Highlights
-
•
Scientific question: Globally, significant advances in chicken gut microbiome studies have been made; however, a comprehensive study on chicken gut bacterial, archaeal, and viral genomes remains unavailable.
-
•
Evidence before this study: Previous studies explored the diversity and abundance of antimicrobial resistance genes in the chicken gut microbiome using metagenomics and state-of-the-art bioinformatics tools. Previous studies constructed an integrated gene catalog and metagenome-assembled genomes (MAGs) using publicly available metagenome data.
-
•
New findings: The study provides the chicken gut multi‑kingdom microbiome catalog (CMKMC), including 18,201 bacterial and 225 archaeal MAGs and 33,411 viral genomes. The study found that 812 and 240 MAGs in the dataset were putative novel species and genera, respectively. Most of the classified species-level viral operational taxonomic units belong to Caudovirales, and diverse auxiliary metabolic and antibiotic resistance genes are carried by viruses.
-
•
Significance of the study: This study provides the most comprehensive multi-kingdom microbiome catalog of the chicken gastrointestinal tract to date, including the largest integrated bacterial and archaeal MAGs and viral genomes from the chicken gut. These results and genomes will be useful as a new reference genome resource and provide new insights into the chicken gut microbiome.
Keywords: Chicken, Microbiome, Metagenome‑assembled genomes, Archaeome, Virome, Antibiotic resistance gene
Abstract
Chicken is an important food animal worldwide and plays an important role in human life by providing meat and eggs. Despite recent significant advances in gut microbiome studies, a comprehensive study of chicken gut bacterial, archaeal, and viral genomes remains unavailable. In this study, we constructed a chicken multi-kingdom microbiome catalog (CMKMC), including 18,201 bacterial, 225 archaeal, and 33,411 viral genomes, and annotated over 6,076,006 protein-coding genes by integrating 135 chicken gut metagenomes and publicly available metagenome-assembled genomes (MAGs) from ten countries. We found that 812 and 240 MAGs in our dataset were putative novel species and genera, respectively, far beyond what was previously reported. The newly unclassified MAGs were predominant in Phyla Firmicutes_A (n = 263), followed by Firmicutes (n = 126), Bacteroidota (n = 121), and Proteobacteria (n = 87). Most of the classified species-level viral operational taxonomic units belong to Caudovirales. Approximately, 63.24 % of chicken gut viromes are predicted to infect two or more hosts, including complete circular viruses. Moreover, we found that diverse auxiliary metabolic genes and antibiotic resistance genes were carried by viruses. Together, our CMKMC provides the largest integrated MAGs and viral genomes from the chicken gut to date, functional insights into the chicken gastrointestinal tract microbiota, and paves the way for microbial interventions for better chicken health and productivity.
1. Introduction
Domestic chicken (Gallus gallus) is an economically important food animal worldwide and plays an integral role in human life by providing meat and eggs. According to the Food and Agriculture Organization of the United Nations, there are more than 1,600 different chicken breeds globally, and Asia ranks among the highest, accounting for approximately 46 % of total breeds (https://www.fao.org/poultry-production-products/en/). There are approximately 25.8 billion living chickens worldwide, and China accounts for more than 5.1 billion of them (https://www.statista.com/statistics/263961/top-countries-worldwide-by-chicken-stock-2007/). Unsurprisingly, China exceeded the global average for the percentage of chickens raised in hotspots of resistance, and India represented the largest hotspots of resistance [1].
The gastrointestinal tract of chickens harbors trillions of bacteria [2] that play vital roles in chicken health, including host metabolism, immunity, nutrition, and productivity [3], [4]. Previous studies have focused on metabolism [5], growth-promoting therapeutics [6], [7], immunity [8], [9], gut microbiota changes [10], [11], [12], [13], host genetics, feed efficiency [14], [15], and diversity and abundance of antibiotic resistance genes (ARGs) [16], [17], [18] in chicken gut microbiota. The chicken gut harbors a diverse multi-kingdom ecosystem comprising bacteria, archaea, and viruses. However, regardless of their abundance, they remain poorly understood.
Metagenomic sequencing of gastrointestinal tract samples combined with the reconstruction of metagenome-assembled genomes (MAGs) enabled us to quickly obtain these unexplored microbiomes and reveal functional interactions between the microbial ecology and gut microbial niches of interest [19]. This strategy has been recently used to reconstruct MAGs from cattle rumen [20], [21], [22], human [23], [24], [25], [26], [27], chicken [28], [29], [30], [31], [32], pig [33], [34], goat [35], [36], ruminants [37], buffalo [38], horse [39], wild animals [40], caprinae [41], pandas [42], glacier [43], ocean [44], soil [45], diverse habitats of Earth [46], [47], and environmental and non-human gastrointestinal samples [48]. For chickens, an integrated gene catalog comprising approximately 16.6 million genes and microbial genomes comprising 12,339 MAGs has also been constructed using 24 chicken cecal content samples from the United Kingdom [28], 495 chicken intestinal digest samples from 7 farms in China [11], 102 chicken samples from 18 live poultry markets (LPMs) in China [49], and 178 broiler farms in 9 European countries [18]. These resources provide an overview of the chicken gut microbiome landscape; however, with increased exploration of the chicken gut microbiome and a better understanding of its structure and function, the size and quality of reference MAGs urgently need expansion and integration.
Recent bioinformatics tools specific for viral identification (e.g., VirSorter2 [50], CheckV [51], and VIBRANT [52]) and classification analysis (e.g., vConTACT2 [53] and GeNomad [54]) significantly facilitate viral identification and taxonomy from metagenomic sequences. Using metagenomic sequencing methods and the above bioinformatics tools, recent virus-focused studies have identified diverse viruses and generated numerous viral genome catalogs and databases for several ecosystems, including the goat gastrointestinal tract [36], ocean viruses [55], human gut [56], [57], [58], [59], [60], rumen [61], soil [62], and activated sludge [63]. Yan et al. (2023) [61] identified 397,180 species-level viral operational taxonomic units (vOTUs) from 975 rumen metagenomes in 13 ruminant species across 8 countries on 5 continents and created a global rumen virome database. They identified the rumen viromes for diversity, function, and virus–host linkages, identified numerous auxiliary metabolic genes (AMGs), and elucidated the ecological influence of phages. However, no similar study on chicken gut or chicken gut-specific virome database exists.
A comprehensive genomic catalog of uncultivated microbiota, including bacteria, archaea, and viruses, is useful for studying gut microbiome although the corresponding microbial entities of MAGs and phages require further confirmation using culture-based methods. However, despite a few studies on the chicken gut microbiome (MAGs and/or gene catalog), systematic exploration of the microbial ecology, including bacteria, archaea, and viruses, with several chickens remains unavailable, severely limiting the deep mining and use of metagenomic data. Motivated by comprehensive virome and MAG studies, we assessed chicken gut multi-kingdom microbial genomes (bacterial, archaeal, and viral) by analyzing 135 metagenomes from 1,215 fecal samples from diverse breeds, both indoor feeding and free-range grazing, across 8 Chinese provinces. We reconstructed 5,974 bacterial and 118 archaeal MAGs and built a chicken MAG catalog using the newly assembled genomes and 12,339 MAGs generated in a previous study [31]. We found that 26.44 % (812/3,071) of the MAGs were candidate novel species under the threshold of 95 % average nucleotide identity (ANI) with public genomic datasets, indicating the novelty of our datasets. We developed a systematically cataloged chicken virome database containing approximately 33,411 species-level vOTUs and annotated 6,076,006 protein-coding genes in the MAGs and virus genomes. Furthermore, we explored the diversity of ARGs, plasmids, and virulence factors carried by MAGs, predicted hosts likely susceptible to the identified viruses, and inferred the potential ecological roles of chicken gut viruses from AMGs and ARGs carried by the chicken gut viral genomes. Altogether, we have filled the gap in chicken gut microbial ecology research by providing a chicken multi-kingdom microbiome catalog, including bacterial, archaeal, and viral genomes and encoding proteins. We believe that these data will be useful as reference genome resources and provide new insights into chicken gut microbiome.
2. Materials and methods
2.1. Sample collection, deoxyribonucleic acid (DNA) extraction, library construction, and metagenomic sequencing
A total of 1,215 chicken fecal samples were collected from 21 broiler farms and 22 LPMs across 8 Chinese provinces; all details regarding sample collection were described in our previous study [17]. Total DNA from chicken fecal samples was extracted using the DNeasy PowerSoil Pro Kit (47,016, Qiagen) following the manufacturer’s instructions. A total of 135 libraries (nine DNA from the same location were mixed) were then built using the KAPA Hyper Prep Kit and shotgun sequenced using the Illumina NovaSeq 6,000 platform.
2.2. Quality control and assembly
Raw sequence reads were filtered to remove residual adapter and low-quality sequences using Trimmomatic v0.39 (https://github.com/usadellab/Trimmomatic) [64] and reads mapped to the chicken genome (National Center of Biotechnology Information [NCBI] Genome ID: GRCg6a; GCF_000002315.6) using Bowtie2 v2.3.4 (https://sourceforge.net/projects/bowtie-bio/files/bowtie2/2.3.0/) [65]. The remaining high-quality paired clean reads were then used for further analyses. All clean reads were assembled using MEGAHIT v1.2.9 (https://github.com/voutcn/megahit) for each sample [66].
2.3. Recovery and quality assessment of microbial MAGs
For metagenomic binning analysis of 135 metagenome data, three different binning tools with default options of MetaBAT2 v.2.12.1, Maxbin2 v2.2.4, and Concoct v.0.4.0 in the metaWRAP v1.3.2 (https://github.com/bxlab/metaWRAP) pipeline [67] were used. MAGs were refined using the bin_refinement module of metaWRAP [67] to improve the bins generated using the three binning tools. The output bins are simply a set of DNA sequence files containing putative genomes. CheckM v1.2.2 (https://github.com/Ecogenomics/CheckM) was used to assess the completeness and contamination of each bin [68]. According to the quality evaluation criteria of CheckM v1.2.2 [68], metagenomic bins with contamination of > 10 % or completeness of < 50 % were not considered. Medium- and high-quality bins were selected as acceptable MAGs (6,087), according to previous reports [22], [23], [24], [33], [37], [48]. They included 1,587 high-quality bins (completeness of > 90 % and contamination of < 5 %) and 4,500 medium-quality bins (completeness of ≥ 50 % and contamination of ≤ 10 %). The remaining were low-quality bins. The mapped reads were reassembled using the reassemble_bin module of the metaWRAP pipeline [67].
2.4. Species-level clustering and dereplication of the MAGs
To dereplicate the MAG collection, dRep v3.4.2 (https://github.com/MrOlm/drep) was used for the dereplication workflow at the threshold of 99 % ANI (the strain level) and 95 % ANI (the species-level) [69]. The 6,087 genomes were dereplicated into 1,834 nonredundant MAGs. To reveal the novelty of the chicken multikingdom microbiome catalog (CMKMC) bacterial and archaeal MAGs, fastANI v1.1 was used to calculate the ANI between the MAGs generated in this study and the 12,339 MAGs in the previous study [31]. Different ANI thresholds (including 95 % and 99 %) were used in this study. Then, 18,426 MAGs were dereplicated into 3,071 nonredundant MAGs, including 3,500 bacterial and 21 archaeal MAGs.
2.5. Taxonomic assignments and phylogenetic analysis of the MAGs
The Genome Taxonomy Database Toolkit (GTDB-Tk v.1.4.1, https://gtdb.ecogenomic.org/) was used to assign taxonomy to the MAGs [70]. MAGs were defined as novel species if the ANI value was < 95 % ANI. MAGs were defined as novel genera if all MAGs clustered at 60 % amino acid identity (AAI) were not assigned a Genus by GTDB-Tk [70]. Average AAI values were calculated using CompareM v0.1.2 (https://github.com/dparks1134/CompareM). Phylogenetic trees were reconstructed using PhyloPhlAn v3.0.60 (https://github.com/biobakery/phylophlan) [71].
2.6. Identification and quality evaluation of viral genomes
Prokaryotic viral genomes were identified using a bioinformatics pipeline similar to that used by Camarillo-Guerrero et al. (2021) [56], Cao et al. (2023) [36], and Yan et al. (2023) [61]. First, viral contigs were identified following the viral sequence identification standard operating procedure (SOP) (https://doi.org/10.17504/protocols.io.bwm5pc86). Contigs of > 5 kb in length were used to identify viral sequences using VirSorter2 v2.2.4 (https://bitbucket.org/MAVERICLab/virsorter2) [50] with the options ‘–includegroups “dsDNAphage, ssDNA”–min-score 0.8′. Contigs that passed the verification procedure were input to CheckV v1.0.1 (https://bitbucket.org/berkeleylab/checkv/src/master/) [51] to trim off host sequences flanking prophages. We then ran the CheckV-trimmed sequences through VirSorter2 again to generate “affi-contigs.tab” files. A total of 59,911 putative viral genomes of > 5 kb in length were identified. The viral sequences were clustered into vOTUs according to 95 % ANI and 85 % alignment fraction of the shorter sequences [49]. The resultant 35,404 vOTUs were further verified using VIBRANT v1.2.1 (https://github.com/AnantharamanLab/VIBRANT, option: --virome) [52]. For conservation, only the vOTUs identified using VirSorter2 v2.2.4, CheckV v1.0.1, and VIBRANT v1.2.1 (33,411) were used to build the chicken virome database and retained for taxonomic annotation and host prediction. VirSorter2, CheckV, and VIBRANT were used for viral identification because they are the most recent bioinformatic tools with the best performance, as recently reported [61]. All predicted viral genomes from eukaryotic viruses and no genes displaying the best hit (Blast) to prokaryotic viruses were removed [72]. vOTU completeness was estimated using CheckV v0.8.1 [51] and VIBRANT v1.2.1 [52].
2.7. Taxonomic classification and host and lifestyle prediction of vOTUs
First, open reading frames (ORFs) of all identified candidate viral genomes were predicted using Prodigal v2.6.3 (https://github.com/hyattpd/Prodigal) [73]. We then used vConTACT2 (https://bitbucket.org/MAVERICLab/vcontact2/src/master/) [53] to assign vOTUs to genus level viral taxa based on the gene-sharing network. The vOTUs were clustered with the reference genome of a viral genus that was assigned to the genus according to the vConTACT2 pipeline. In addition, we used GeNomad for the taxonomic classification (default parameters) of each vOTU. The probable hosts of the chicken gut viruses were predicted using iPHoP v1.3.3 (https://bitbucket.org/srouxjgi/iphop/src/main/, --min_score 90) [54], which integrated host-based (Blast, CRISPR, VirHostMatcher, WIsH, and PHP) and phage-based methods (RaFAH) to reliably predict host taxonomy at the genera level for various viruses infecting bacteria and archaea. VIBRANT v1.2.1 [52] was used to predict the lifestyles of the CMKMC viruses. According to the VIBRANT prediction, the virus types were classified as either lysogenic or lytic.
2.8. Functional analyses of MAGs and viral genomes
The virulent amino acid sequences were identified against the virulence factor database (VFDB) [74] using ABRicate v0.9.7 (https://github.com/tseemann/abricate/). Plasmid types were identified using PlasmidFinder v2.0.1 (https://github.com/kcri-tz/plasmidfinder) [75]. The resistance mechanisms and antimicrobial classes of potential ARGs were further annotated by running RGI v6.0.3 (https://github.com/arpcard/rgi) [76] against the CARD database. Acquired ARGs were predicted using ResFinder v4.0 (https://bitbucket.org/genomicepidemiology/resfinder/src/master/) [77]. MAGs were also functionally annotated using the Distilled and Refined Annotation of Metabolism (DRAM, https://github.com/shafferm/DRAM) [78]. Genome annotation of MAGs (including the prediction of protein-coding regions, t-ribonucleic acid [tRNA], and rRNA) was performed using Prokka [79] with the annotate_bins module of the metaWRAP pipeline [67].
2.9. Identification of ARGs and AMGs carried by viruses
The viral sequences were searched for ARGs using strict criteria. The CARD database (v3.2.5) was downloaded and searched for candidate ARGs carried by the viral genomes of CMKMC with a threshold of 90 % sequence identity and a strict and perfect cutoff. Acquired ARGs in the CMKMC virome were also searched for using the ResFinder 4.0 database [77], which was validated with datasets of MIC values and WGS data. A total of 153 ARG-carrying viral contigs were found. VIBRANT v1.2.1 [52] and DRAMv [78] were used to identify vAMGs from viral genomes. VIBRANT v1.2.1 [52] was used to predict the lifestyles of the CMKMC viruses. According to the VIBRANT results, the virus type was classified as either lysogenic or lytic. Virus quality was classified as complete circular, high-quality draft, medium-quality draft, or low-quality draft. Then, detailed classifications of the genes from carbohydrate-active enzyme (CAZyme, http://www.cazy.org/), protein family (Pfam, http://pfam.xfam.org/), and virus orthologous group (VOG, https://vogdb.org/) databases were obtained.
2.10. Statistical analyses and visualization
The phylogenetic trees of MAGs were visualized using iTOL v6.1.1 (https://itol.embl.de/) [80]. Multiple comparison tests, including the Kruskal–Wallis and Mann–Whitney U tests, were performed using GraphPad Prism v9.0. Statistical significance was considered at P < 0.05. Stack columns and bar charts were created using GraphPad Prism v9.0. Figures were generated using the ImageGP platform (http://www.ehbio.com/ImageGP/) [81].
3. Results
3.1. Construction of the chicken multi-kingdom microbiome catalog
To provide a comprehensive overview of the microorganisms associated with the gastrointestinal tract of chicken, 1,215 fecal samples from 21 broiler farms and 22 LPMs, across eight geographical locations (Guangxi Zhuang Autonomous Region, Guangdong, Hunan, Zhejiang, Jiangsu, Anhui, Henan, and Shandong Provinces of China), involving 14 chicken breeds and two feeding styles (free-range and battery-cage) were collected (Fig. 1A and Supplementary Table 1). After removing adapter sequences, low-quality sequences, and the chicken host genomes (GRCg6a), high-quality clean reads were assembled and the contigs/scaffolds were binned into 6,087 bins (also known as MAGs) using three binning approaches with the default options of MetaBAT2, Maxbin2, and Concoct in the metaWRAP pipeline [67]. Detailed information on completeness, contamination, genome size, contig numbers, N50, and GC content is summarized in Supplementary Table 2. We then removed redundancy at a 95 % ANI threshold screened for completeness of ≥ 50 % or contamination of ≤ 10 %, and 1,833 MAGs were obtained. They were classified using GTDB-Tk, and 5,974 bacterial and 118 archaeal MAGs were identified (Supplementary Table 3). Among these, approximately 26.1 % (1,586) were of high quality (with the cutoff: completeness > 90 % and contamination < 5 %) according to the criteria used in a previous study [36]. At ANI thresholds of 95 %, 61.31 % of the CMKMC bacterial/archaeal MAGs were novel candidate species. The novelty of the newly generated chicken MAGs was then analyzed by comparing them with the 12,339 MAGs from 799 chicken gut metagenomes reported previously [31] and annotated using GTDB-Tk [70] and dRep [69]. Among these, 7,383 and 134 high-quality bacterial and archaeal MAGs, respectively, were identified. At ANI thresholds of 95 %, 3,071 nonredundant MAGs were obtained, including 3,050 and 21 bacterial and archaeal MAGs, respectively (Fig. 1B & C and Supplementary Table 4). Notably, 812 species-level MAGs (74.63 % of 1,088) were novel putative species (i.e., their ANI was below the cutoff value with sequences in any of the public databases and the 12,339 MAGs), and 240 genera were candidate novel genera distributed in 42 families and 13 phyla (Supplementary Table 5). Therefore, we referred to the 18,201 bacterial and 225 archaeal MAGs as the chicken MAG catalog.
Fig. 1.
Pipeline for the construction of the chicken multi-kingdom microbiome catalog, including bacterial, archaeal, and viral genomes. A) Overview of the overall strategy and datasets employed for CMKMC. Metagenomic sequencing data from the samples spanning origin, geography, breed, and farming system as well as 12,339 chicken MAGs from 799 metagenome data [31] were integrated and used to construct the CMKMC. The reconstructed microbial genomes were clustered to strain- and species-level genome bins at 99 % and 95 % of the ANI, respectively. A total of 6,087 novel MAGs (1,883 nonredundant MAGs) divided into medium-quality MAGs (≥50 % completeness and ≤ 10 % contamination) and high-quality MAGs (>90 % completeness and < 5 % contamination) were reconstructed. SGBs containing at least one reference genome in the GTDB were considered kSGB, and the SGBs without reference genomes were considered uSGBs. B) Quality of the 18,201 bacterial genomes in the CMKMC. C) Quality of the 225 archaeal genomes in the CMKMC. D) Quality of the 33,411 viral genomes in the CMKMC. E) Composition of the genomes in the CMKMC. For CMKMC MAGs, the quality criteria are selected as near complete: > 90 % completeness and ≤ 5 % contamination according to CheckM and at least 18 tRNA, high-quality: > 90 % completeness and < 5 % contamination, and medium-quality: ≥ 50 % completeness and ≤ 10 % contamination. For viral genomes, the quality was evaluated using VIBRANT. Abbreviations: CMKMC, chicken multi-kingdom microbiome catalog; MAGs, metagenome-assembled genomes; SGBs, species-level genome bins; ANI, average nucleotide identity; VFs, virulence factors; VFDB, virulence factor database; kSGB, known SGBs; uSGBs, unknown SGBs; GTDB, Genome Taxonomy Database LPM, live poultry markets; vOTU, viral operational taxonomic units.
In addition, 59,911 candidate viral genomes (mostly bacteriophages) with a minimal length of 5 kb were identified using state-of-the-art bioinformatics methods (Fig. 1A) and were clustered into 35,404 nonredundant vOTU at an ANI of 95 %. VIBRANT was used to verify the reliability of these viral genomes, and a chicken virome database representing 33,411 vOTUs was constructed (Fig. 1D and Supplementary Table 6). Among these, 3.01 % were of high-quality and 0.28 % were of complete circular genomes (Fig. 1D and Supplementary Table 7).
Altogether, we built a CMKMC catalog (Fig. 1E), including 18,201 bacterial and 225 archaeal MAGs and 33,411 viral genomes that better represent the chicken gut microbiome while containing a large proportion of novel candidate genomes.
3.2. Taxonomic annotation of the CMKMC genomes
First, we annotated the classifications of these MAGs in the CMKMC using GTDB-Tk [70]. The 18,426 MAGs were classified into bacterial (n = 18,201) and archaeal (n = 225) kingdoms, and most were classified into known taxonomy at the phylum, class, order, family, and genus levels (Fig. 2A). A total of 11,885, 3,304, 403, and 13 species-level genome bins (SGBs) could not be classified into known taxonomy at the species, genera, families, and order levels (Fig. 2A). Notably, at the species-level, only 3,732/6,087 MAGs (61.31 %) were classified into known species, indicating that many MAGs were previously unknown (i.e., excluded from the GTDB database). At the phylum level, the bacterial MAGs were predominated by Firmicutes_A (n = 7,067) and Firmicutes (n = 3,448), followed by Bacteroidota (n = 2,998), Actinobacteriota (n = 1,347), and Proteobacteria (n = 1,181) (Fig. 2B). The newly generated bacterial SGBs were predominated by Firmicutes, Firmicutes_A, and Bacteroidota, whereas the percentage of new species at each phylum level was inconsistent (Fig. 2C - E), greatly expanding our understanding of unknown species. All Firmicutes_A members belonged to the class Clostridia, which comprises the orders Oscillospirales (n = 2,780), Lachnospirales (n = 2,419), 4C28d-15 (n = 1,140), Christensenellales (n = 336), Peptostreptococcales (n = 232), Clostridiales (n = 81), TANB77 (n = 45), Monoglobales (n = 18), Tissierellales (n = 9), UBA1212 (n = 6), and Eubacteriales (n = 1). All Bacteroidota members belonged to the class Bacilli, which included the orders Lactobacillales (n = 2,518), Erysipelotrichales (n = 310), Staphylococcales (n = 226), RF39 (n = 142), Mycoplasmatales (n = 97), RFN20 (n = 78), Haloplasmatales (n = 28), Bacillales_A (n = 17), Bacillales (n = 16), Acholeplasmatales (n = 9), Exiguobacterales (n = 6), and one unknown order. All Bacteroidota members belonged to the class Bacteroidia, which included the orders Bacteroidales (n = 2,885), Flavobacteriales (n = 77), Sphingobacteriales (n = 34), Chitinophagales (n = 1), and Cytophagales (n = 1). At the species-level, bacterial SGBs were dominated by Escherichia flexneri (n = 204) and Lactobacillus_B salivarius (n = 203), followed by Lactobacillus_H ingluviei (n = 183), Lactobacillus_H vaginalis (n = 171), and Corynebacterium stationis (n = 165).
Fig. 2.
Taxonomic annotation and phylogenetic tree of the CMKMC genomes. A) Taxonomic classification of 18,426 MAGs at different levels. B) The number of SGBs in each phylum. C) The number of newly generated SGBs in each phylum. D) The percentage of uSGBs in each phylum. Classification rates of newly generated SGB (bacterial and archaeal MAGs) in GTDB in each phylum. E) The phylogenetic relationship between the 3,050 bacterial and 21 archaeal MAGs in the CMKMC and their taxonomic classification according to GTDB–Tk. The annotations from inside to outside represent annotations at the species-level (different colors represent different phyla), newly generated (in blue), unclassified family (in brown), unclassified genus (in dark blue), and unclassified species (in purple). The three phyla are from Archaea and the others belong to bacteria. Abbreviations: SGBs, species-level genome bins; kSGB, known SGBs; uSGBs, unknown SGBs; MAGs, metagenome-assembled genomes; CMKMC, chicken multi-kingdom microbiome catalog; GTDB, Genome Taxonomy Database.
All archaeal MAGs produced methane belonging to the phyla Halobacterota (n = 102), Euryarchaeota (n = 78), and Thermoplasmatota (n = 45). At the species-level, 80 previously unknown SGBs (uSGBs) were newly generated, accounting for 35.56 % of the total archaeal MAGs. Among these, 57, 20, and 3 uSGBs were classified into Halobacterota, Thermoplasmatota, and Euryarchaeota. These data imply that Halobacterota and Thermoplasmatota have higher strain diversity in the chicken gut than Euryarchaeota. At the genus level, the number of Methanocorpusculum MAGs (n = 102) was higher than that of any of the other four genera (Supplementary Fig. 1), inconsistent with the prevailing notion of Methanobrevibacter in ruminant animals [36]. Overall, these results demonstrate that our CMKMC MAGs significantly expand the public database with functionally important microbial communities.
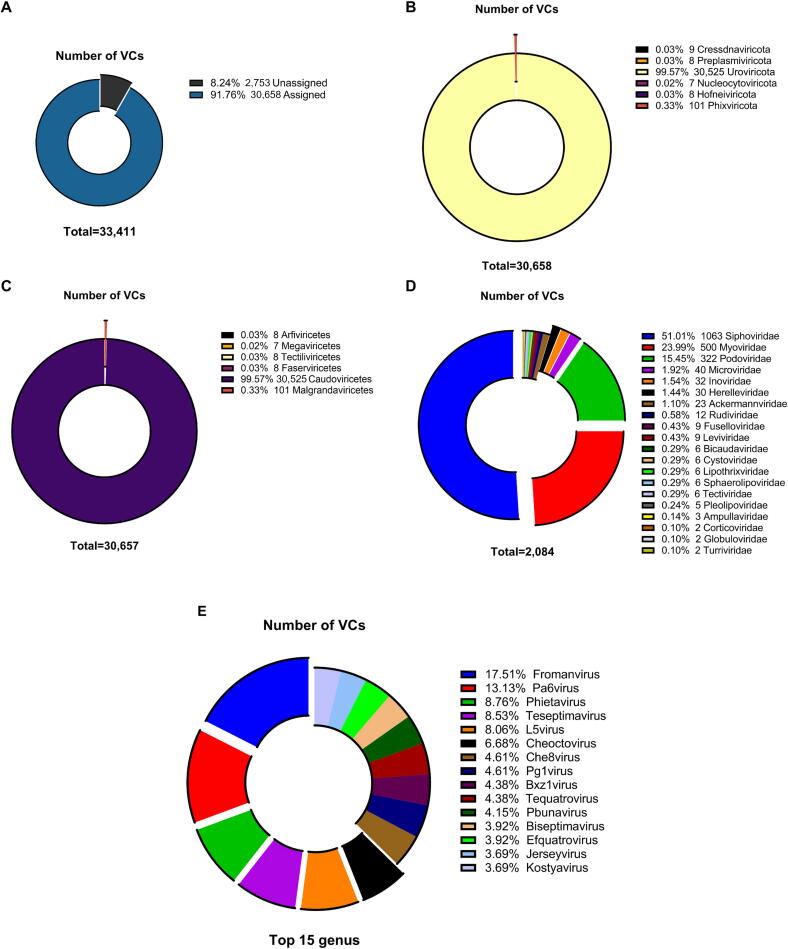
Next, we annotated these viral genomes in the CMKMC using the GeNomad tool, which classifies a marker gene taxonomy. We assigned 91.76 % of the classified DNA vOTUs to known taxonomical clades (Fig. 3A and Supplementary Table 8) and 30,658 and 30,657 vOTUs to known phylum and class levels (Fig. 3B & C). Next, 30,525 viral clusters (VCs) were assigned to the class Caudoviricetes (i.e., tailed dsDNA bacteriophages), followed by Malgrandaviricetes (n = 101), Arfiviricetes (n = 8), Faserviricetes (n = 8), Tectiliviricetes (n = 8), and Megaviricetes (n = 7) (Fig. 3C). We also classified the CMKMC viral genomes by clustering network analysis using vConTACT2. Of the 33,411 vOTUs, 19,503 (approximately 41.1 %) were clustered, resulting in 4,895 VCs (Supplementary Table 9). Among them, only 2,613 vOTUs could be classified by linking viral proteins to existing viruses in the NCBI RefSeq database, generating 446 VCs. We classified 1,636 vOTUs as belonging to known genera and 2,084 as belonging to known families. Most of the genera- and families- assignable vOTUs (90.45 %) are classified into Siphoviridae, Myoviridae, or Podoviridae in the order Caudovirales, which are also the most abundant viral families in the human gut and rumen virome [61]. The remaining vOTUs (8.24 %) could not be classified into any existing genera or families; thus, they represent novel clades. In summary, these findings represent the high diversity of both bacterial and archaeal MAGs and DNA viral communities in the chicken gut microbiome, with an unexpectedly high richness of new microbial genomes.
Fig. 3.
Taxonomic classifications of the 33,411 viral genomes. A) Virus taxonomy for DNA VCs. Virus taxonomy is genome-based. Most of the vOTUs (91.76%) were assigned to the known virus. B)–E) phylum (B), class (C), family (D), and genus (E) level taxonomy and virus proportion in the chicken virome database. Most of the vOTUs (99.76%) classified into existing classes were under the order Caudovirales. Detailed taxonomic assignments of individual vOTUs are presented in Supplementary Table 9. Genus level taxonomy and proportion of the 1,636 vOTUs that could be assigned to existing genera or families using vConTACT2. Abbreviations: VCs, viral clusters; vOTUs, viral operational taxonomic units; DNA, deoxyribonucleic acid.
3.3. Functional annotation of the CMKMC genomes
We further annotated the CMKMC genomes with the DRAM, VFDB, PlasmidFinder, and CARD/ResFinder databases to reveal CAZymes, virulence genes, plasmid patterns, and antimicrobial resistance (AMR) profiles in the chicken gut microbiome. A total of 21,765 classified ARGs were found in the CMKMC genomes. The most widely distributed ARGs were aminoglycoside (n = 3,209) and fluoroquinolone (n = 3,023), followed by tetracycline (n = 2,217), peptide (n = 1,259), and beta-lactam (n = 2,740) (Fig. 4A and Supplementary Table 10). The top 10 AMR gene families accounted for 71.11 % of the total AMR (Fig. 4B). The common ARGs in these MAGs were acrD (n = 295), acrB (n = 290), and lnu(C) (n = 288). These ARGs belonged to two major resistance mechanisms: antibiotic efflux and inactivation (Fig. 4C). Of the antibiotics inactivated, 49.07 % were beta-lactamase, and OXA beta-lactamase (n = 388), EC beta-lactamase (n = 189), and TEM beta-lactamase (n = 173) were more abundant than the others (Fig. 4D).
Fig. 4.
Functional annotation of the CMKMC genomes. A) Overview of ARGs identified in the CMKMC genomes. B) The diversity of the AMR gene family. C) The antibiotic resistance mechanisms of the detected ARGs. D) Overview of beta-lactamase ARGs identified in the CMKMC genomes. E) The top ten most abundant plasmids found in the CMKMC genomes. F) The top 15 most abundant VFs found in the CMKMC genomes. G) The prevalence of the 409 CAZyme subclasses found in the CMKMC genomes. H) The top 20 most abundant CAZyme subclasses found in the CMKMC genomes. I) Distribution of ARGs, plasmids, and VFs in the CMKMC genomes. Abbreviations: CMKMC, chicken multi-kingdom microbiome catalog; ARGs, antibiotic resistance genes; AMR, antimicrobial resistance; VFs, virulence factors; CAZyme, carbohydrate-active enzyme; AA, auxiliary activities; CBM, carbohydrate-binding module; CE, carbohydrate esterase; GH, glycoside hydrolase; GT, glycosyl transferase; PL, polysaccharide lyase.
Plasmid typing results showed that 124 types (a total of 1,696) of plasmids were present in 649 MAGs (3.52 % of the total). The top five plasmid types in the CMKMC genomes were Col(pHAD28), Col(MG828), ColRNAI, p0111, and IncFIB (AP001918) (Fig. 4E and Supplementary Table 11), inconsistent with a previous study [31]. The common virulence factor (VF) genes carried by MAGs in the chicken gut were tssH-5/clpV, algI, cheY, clpP, and algW (Fig. 4F). Importantly, 45.15 % and 34.88 % of them (MAGs carried plasmid and VF) were newly generated. Many ARGs and VFs were detected by uSGBs (Fig. 4G), thereby enlarging our insight into functionally important microbial taxa. Plasmid and VF profiles are highly associated with horizontal gene transfer and in-depth and precise mining of mobile genetic elements (MGEs) in metagenomes is required.
A total of 5,431,553 protein-coding genes were annotated from the CMKMC genomes. We annotated 48.47 % (Supplementary Fig. 2) and 3.08 % of these genes according to the Kyoto Encyclopedia of Genes and Genomes (KEGG) and CAZyme databases, respectively. Notably, half of these genes were not annotated to known functions by either database, indicating that they may code for new functions. A total of 167,547 CAZyme-encoding sequences were annotated in the CMKMC genomes, corresponding to eight classes (409 CAZyme subclasses) (Fig. 4H & I and Supplementary Table 12). The glycoside hydrolase (GH) class was the most dominant in the CMKMC genomes, followed by glycosyltransferase (GT), carbohydrate esterase (CE), and carbohydrate-binding module (CBM). CAZymes from the GH and GT classes were the most predominant in the CMKMC genomes, with GT2_Glycos_transf_2 and GT4 displaying the highest richness among chickens (Fig. 4I). These results may help us better understand the differences in carbohydrate metabolism in the chicken gut microbiota.
3.4. Host prediction of the CMKMC virome
Host prediction is important for understanding the potential role of DNA viruses (phages) in the chicken gut ecosystem. Thus, we predicted these hosts for the CMKMC viruses using six different methods, including Blast, CRISPR, VirHostMatcher, WIsH, PHP, and RaFAH, in iPHop [54]. In total, 88.89 % of the 33,411 genomes were putative lytic viruses (virulent or uncertain virulent), and the remaining were lysogenic viruses (Fig. 5A). Overall, 4,872 of the 13,256 viruses with predicted hosts (36.75 %) were classified as specialist, and the remaining viruses (n = 8,384) were classified as generalists (Fig. 5B & C and Supplementary Table 13 & 14). By predicting the putative hosts of the identified chicken gut viruses, we identified 75 archaeophages and 16,505 bacteriophages (Fig. 5D). These viruses were predicted to infect 28 phyla, 45 classes, 103 orders, 204 families, and 754 genera (Supplementary Fig. 3). Surprisingly, a large proportion of phages were predicted to infect multiple host genomes. The number of phages per host genome varied, with most of the hosts belonging to Firmicutes (Fig. 5E), whereas most of the Proteobacteria hosts had more than one phage per host genome. The archaeaphages were predicted to infect 17 known and 4 unknown genera of archaeaphages, and the bacteriophages could infect 737 known and 52 unknown genera of bacteria (Fig. 5F).
Fig. 5.
Host prediction of the CMKMC virome. A) The prevalence of the CMKMC virome lifestyle. B) Virus diversity as a function of the number of predicted hosts. The viruses could be divided into specialist (number of hosts = 1) and generalist (number of hosts > 1). C) Virus distributions as a function of their number of predicted hosts. D) Overview of known viral hosts grouped at the kingdom level. E) Distribution of putative viral hosts grouped at the phylum level. F) Distribution of the viruses as a function of the taxonomic classification of their hosts in top 20 genera. Abbreviations: CMKMC, chicken multi-kingdom microbiome catalog.
3.5. Analysis of viral auxiliary metabolic genes (vAMGs) and virus‑carried ARGs
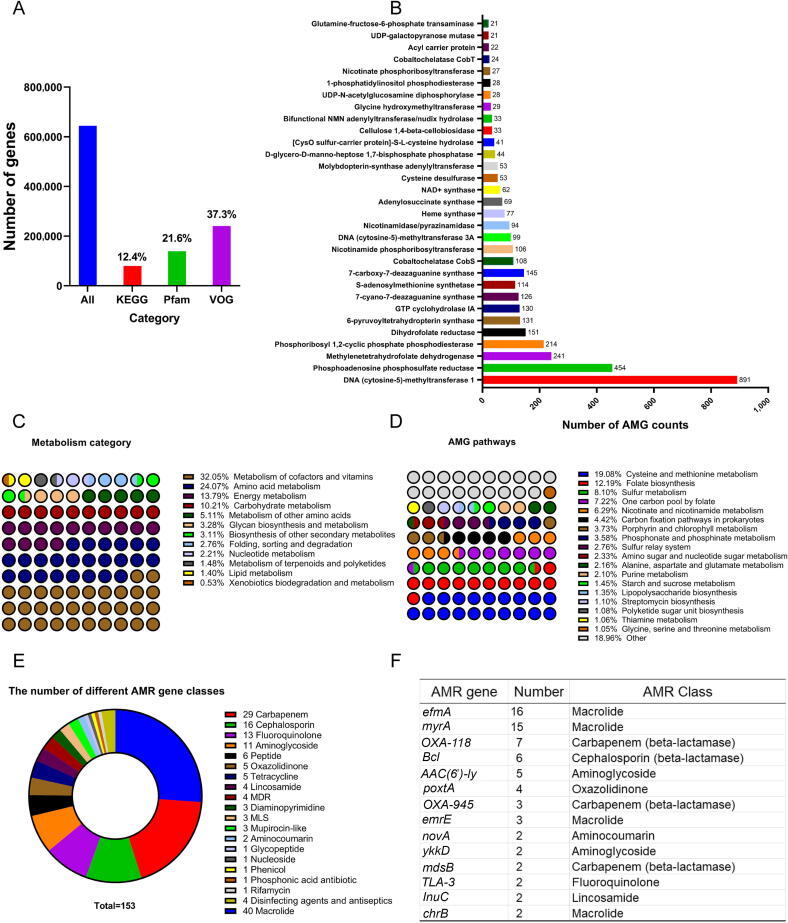
Among the 33,411 viral contigs, 644,453 ORFs were identified and annotated by 12.38 %, 21.64%, and 37.30 % of them according to the KEGG, Pfam, and VOG databases, respectively (Fig. 6A). To explore the mechanisms of chicken gut virus interaction between viruses and their hosts and their effect on biochemical cycles, vAMGs were predicted. By searching the viral contigs (4,602), we found 3,344 viral genomes carrying more than one AMG (Supplementary Table 15). The most prevalent AMG was found in 891 of these genomes, encoding DNMT1, a DNA (cytosine-5)-methyltransferase 1 that protects phages from the host’s antiviral restriction-modification systems (Fig. 6B and Supplementary Table 16). The second most prevalent AMG cysH was found in 454 genomes, encoding phosphoadenosine phosphosulfate reductase (a key enzyme in the assimilatory sulfate reduction pathway). These AMGs encode enzymes representing twelve metabolism categories involved in various metabolic processes, including the metabolism of carbohydrates, vitamins, nitrogen, and nucleotides. Among them, chicken gut phages tended to encode AMGs for the metabolism of cofactors and vitamins (32.05 %), amino acid metabolism (24.07 %), energy metabolism (13.79 %), and carbohydrate metabolism (10.21 %) (Fig. 6C). To explore the impact of viruses on gut microbiome biochemical cycling, the vAMG pathways were examined and summarized. A total of 1,147 vAMGs were classified as cysteine and methionine metabolism genes based on the KEGG database. For sulfur metabolism, 733 vAMGs were identified (Fig. 6D). Of these AMG-carrying vAMGs, we annotated 932 C-5 cytosine-specific DNA methylases. By providing diverse and unique AMGs, chicken gut viruses may play an important role in nutrient acquisition via their hosts or metabolic pathways.
Fig. 6.
AMGs and ARGs carried by chicken gut viruses. A) Number of genes annotated by KEGG, Pfam, and VOG in the CMKMC virome. B) A bar plot showing the AMG categories identified in the rumen virome. See Supplementary Table 15 for the detailed AMGs, full annotation of the final AMG-carrying contigs, and AMG functional category annotation. C) The metabolism categories of the detected AMGs. D) Overview of the metabolism pathways of the detected AMGs. E) The number of ARG-carrying viruses and their ARG classes identified in the vial contigs. F) The top 14 most abundant ARGs found in the CMKMC virome. Abbreviations: AMGs, auxiliary metabolic genes; ARGs, antibiotic resistance genes; KEGG, Kyoto Encyclopedia of Genes and Genomes; VOG, virus orthologous group; CMKMC, chicken multi-kingdom microbiome catalog; Pfam, protein family; AMR, antimicrobial resistance; MDR, multidrug resistance; MLS, macrolide-lincosamide-streptogramin.
Among the 33,411 viral contigs, 153 carried ARGs, indicating that ARGs are uncommon among chicken gut phage genomes. Several major ARG classes were identified, including macrolide, beta-lactam, fluoroquinolone, and aminoglycoside (Fig. 6E and Supplementary Table 17), all of which are prevalent and highly expressed in chicken gut microbiomes [82]. The top six ARGs prevalent in chicken gut viral genomes were efmA, myrA, OXA-118, BcI, AAC(6′)-Iy, and poxtA (Fig. 6F). Given the limited number and variety of identified ARG-encoding viruses, further studies are required to investigate the potential role of phages as a reservoir and dissemination vector of ARGs.
4. Discussion
Chicken meat is white meat, and worldwide chicken meat production and consumption has shown the fastest growing trend in recent decades [83]. The microbiome in the gastrointestinal tract plays an important role in digestion and absorption [3], [4]. Feng et al. (2023) [84] revealed that the development of the chicken gastrointestinal tract microbiome was associated with changes in chicken serum metabolites using quantitative microbiome profiling. Despite recent significant advances in microbiome-related studies, a comprehensive study on the chicken multi-kingdom microbiome involving multiple origins, breeds, feeding styles, and geographical factors remains lacking. In this study, we filled this gap by analyzing 135 chicken gut metagenomes [17], covering farm and LPM origins, 14 breeds, 2 feeding styles, and 43 geographical locations combined with 12,339 MAGs generated in our previous study [31]. On the basis of this dataset, the first chicken multi‑kingdom microbiome catalog (CMKMC, Fig. 1) comprising 18,201 bacterial, 225 archaeal, and 33,411 viral genomes was built, and 6,076,006 nonredundant protein-coding genes were annotated. Importantly, the CMKMC contained large proportions of novel genomes compared with those in a previous study [31]. Besides the dataset novelty, the CMKMC genome analysis indicated some important implications that can be generalized to other animals.
First, 812 nonredundant uSGBs, including 799 bacterial and 13 archaeal SGBs at 95 % ANI (Fig. 2E), which could not be classified into known species, were assembled using GTDB-Tk [70] and the public dataset [31]. The number of the 812 novel species belonging to 240 genera is 6.3 times that of the 38 new candidate genera mentioned in the previous study [31], greatly expanding our understanding of uncultured microorganisms. The uSGBs were dominant in the phyla Firmicutes_A (n = 263), followed by Firmicutes (n = 126), Bacteroidota (n = 121), and Proteobacteria (n = 87). This study provides a useful resource for future studies on the chicken gut microbiome. However, the CMKMC catalog is far from complete, as it still lacks or inadequately represents some of the low abundance microbial genomes in the chicken gut.
Second, several ARGs in uncultured bacterial genomes were identified. Some MAGs carried ARGs resistant to last-line antibiotics, such as colistin, carbapenem, tigecycline, and oxazolidinone (Fig. 4I). Moreover, 3.57 % and 34.04 % of the MAGs carried plasmids and VFs, respectively. Using MGEs, horizontal gene transfer of ARGs and virulence genes occurred between and/or within intestinal bacteria, even from symbiotic bacteria to pathogenic or opportunistic bacteria [85], [86].
Finally, by deep mining the chicken gut metagenomes, 33,411 species-level vOTUs, including 72 lytic and 23 lysogenic complete circular viruses, were obtained (Fig. 3). Consistent with previous studies of viromes in the human gut [56] and rumen [61], we found chicken gut viruses that may infect multiple species (Fig. 5). Therefore, these results provide a viable approach and help researchers recover similar resources for other food animals such as duck [49] and pig [18], [33], [34], [82]. Viruses play an essential role in ARG acquisition, maintenance, and dissemination in different environments [87]. Previous studies have focused on the reservoirs and contribution of viruses to AMR dissemination, for example, in rumen [61] and activated sludge systems [63]. In this study, only 153 viruses carried ARGs. We also found that 3,344 genomes carried 4,601 AMGs. The most common and abundant putative AMGs were DNMT1 (which catalyzes methionine degradation) and cysH, both of which are frequently reported to be carried by viruses in rumen ecosystems [88], [89].
The uniqueness of this study was in its provision of a reference genome set of the chicken gut microbiome using state-of-the-art metagenomic approaches. The genome set could be used to mine biosynthetic clusters [45], [90], novel toxin-metabolizing genes [40], antimicrobial peptides [91], microbial dark matter [92], [93], and ARGs [94], [95], [96].
This study had several limitations. First, it mainly focused on bacterial and archaeal MAGs and viral genomes, whereas eukaryotic MAGs were excluded. Moreover, the assembled genomes rely on sufficient sequencing coverage depth, and low abundance cannot be captured. Furthermore, short read-based methods have difficulties in recovering low abundance and complex genome sequences. For example, recent studies using high-fidelity long-read sequencing technology [97] have shown significant improvements in metagenome assemblies and the advantages of generating higher proportions of complete MAGs, as observed in a study on chicken [30] and human gut microbiomes [89]. Although our study obtained a significant proportion of high-quality bacterial and archaeal MAGs (40.80 %) and viral genomes (3.29 %), there is potential for further improvement using third-generation sequencing platforms. Finally, new methods for the reconstruction of MAGs and viral genomes (e.g., ViWRAP) [98], taxonomic classification, and host prediction will continue to be developed to overcome these limitations, and improved MAG and viral genome quality will be generated in the future.
5. Conclusions
In summary, we built and analyzed a comprehensive CMKMC, including bacterial, archaeal, and viral genomes, and provided functional insights into the chicken gut microbiota, paving the way for microbial interventions to improve chicken gut health and productivity.
Acknowledgements
This study was supported in part by grants from the National Key Research and Development Program of China (2023YFC2307101, 2020YFA0509202), the Young TopNotch Talents Foundation of Henan Agricultural University (30501278), the major Scientific and Technological Project of Henan Province (221100110600), the Major Program of the National Natural Science Foundation of China (81991534), CAS Southeast Asia Biodiversity Research Institute (151C53KYSB20210023), the Self-supporting Program of Guangzhou Laboratory (SRPG22-001), and the National Science and Technology Infrastructure of China (National Pathogen Resource Center-NPRC-32). Y.B. is supported by the Youth Innovation Promotion Association of CAS (Y2021034).
We are grateful to Mr. Zewu Zhang (Dongguan Municipal Center for Disease Control and Prevention), professor Yongchun Yang (College of Animal Science and Technology, Zhejiang Agriculture and Forestry University), and Dr. Juan Li (Key Laboratory of Etiology and Epidemiology of Emerging Infectious Diseases in Universities of Shandong, Shandong First Medical University and Shandong Academy of Medical Sciences) for their help in collecting chicken fecal samples. We also thank Dr. Canping Huang, Dr. Haiyuan Wang, Dr Pengtao Jiao, Mr. Liwei Yin, Mr. Cheng Zhang, and Mr. Wenlv Zheng for their help in collecting chicken fecal samples. We thank all participants for their help in collecting farm and LPM samples. We also thank the Veterinary Big Data and Bioinformatics Center, Henan Agricultural University, for their support and help.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Author contributions
Yanan Wang: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Funding acquisition. Mengqi Qu: Data curation, Writing – review & editing. Yuhai Bi: Methodology, Funding acquisition, Writing – review & editing. William J. Liu: Methodology, Data curation, Writing – review & editing. Sufang Ma: Writing – review & editing. Bo Wan: Writing – review & editing. Yongfei Hu: Writing – review & editing. Baoli Zhu: Writing – review & editing. Gaiping Zhang: Conceptualization, Supervision, Project administration, Funding acquisition, Writing – review & editing. George F. Gao: Conceptualization, Supervision, Project administration, Funding acquisition, Writing – review & editing.
Data availability
The multikingdom microbiome catalog of the chicken gastrointestinal tract, including bacterial and archaeal MAGs, viral genomes, annotation of MAGs, and viruses generated in the present study, is available in the figshare repository with the identifiers https://doi.org/10.6084/m9.figshare.24681096.v1. Public chicken MAGs are available from https://doi.org/10.6084/m9.figshare.15911964.
Code availability
The source code used for the CMKMC analysis in this study is available at https://github.com/NANYW123/Chicken-CMKMC.git.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bsheal.2024.02.006.
Supplementary data
The following are the Supplementary data to this article:
References
- 1.Van Boeckel T.P., Pires J., Silvester R., Zhao C., Song J., Criscuolo N.G., Gilbert M., Bonhoeffer S., Laxminarayan R. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science. 2019;365:eaaw1944. doi: 10.1126/science.aaw1944. [DOI] [PubMed] [Google Scholar]
- 2.Apajalahti J., Kettunen A. Avian Gut Function in Health and Disease. CABI; Wallingford UK: 2006. Microbes of the chicken gastrointestinal tract; pp. 124–137. [DOI] [Google Scholar]
- 3.Oakley B.B., Lillehoj H.S., Kogut M.H., Kim W.K., Maurer J.J., Pedroso A., Lee M.D., Collett S.R., Johnson T.J., Cox N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014;360:100–112. doi: 10.1111/1574-6968.12608. [DOI] [PubMed] [Google Scholar]
- 4.Shang Y., Kumar S., Oakley B., Kim W.K. Chicken gut microbiota: importance and detection technology. Front. Vet. Sci. 2018;5:254. doi: 10.3389/fvets.2018.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torok V.A., Ophel-Keller K., Loo M., Hughes R.J. Application of methods for identifying broiler chicken gut bacterial species linked with increased energy metabolism. Appl. Environ. Microbiol. 2008;74:783–791. doi: 10.1128/AEM.01384-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta C.L., Blum S.E., Kattusamy K., Daniel T., Druyan S., Shapira R., Krifucks O., Zhu Y.G., Zhou X.Y., Su J.Q., et al. Longitudinal study on the effects of growth-promoting and therapeutic antibiotics on the dynamics of chicken cloacal and litter microbiomes and resistomes. Microbiome. 2021;9:178. doi: 10.1186/s40168-021-01136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou A., Nadeau K., Xiong X., Wang P.W., Copeland J.K., Lee J.Y., Pierre J.S., Ty M., Taj B., Brumell J.H., Guttman D.S., et al. Systematic profiling of the chicken gut microbiome reveals dietary supplementation with antibiotics alters expression of multiple microbial pathways with minimal impact on community structure. Microbiome. 2022;10:127. doi: 10.1186/s40168-022-01319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y., Feng Y., Yang X., Lv Z., Li P., Zhang M., Wei F., Jin X., Hu Y., Guo Y., et al. Mining chicken ileal microbiota for immunomodulatory microorganisms. I.S.M.E. J. 2023;17:758–774. doi: 10.1038/s41396-023-01387-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X., Akhtar M., Chen Y., Ma Z., Liang Y., Shi D., Cheng R., Cui L., Hu Y., Nafady A.A., et al. Chicken jejunal microbiota improves growth performance by mitigating intestinal inflammation. Microbiome. 2022;10:107. doi: 10.1186/s40168-022-01299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao P., Ma C., Sun Z., Wang L., Huang S., Su X., Xu J., Zhang H. Feed-additive probiotics accelerate yet antibiotics delay intestinal microbiota maturation in broiler chicken. Microbiome. 2017;5:91. doi: 10.1186/s40168-017-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang P., Zhang Y., Xiao K., Jiang F., Wang H., Cao H., Huang S., Guo Y., Fan W., Zeng J., et al. The chicken gut metagenome and the modulatory effects of plant-derived benzylisoquinoline alkaloids. Microbiome. 2018;6:211. doi: 10.1186/s40168-018-0590-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandit R.J., Hinsu A.T., Patel N.V., Koringa P.G., Jakhesara S.J., Rank D.N., Raman M., Tirumurugaan K.G., Blake D.P., Joshi C.G., et al. Microbial diversity and community composition of caecal microbiota in commercial and indigenous Indian chickens determined using 16S rDNA amplicon sequencing. Microbiome. 2018;6:115. doi: 10.1186/s40168-018-0501-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiong W., Wang Y., Sun Y., Ma L., Zeng Q., Jiang X., Li A., Zeng Z., Zhang T. Antibiotic-mediated changes in the fecal microbiome of broiler chickens define the incidence of antibiotic resistance genes. Microbiome. 2018;6:34. doi: 10.1186/s40168-018-0419-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen C., Yan W., Mai C., Duan Z., Zheng J., Sun C., Yang N. Joint contributions of the gut microbiota and host genetics to feed efficiency in chickens. Microbiome. 2021;9:126. doi: 10.1186/s40168-021-01040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He Z., Liu R., Wang M., Wang Q., Zheng J., Ding J., Wen J., Fahey A.G., Zhao G. Combined effect of microbially derived cecal SCFA and host genetics on feed efficiency in broiler chickens. Microbiome. 2023;11:198. doi: 10.1186/s40168-023-01627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker M., Zhang X., Maciel-Guerra A., Dong Y., Wang W., Hu Y., Renney D., Hu Y., Liu L., Li H., et al. Machine learning and metagenomics reveal shared antimicrobial resistance profiles across multiple chicken farms and abattoirs in China. Nat. Food. 2023;4:707–720. doi: 10.1038/s43016-023-00814-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y., Lyu N., Liu F., Liu W.J., Bi Y., Zhang Z., Ma S., Cao J., Song X., Wang A., et al. More diversified antibiotic resistance genes in chickens and workers of the live poultry markets. Environ. Int. 2021;153:106534. doi: 10.1016/j.envint.2021.106534. [DOI] [PubMed] [Google Scholar]
- 18.P. Munk, B.E. Knudsen, O. Lukjancenko, A.S.R. Duarte, L. Van Gompel, EFFORT Group, D. Heederik, J.A. Wagenaar, D. Mevius, F.M. Aarestrup, et al., Abundance and diversity of the faecal resistome in slaughter pigs and broilers in nine European countries, Nat. Microbiol. 3 (2018) 898–908, https://doi.org/10.1038/s41564-018-0192-9. [DOI] [PubMed]
- 19.New F.N., Brito I.L. What is metagenomics teaching us, and what is missed? Annu. Rev. Microbiol. 2020;74:117–135. doi: 10.1146/annurev-micro-012520-072314. [DOI] [PubMed] [Google Scholar]
- 20.Solden L.M., Naas A.E., Roux S., Daly R.A., Collins W.B., Nicora C.D., Purvine S.O., Hoyt D.W., Schückel J., Jørgensen B., et al. Interspecies cross-feeding orchestrates carbon degradation in the rumen ecosystem. Nat. Microbiol. 2018;3:1274–1284. doi: 10.1038/s41564-018-0225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart R.D., Auffret M.D., Warr A., Wiser A.H., Press M.O., Langford K.W., Liachko I., Snelling T.J., Dewhurst R.J., Walker A.W., et al. Assembly of 913 microbial genomes from metagenomic sequencing of the cow rumen. Nat. Commun. 2018;9:870. doi: 10.1038/s41467-018-03317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart R.D., Auffret M.D., Warr A., Walker A.W., Roehe R., Watson M. Compendium of 4,941 rumen metagenome-assembled genomes for rumen microbiome biology and enzyme discovery. Nat. Biotechnol. 2019;37:953–961. doi: 10.1038/s41587-019-0202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almeida A., Mitchell A.L., Boland M., Forster S.C., Gloor G.B., Tarkowska A., Lawley T.D., Finn R.D. A new genomic blueprint of the human gut microbiota. Nature. 2019;568:499–504. doi: 10.1038/s41586-019-0965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nayfach S., Shi Z.J., Seshadri R., Pollard K.S., Kyrpides N.C. New insights from uncultivated genomes of the global human gut microbiome. Nature. 2019;568:505–510. doi: 10.1038/s41586-019-1058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasolli E., Asnicar F., Manara S., Zolfo M., Karcher N., Armanini F., Beghini F., Manghi P., Tett A., Ghensi P., et al. Extensive unexplored human microbiome diversity revealed by over 150,000 genomes from metagenomes spanning age, geography, and lifestyle. Cell. 2019;176:649–662.e20. doi: 10.1016/j.cell.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chibani C.M., Mahnert A., Borrel G., Almeida A., Werner A., Brugère J.F., Gribaldo S., Finn R.D., Schmitz R.A., Moissl-Eichinger C. A catalogue of 1,167 genomes from the human gut archaeome. Nat. Microbiol. 2022;7:48–61. doi: 10.1038/s41564-021-01020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leviatan S., Shoer S., Rothschild D., Gorodetski M., Segal E. An expanded reference map of the human gut microbiome reveals hundreds of previously unknown species. Nat. Commun. 2022;13:3863. doi: 10.1038/s41467-022-31502-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glendinning L., Stewart R.D., Pallen M.J., Watson K.A., Watson M. Assembly of hundreds of novel bacterial genomes from the chicken caecum. Genome Biol. 2020;21:34. doi: 10.1186/s13059-020-1947-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilroy R., Ravi A., Getino M., Pursley I., Horton D.L., Alikhan N.F., Baker D., Gharbi K., Hall N., Watson M., et al. Extensive microbial diversity within the chicken gut microbiome revealed by metagenomics and culture. PeerJ. 2021;9 doi: 10.7717/peerj.10941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y., Jiang F., Yang B., Wang S., Wang H., Wang A., Xu D., Fan W. Improved microbial genomes and gene catalog of the chicken gut from metagenomic sequencing of high-fidelity long reads. GigaScience. 2022;11 doi: 10.1093/gigascience/giac116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng Y., Wang Y., Zhu B., Gao G.F., Guo Y., Hu Y. Metagenome-assembled genomes and gene catalog from the chicken gut microbiome aid in deciphering antibiotic resistomes. Commun. Biol. 2021;4:1305. doi: 10.1038/s42003-021-02827-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segura-Wang M., Grabner N., Koestelbauer A., Klose V., Ghanbari M. Genome-resolved metagenomics of the chicken gut microbiome. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.726923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen C., Zhou Y., Fu H., Xiong X., Fang S., Jiang H., Wu J., Yang H., Gao J., Huang L. Expanded catalog of microbial genes and metagenome-assembled genomes from the pig gut microbiome. Nat. Commun. 2021;12 doi: 10.1038/s41467-021-21295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holman D.B., Kommadath A., Tingley J.P., Abbott D.W. Novel insights into the pig gut microbiome using metagenome-assembled genomes. Microbiol. Spectr. 2022;10 doi: 10.1128/spectrum.02380-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng X., Wilken S.E., Lankiewicz T.S., Gilmore S.P., Brown J.L., Barry K., Grigoriev I.V., Theodorou M.K., Valentine D.L., O'Malley M.A., et al. Genomic and functional analyses of fungal and bacterial consortia that enable lignocellulose breakdown in goat gut microbiomes. Nat. Microbiol. 2021;6:499–511. doi: 10.1038/s41564-020-00861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao Y., Feng T., Wu Y., Xu Y., Du L., Wang T., Luo Y., Wang Y., Li Z., Xuan Z., et al. The multi-kingdom microbiome of the goat gastrointestinal tract. Microbiome. 2023;11:219. doi: 10.1186/s40168-023-01651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie F., Jin W., Si H., Yuan Y., Tao Y., Zhu W., Wang M., Qiu Q., Li Z., Mao S., et al. An integrated gene catalog and over 10,000 metagenome-assembled genomes from the gastrointestinal microbiome of ruminants. Microbiome. 2021;9:137. doi: 10.1186/s40168-021-01078-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tong F., Wang T., Gao N.L., Liu Z., Cui K., Duan Y., Wu S., Luo Y., Li Z., Yang C., et al. The microbiome of the buffalo digestive tract. Nat. Commun. 2022;13:823. doi: 10.1038/s41467-022-28402-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.C. Li, X. Li, R. Guo, W. Ni, K. Liu, Z. Liu, J. Dai, Y. Xu, S. Abduriyim, Z. Wu, et al., Expanded catalogue of metagenome-assembled genomes reveals resistome characteristics and athletic performance-associated microbes in horse, Microbiome. 11 (2023) 7, 10.1186/s40168-022-01448-z. [DOI] [PMC free article] [PubMed]
- 40.Levin D., Raab N., Pinto Y., Rothschild D., Zanir G., Godneva A., Mellul N., Futorian D., Gal D., Leviatan S., et al. Diversity and functional landscapes in the microbiota of animals in the wild. Science. 2021;372:eabb5352. doi: 10.1126/science.abb5352. [DOI] [PubMed] [Google Scholar]
- 41.X.X. Zhang, Q.B. Lv, Q.L. Yan, Y. Zhang, R.C. Guo, J.X. Meng, H. Ma, S.Y. Qin, Q.H. Zhu, C.Q. Li, et al., A catalog of over 5,000 metagenome-assembled microbial genomes from the caprinae gut microbiota, Microbiol. Spectr. 10 (2022) e0221122, 10.1128/spectrum.02211-22. [DOI] [PMC free article] [PubMed]
- 42.Deng F., Wang C., Li D., Peng Y., Deng L., Zhao Y., Zhang Z., Wei M., Wu K., Zhao J., et al. The unique gut microbiome of giant pandas involved in protein metabolism contributes to the host’s dietary adaption to bamboo. Microbiome. 2023;11:180. doi: 10.1186/s40168-023-01603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y., Ji M., Yu T., Zaugg J., Anesio A.M., Zhang Z., Hu S., Hugenholtz P., Liu K., Liu P., et al. A genome and gene catalog of glacier microbiomes. Nat. Biotechnol. 2022;40:1341–1348. doi: 10.1038/s41587-022-01367-2. [DOI] [PubMed] [Google Scholar]
- 44.Royo-Llonch M., Sánchez P., Ruiz-González C., Salazar G., Pedrós-Alió C., Sunagawa S., Wincker P., Karp-Boss L., Bowler C., Acinas S.G., et al. Compendium of 530 metagenome-assembled bacterial and archaeal genomes from the polar Arctic Ocean. Nat. Microbiol. 2021;6:1561–1574. doi: 10.1038/s41564-021-00979-9. [DOI] [PubMed] [Google Scholar]
- 45.Ma B., Lu C., Wang Y., Yu J., Zhao K., Xue R., Ren H., Lv X., Pan R., Zhang J., et al. A genomic catalogue of soil microbiomes boosts mining of biodiversity and genetic resources. Nat. Commun. 2023;14:7318. doi: 10.1038/s41467-023-43000-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nayfach S., Roux S., Seshadri R., Udwary D., Varghese N., Schulz F., Wu D., Paez-Espino D., Chen I.M., Huntemann M., et al. A genomic catalog of Earth’s microbiomes. Nat. Biotechnol. 2021;39:499–509. doi: 10.1038/s41587-020-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt T.S.B., Fullam A., Ferretti P., Orakov A., Maistrenko O.M., Ruscheweyh H.J., Letunic I., Duan Y., Van Rossum T., Sunagawa S., et al. SPIRE: a searchable, planetary-scale microbiome resource. Nucleic Acids Res. 2023;52:D777–D783. doi: 10.1093/nar/gkad943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parks D.H., Rinke C., Chuvochina M., Chaumeil P.A., Woodcroft B.J., Evans P.N., Hugenholtz P., Tyson G.W. Recovery of nearly 8,000 metagenome-assembled genomes substantially expands the tree of life. Nat. Microbiol. 2017;2:1533–1542. doi: 10.1038/s41564-017-0012-7. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y., Hu Y., Cao J., Bi Y., Lv N., Liu F., Liang S., Shi Y., Jiao X., Gao G.F., et al. Antibiotic resistance gene reservoir in live poultry markets. J. Infect. 2019;78:445–453. doi: 10.1016/j.jinf.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 50.J. Guo, B. Bolduc, A.A. Zayed, A. Varsani, G. Dominguez-Huerta, T.O. Delmont, A.A. Pratama, M.C. Gazitúa, D. Vik, M.B. Sullivan, et al., VirSorter2: a multi-classifier, expert-guided approach to detect diverse DNA and RNA viruses, Microbiome 9 (2021) 37, 10.1186/s40168-020-00990-y. [DOI] [PMC free article] [PubMed]
- 51.Nayfach S., Camargo A.P., Schulz F., Eloe-Fadrosh E., Roux S., Kyrpides N.C. CheckV assesses the quality and completeness of metagenome-assembled viral genomes. Nat. Biotechnol. 2021;39:578–585. doi: 10.1038/s41587-020-00774-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kieft K., Zhou Z., Anantharaman K. VIBRANT: automated recovery, annotation and curation of microbial viruses, and evaluation of viral community function from genomic sequences. Microbiome. 2020;8:90. doi: 10.1186/s40168-020-00867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bin Jang H., Bolduc B., Zablocki O., Kuhn J.H., Roux S., Adriaenssens E.M., Brister J.R., Kropinski A.M., Krupovic M., Lavigne R., et al. Taxonomic assignment of uncultivated prokaryotic virus genomes is enabled by gene-sharing networks. Nat. Biotechnol. 2019;37:632–639. doi: 10.1038/s41587-019-0100-8. [DOI] [PubMed] [Google Scholar]
- 54.Roux S., Camargo A.P., Coutinho F.H., Dabdoub S.M., Dutilh B.E., Nayfach S., Tritt A. iPHoP: an integrated machine learning framework to maximize host prediction for metagenome-derived viruses of archaea and bacteria. PLoS Biol. 2023;21 doi: 10.1371/journal.pbio.3002083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gregory A.C., Zayed A.A., Conceição-Neto N., Temperton B., Bolduc B., Karp-Boss L., Roux S., Sunagawa S., Wincker P., Sullivan M.B., et al. Marine DNA viral macro- and microdiversity from pole to pole. Cell. 2019;177:1109–1123.e14. doi: 10.1016/j.cell.2019.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Camarillo-Guerrero L.F., Almeida A., Rangel-Pineros G., Finn R.D., Lawley T.D. Massive expansion of human gut bacteriophage diversity. Cell. 2021;184:1098–1109.e9. doi: 10.1016/j.cell.2021.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nayfach S., Páez-Espino D., Call L., Low S.J., Sberro H., Ivanova N.N., Proal A.D., Fischbach M.A., Bhatt A.S., Hugenholtz P., et al. Metagenomic compendium of 189,680 DNA viruses from the human gut microbiome. Nat. Microbiol. 2021;6:960–970. doi: 10.1038/s41564-021-00928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johansen J., Plichta D.R., Nissen J.N., Jespersen M.L., Shah S.A., Deng L., Stokholm J., Bisgaard H., Nielsen D.S., Sørensen S.J., et al. Genome binning of viral entities from bulk metagenomics data. Nat. Commun. 2022;13:965. doi: 10.1038/s41467-022-28581-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li R., Wang Y., Hu H., Tan Y., Ma Y. Metagenomic analysis reveals unexplored diversity of archaeal virome in the human gut. Nat. Commun. 2022;13 doi: 10.1038/s41467-022-35735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gregory A.C., Zablocki O., Zayed A.A., Howell A., Bolduc B., Sullivan M.B. The gut virome database reveals age-dependent patterns of virome diversity in the human gut. Cell Host Microbe. 2020;28:724–740.e8. doi: 10.1016/j.chom.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan M., Pratama A.A., Somasundaram S., Li Z., Jiang Y., Sullivan M.B., Yu Z. Interrogating the viral dark matter of the rumen ecosystem with a global virome database. Nat. Commun. 2023;14 doi: 10.1038/s41467-023-41075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coclet C., Sorensen P.O., Karaoz U., Wang S., Brodie E.L., Eloe-Fadrosh E.A., Roux S. Virus diversity and activity is driven by snowmelt and host dynamics in a high-altitude watershed soil ecosystem. Microbiome. 2023;11:237. doi: 10.1186/s40168-023-01666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fan X., Ji M., Mu D., Zeng X., Tian Z., Sun K., Gao R., Liu Y., He X., Wu L., et al. Global diversity and biogeography of DNA viral communities in activated sludge systems. Microbiome. 2023;11:234. doi: 10.1186/s40168-023-01672-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li D., Liu C.M., Luo R., Sadakane K., Lam T.W. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics. 2015;31:1674–1676. doi: 10.1093/bioinformatics/btv033. [DOI] [PubMed] [Google Scholar]
- 67.Uritskiy G.V., DiRuggiero J., Taylor J. MetaWRAP-a flexible pipeline for genome-resolved metagenomic data analysis. Microbiome. 2018;6:158. doi: 10.1186/s40168-018-0541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parks D.H., Imelfort M., Skennerton C.T., Hugenholtz P., Tyson G.W. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015;25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olm M.R., Brown C.T., Brooks B., Banfield J.F. dRep: a tool for fast and accurate genomic comparisons that enables improved genome recovery from metagenomes through de-replication, I.S.M.E. J. 2017;11:2864–2868. doi: 10.1038/ismej.2017.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chaumeil P.A., Mussig A.J., Hugenholtz P., Parks D.H. GTDB-Tk: a toolkit to classify genomes with the genome taxonomy database. Bioinformatics. 2019;36:1925–1927. doi: 10.1093/bioinformatics/btz848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Asnicar F., Thomas A.M., Beghini F., Mengoni C., Manara S., Manghi P., Zhu Q., Bolzan M., Cumbo F., May U., et al. Precise phylogenetic analysis of microbial isolates and genomes from metagenomes using PhyloPhlAn 3.0. Nat. Commun. 2020;11:2500. doi: 10.1038/s41467-020-16366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao S., Paez-Espino D., Li J., Ai H., Liang J., Luo Z., Zheng J., Chen H., Shu W., Huang L. Patterns and ecological drivers of viral communities in acid mine drainage sediments across Southern China. Nat. Commun. 2022;13:2389. doi: 10.1038/s41467-022-30049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hyatt D., Chen G.L., Locascio P.F., Land M.L., Larimer F.W., Hauser L.J. Prodigal: prokaryotic gene recognition and translation initiation site identification. B.M.C. Bioinf. 2010;11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu B., Zheng D., Zhou S., Chen L., Yang J., Vfdb, a general classification scheme for bacterial virulence factors. Nucleic Acids Res. 2022;50(2022):D912–D917. doi: 10.1093/nar/gkab1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carattoli A., Zankari E., García-Fernández A., Voldby Larsen M., Lund O., Villa L., Møller Aarestrup F., Hasman H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014;58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alcock B.P., Huynh W., Chalil R., Smith K.W., Raphenya A.R., Beiko R.G., Hsiao W.W.L., Brinkman F.S.L., Van Domselaar G., McArthur A.G., Card, et al. expanded curation, support, for machine learning, and resistome prediction at the comprehensive antibiotic resistance database. Nucleic Acids Res. 2023;51(2023):D690–D699. doi: 10.1093/nar/gkac920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bortolaia V., Kaas R.S., Ruppe E., Roberts M.C., Schwarz S., Cattoir V., Philippon A., Allesoe R.L., Rebelo A.R., Florensa A.F., et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020;75:3491–3500. doi: 10.1093/jac/dkaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shaffer M., Borton M.A., McGivern B.B., Zayed A.A., La Rosa S.L., Solden L.M., Liu P., Narrowe A.B., Rodríguez-Ramos J., Bolduc B., et al. DRAM for distilling microbial metabolism to automate the curation of microbiome function. Nucleic Acids Res. 2020;48:8883–8900. doi: 10.1093/nar/gkaa621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 80.Letunic I., Bork P. Interactive tree of life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49:W293–W296. doi: 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen T., Liu Y., Huang L. ImageGP: an easy-to-use data visualization web server for scientific researchers. iMeta. 2022;1 doi: 10.1002/imt2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Y., Hu Y., Liu F., Cao J., Lv N., Zhu B., Zhang G., Gao G.F. Integrated metagenomic and metatranscriptomic profiling reveals differentially expressed resistomes in human, chicken, and pig gut microbiomes. Environ. Int. 2020;138 doi: 10.1016/j.envint.2020.105649. [DOI] [PubMed] [Google Scholar]
- 83.Mottet A., Tempio G. Global poultry production: current state and future outlook and challenges. Worlds Poult. Sci. J. 2017;73:245–256. doi: 10.1017/S0043933917000071. [DOI] [Google Scholar]
- 84.Feng Y., Zhang M., Liu Y., Yang X., Wei F., Jin X., Liu D., Guo Y., Hu Y. Quantitative microbiome profiling reveals the developmental trajectory of the chicken gut microbiota and its connection to host metabolism. iMeta. 2023;2 doi: 10.1002/imt2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu Y., Yang X., Li J., Lv N., Liu F., Wu J., Lin I.Y., Wu N., Weimer B.C., Gao G.F. et al.,The bacterial mobile resistome transfer network connecting the animal and human microbiomes. Appl. Environ. Microbiol. 2016;82:6672–6681. doi: 10.1128/AEM.01802-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Frost L.S., Leplae R., Summers A.O., Toussaint A. Mobile genetic elements: the agents of open source evolution. Nat. Rev. Microbiol. 2005;3:722–732. doi: 10.1038/nrmicro1235. [DOI] [PubMed] [Google Scholar]
- 87.Calero-Cáceres W., Ye M., Balcázar J.L. Bacteriophages as environmental reservoirs of antibiotic resistance. Trends Microbiol. 2019;27:570–577. doi: 10.1016/j.tim.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 88.Anderson C.L., Sullivan M.B., Fernando S.C. Dietary energy drives the dynamic response of bovine rumen viral communities. Microbiome. 2017;5:155. doi: 10.1186/s40168-017-0374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jin H., Quan K., He Q., Kwok L.Y., Ma T., Li Y., Zhao F., You L., Zhang H., Sun Z. A high-quality genome compendium of the human gut microbiome of Inner Mongolians. Nat. Microbiol. 2023;8:150–161. doi: 10.1038/s41564-022-01270-1. [DOI] [PubMed] [Google Scholar]
- 90.B. Wei, G.A. Hu, Z.Y. Zhou, W.C. Yu, A.Q. Du, C.L. Yang, Y.L. Yu, J.W. Chen, H.W. Zhang, Q. Wu, et al., Global analysis of the biosynthetic chemical space of marine prokaryotes, Microbiome 11 (2023) 144, 10.1186/s40168-023-01573-3. [DOI] [PMC free article] [PubMed]
- 91.Ma Y., Guo Z., Xia B., Zhang Y., Liu X., Yu Y., Tang N., Tong X., Wang M., Ye X., et al. Identification of antimicrobial peptides from the human gut microbiome using deep learning. Nat. Biotechnol. 2022;40:921–931. doi: 10.1038/s41587-022-01226-0. [DOI] [PubMed] [Google Scholar]
- 92.Rodríguez Del Río Á., Giner-Lamia J., Cantalapiedra C.P., Botas J., Deng Z., Hernández-Plaza A., Munar-Palmer M., Santamaría-Hernando S., Rodríguez-Herva J.J., Ruscheweyh H.J., et al. Functional and evolutionary significance of unknown genes from uncultivated taxa. Nature. 2024;626:377–384. doi: 10.1038/s41586-023-06955-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pavlopoulos G.A., Baltoumas F.A., Liu S., Selvitopi O., Camargo A.P., Nayfach S., Azad A., Roux S., Call L., Ivanova N.N., et al. Unraveling the functional dark matter through global metagenomics. Nature. 2023;622:594–602. doi: 10.1038/s41586-023-06583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Inda-Díaz J.S., Lund D., Parras-Moltó M., Johnning A., Bengtsson-Palme J., Kristiansson E. Latent antibiotic resistance genes are abundant, diverse, and mobile in human, animal, and environmental microbiomes. Microbiome. 2023;11:44. doi: 10.1186/s40168-023-01479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang Y., Liu F., Zhu B., Gao G.F. Metagenomic data screening reveals the distribution of mobilized resistance genes tet(X), mcr and carbapenemase in animals and humans. J. Infect. 2020;80:121–142. doi: 10.1016/j.jinf.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 96.Cao J., Hu Y., Liu F., Wang Y., Bi Y., Lv N., Li J., Zhu B., Gao G.F. Metagenomic analysis reveals the microbiome and resistome in migratory birds. Microbiome. 2020;8:26. doi: 10.1186/s40168-019-0781-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu L., Yang Y., Deng Y., Zhang T. Nanopore long-read-only metagenomics enables complete and high-quality genome reconstruction from mock and complex metagenomes. Microbiome. 2022;10:209. doi: 10.1186/s40168-022-01415-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou Z., Martin C., Kosmopoulos J.C., Anantharaman K. ViWrap: A modular pipeline to identify, bin, classify, and predict viral-host relationships for viruses from metagenomes. Imeta. 2023;2 doi: 10.1002/imt2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The multikingdom microbiome catalog of the chicken gastrointestinal tract, including bacterial and archaeal MAGs, viral genomes, annotation of MAGs, and viruses generated in the present study, is available in the figshare repository with the identifiers https://doi.org/10.6084/m9.figshare.24681096.v1. Public chicken MAGs are available from https://doi.org/10.6084/m9.figshare.15911964.