;80(5):845–853. doi: 10.3168/jds.S0022-0302(97)76006-5. [DOI] [PubMed] [Google Scholar]
- Hasegawa N., San Clemente C. L. Virulence and immunity of Staphylococcus aureus BB and certain deficient mutants. Infect Immun. 1978 Nov;22(2):473–479. doi: 10.1128/iai.22.2.473-479.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Betley M. J., Hopkins C. A., Perez N. E., Pier G. B. Virulence studies, in mice, of transposon-induced mutants of Staphylococcus aureus differing in capsule size. J Infect Dis. 1987 Nov;156(5):741–750. doi: 10.1093/infdis/156.5.741. [DOI] [PubMed] [Google Scholar]
- Lopes J. D., dos Reis M., Brentani R. R. Presence of laminin receptors in Staphylococcus aureus. Science. 1985 Jul 19;229(4710):275–277. doi: 10.1126/science.3160113. [DOI] [PubMed] [Google Scholar]
- Mamo W., Lindahl M., Jonsson P. Enhanced virulence of Staphylococcus aureus from bovine mastitis induced by growth in milk whey. Vet Microbiol. 1991 May;27(3-4):371–384. doi: 10.1016/0378-1135(91)90161-8. [DOI] [PubMed] [Google Scholar]
- Odierno L., Risatti G., Calzolari A., Giraudo J. A., González Quintana H., Nagel R. Pathogenicity in mice of Staphylococcus aureus mutants deficient in exoprotein synthesis. Vet Microbiol. 1994 Aug 1;41(3):249–258. doi: 10.1016/0378-1135(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Odierno L., Zandarín A., Ferrari M., Rampone A., Giraudo J., Calzolari A. Comparación de métodos de detección de cápsula de Staphylococcus aureus. Rev Latinoam Microbiol. 1995 Jul-Sep;37(3):245–255. [PubMed] [Google Scholar]
- Opdebeeck J. P., Frost A. J., O'Boyle D., Norcross N. L. The expression of capsule in serum-soft agar by Staphylococcus aureus isolated from bovine mastitis. Vet Microbiol. 1987 Mar;13(3):225–234. doi: 10.1016/0378-1135(87)90085-x. [DOI] [PubMed] [Google Scholar]
- Patel A. H., Nowlan P., Weavers E. D., Foster T. Virulence of protein A-deficient and alpha-toxin-deficient mutants of Staphylococcus aureus isolated by allele replacement. Infect Immun. 1987 Dec;55(12):3103–3110. doi: 10.1128/iai.55.12.3103-3110.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampone H., Martínez G. L., Giraudo A. T., Calzolari A., Nagel R. In vivo expression of exoprotein synthesis with a Sae mutant of Staphylococcus aureus. Can J Vet Res. 1996 Jul;60(3):237–240. [PMC free article] [PubMed] [Google Scholar]
- Seki K., Ogasawara M., Sakurada J., Murai M., Masuda S. Altered virulence of a pleiotropic Staphylococcus aureus mutant with a low producibility of coagulase and other factors in mice. Microbiol Immunol. 1989;33(12):981–990. doi: 10.1111/j.1348-0421.1989.tb03156.x. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Nagano Y. Rapid procedure for isolation of plasmid DNA and application to epidemiological analysis. J Clin Microbiol. 1984 Oct;20(4):608–613. doi: 10.1128/jcm.20.4.608-613.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R., Rogolsky M., Wiley B. B., Glasgow L. A. Isolation of extrachromosomal deoxyribonucleic acid for exfoliative toxin production from phage group II Staphylococcus aureus. J Bacteriol. 1975 Apr;122(1):99–105. doi: 10.1128/jb.122.1.99-105.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. L., Kennedy J. W. Immunisation against experimental staphylococcal mastitis in sheep - effect of challenge with a heterologous strain of Staphylococcus aureus. Aust Vet J. 1981 Jul;57(7):309–313. doi: 10.1111/j.1751-0813.1981.tb05834.x. [DOI] [PubMed] [Google Scholar]
- Watson D. L. Staphylococcal mastitis vaccine. Vaccine. 1992;10(5):359–359. doi: 10.1016/0264-410x(92)90387-y. [DOI] [PubMed] [Google Scholar]
- Yoshida K. Demonstration of Serologically Different Capsular Types Among Strains of Staphylococcus aureus by the Serum-Soft Agar Technique. Infect Immun. 1971 Apr;3(4):535–539. doi: 10.1128/iai.3.4.535-539.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., Kessler R. E. Growth characteristics of group A streptococci in a new chemically defined medium. Infect Immun. 1980 Feb;27(2):444–448. doi: 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]