Abstract
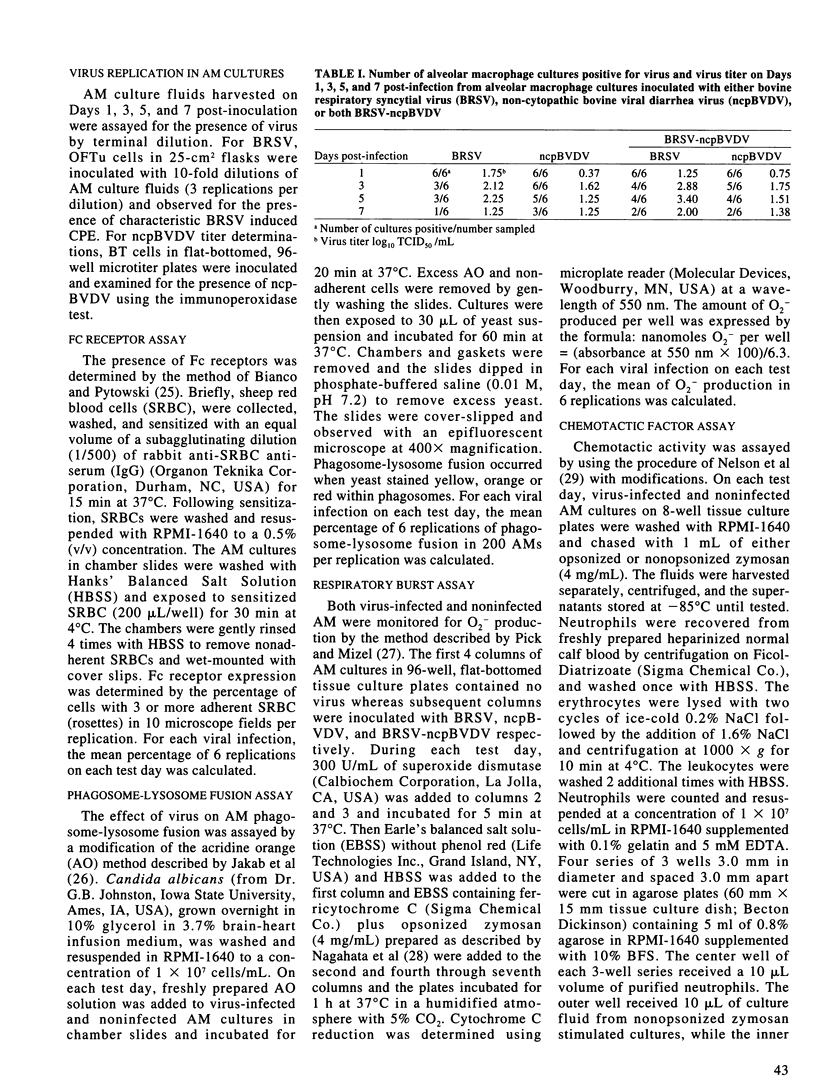
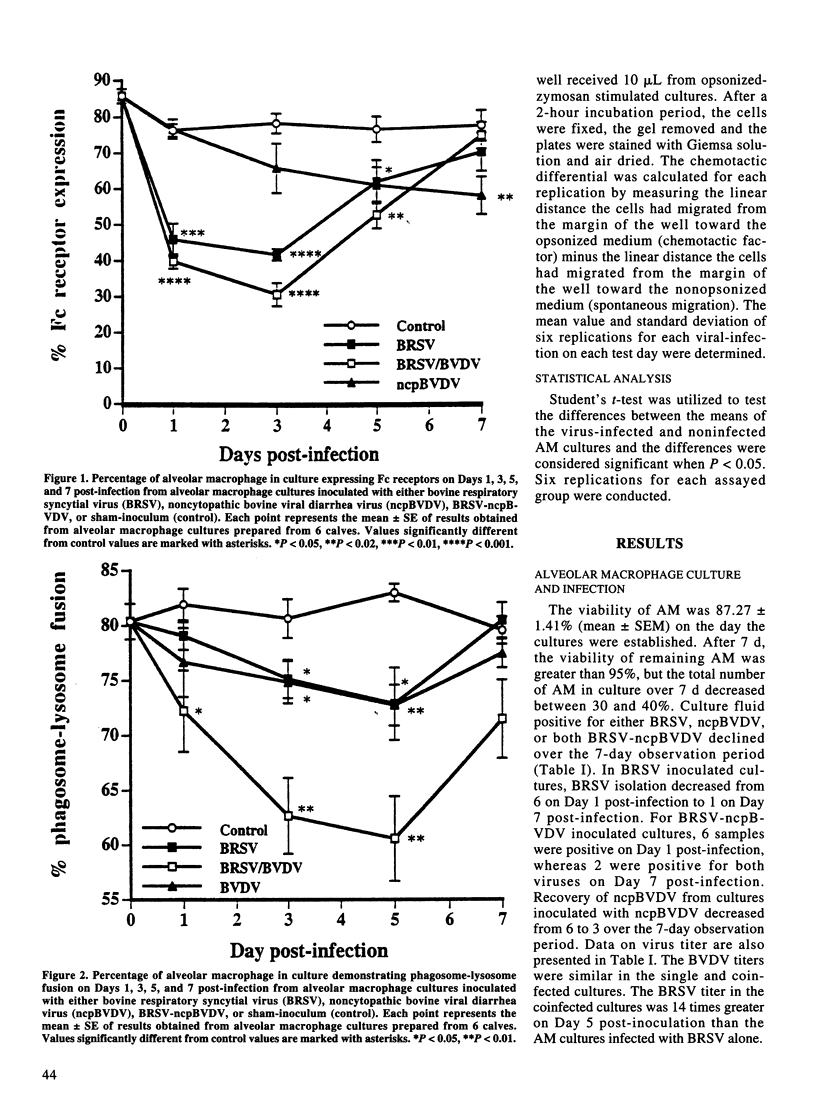
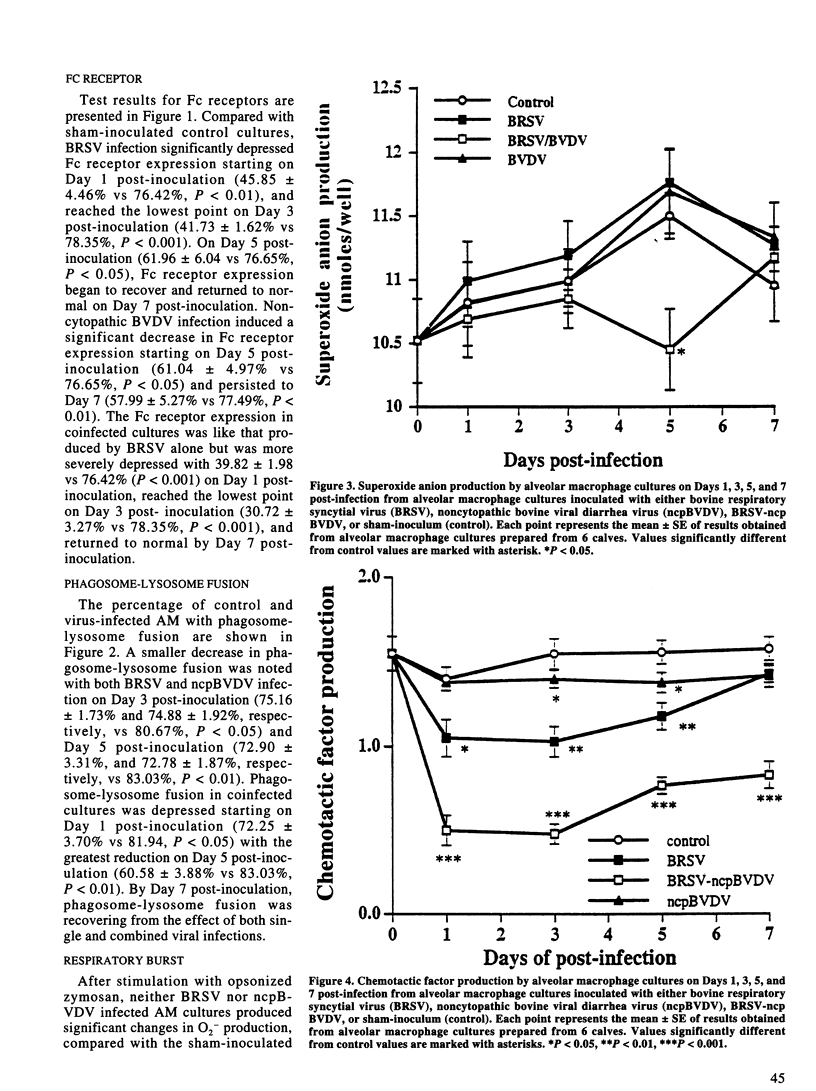
The effect of bovine respiratory syncytial virus (BRSV) and non-cytopathic bovine viral diarrhea virus (ncpBVDV) infection on selected bovine alveolar macrophage (AM) functions was investigated. Alveolar macrophages were harvested from 2- to 6-month-old calves seronegative for BRSV and BVDV and inoculated with approximately 1 median cell culture infective dose of virus per AM. Control, BRSV infected, ncpBVDV-infected and BRSV-ncpBVDV coinfected AM cultures were evaluated for Fc receptor expression, phagosome-lysosome fusion, superoxide anion (O2-) production, and chemotactic activity on Days 1, 3, 5, and 7 post-infection. Both single and combined viral infections significantly depressed AM Fc receptor expression, phagosome-lysosome fusion, and secretion of chemotactic factors with a more significant synergistic depression seen in BRSV-ncpBVDV coinfection. Production of O2- by AM was not decreased by either BRSV or ncpBVDV infection, but was significantly decreased by coinfection with BRSV-ncpBVDV. The present study confirms previous reports of BRSV effects on AM functions and indicate that ncpBVDV affects AM functions in vitro. Coinfection with BRSV-ncpBVDV produced a synergistic depression on AM functions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair B. M., McNulty M. S. Effect of "in vitro" exposure of bovine alveolar macrophages to different strains of bovine respiratory syncytial virus. Vet Immunol Immunopathol. 1992 Jan 15;30(2-3):193–206. doi: 10.1016/0165-2427(92)90138-g. [DOI] [PubMed] [Google Scholar]
- Babiuk L. A., Lawman M. J., Ohmann H. B. Viral-bacterial synergistic interaction in respiratory disease. Adv Virus Res. 1988;35:219–249. doi: 10.1016/S0065-3527(08)60713-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J. C., Ellis J. A., Clark E. G. Bovine respiratory syncytial virus. Vet Clin North Am Food Anim Pract. 1997 Nov;13(3):425–454. doi: 10.1016/s0749-0720(15)30307-8. [DOI] [PubMed] [Google Scholar]
- Becker S., Quay J., Soukup J. Cytokine (tumor necrosis factor, IL-6, and IL-8) production by respiratory syncytial virus-infected human alveolar macrophages. J Immunol. 1991 Dec 15;147(12):4307–4312. [PubMed] [Google Scholar]
- Bolin S. R., Matthews P. J., Ridpath J. F. Methods for detection and frequency of contamination of fetal calf serum with bovine viral diarrhea virus and antibodies against bovine viral diarrhea virus. J Vet Diagn Invest. 1991 Jul;3(3):199–203. doi: 10.1177/104063879100300302. [DOI] [PubMed] [Google Scholar]
- Bryson D. G., McFerran J. B., Ball H. J., Neill S. D. Observations on outbreaks of respiratory disease in calves associated with parainfluenza type 3 virus and respiratory syncytial virus infection. Vet Rec. 1979 Jan 20;104(3):45–49. doi: 10.1136/vr.104.3.45. [DOI] [PubMed] [Google Scholar]
- Fels A. O., Cohn Z. A. The alveolar macrophage. J Appl Physiol (1985) 1986 Feb;60(2):353–369. doi: 10.1152/jappl.1986.60.2.353. [DOI] [PubMed] [Google Scholar]
- Jakab G. J. Immune impairment of alveolar macrophage phagocytosis during influenza virus pneumonia. Am Rev Respir Dis. 1982 Nov;126(5):778–782. doi: 10.1164/arrd.1982.126.5.778. [DOI] [PubMed] [Google Scholar]
- Jakab G. J., Warr G. A., Sannes P. L. Alveolar macrophage ingestion and phagosome-lysosome fusion defect associated with virus pneumonia. Infect Immun. 1980 Mar;27(3):960–968. doi: 10.1128/iai.27.3.960-968.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadom N. J., Dedieu J. F., Viso M. Bovine alveolar macrophage: a review. Ann Rech Vet. 1985;16(3):175–183. [PubMed] [Google Scholar]
- Kobzik L., Godleski J. J., Brain J. D. Selective down-regulation of alveolar macrophage oxidative response to opsonin-independent phagocytosis. J Immunol. 1990 Jun 1;144(11):4312–4319. [PubMed] [Google Scholar]
- Lehmkuhl H. D., Gough P. M., Reed D. E. Characterization and identification of a bovine respiratory syncytial virus isolated from young calves. Am J Vet Res. 1979 Jan;40(1):124–126. [PubMed] [Google Scholar]
- Martin S. W., Bateman K. G., Shewen P. E., Rosendal S., Bohac J. G., Thorburn M. A group level analysis of the associations between antibodies to seven putative pathogens and respiratory disease and weight gain in Ontario feedlot calves. Can J Vet Res. 1990 Jun;54(3):337–342. [PMC free article] [PubMed] [Google Scholar]
- Midulla F., Huang Y. T., Gilbert I. A., Cirino N. M., McFadden E. R., Jr, Panuska J. R. Respiratory syncytial virus infection of human cord and adult blood monocytes and alveolar macrophages. Am Rev Respir Dis. 1989 Sep;140(3):771–777. doi: 10.1164/ajrccm/140.3.771. [DOI] [PubMed] [Google Scholar]
- Morahan P. S., Connor J. R., Leary K. R. Viruses and the versatile macrophage. Br Med Bull. 1985 Jan;41(1):15–21. doi: 10.1093/oxfordjournals.bmb.a072017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahata H., Yatsu A., Noda H. The evaluation of a quantitative assay for estimating the bacterial activity of bovine neutrophils by nitroblue tetrazolium reduction. Br Vet J. 1986 Nov-Dec;142(6):578–584. doi: 10.1016/0007-1935(86)90117-x. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Olchowy T. W., Ames T. R., Molitor T. W. Interaction of bovine respiratory syncytial virus with bovine alveolar macrophages in vivo: effects of virus infection upon selected cell functions. Can J Vet Res. 1994 Jan;58(1):42–48. [PMC free article] [PubMed] [Google Scholar]
- Panuska J. R., Cirino N. M., Midulla F., Despot J. E., McFadden E. R., Jr, Huang Y. T. Productive infection of isolated human alveolar macrophages by respiratory syncytial virus. J Clin Invest. 1990 Jul;86(1):113–119. doi: 10.1172/JCI114672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick E., Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46(2):211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Potgieter L. N. Bovine respiratory tract disease caused by bovine viral diarrhea virus. Vet Clin North Am Food Anim Pract. 1997 Nov;13(3):471–481. doi: 10.1016/s0749-0720(15)30309-1. [DOI] [PubMed] [Google Scholar]
- Potgieter L. N., McCracken M. D., Hopkins F. M., Walker R. D. Effect of bovine viral diarrhea virus infection on the distribution of infectious bovine rhinotracheitis virus in calves. Am J Vet Res. 1984 Apr;45(4):687–690. [PubMed] [Google Scholar]
- Rankin J. A., Sylvester I., Smith S., Yoshimura T., Leonard E. J. Macrophages cultured in vitro release leukotriene B4 and neutrophil attractant/activation protein (interleukin 8) sequentially in response to stimulation with lipopolysaccharide and zymosan. J Clin Invest. 1990 Nov;86(5):1556–1564. doi: 10.1172/JCI114875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richer L., Marois P., Lamontagne L. Association of bovine viral diarrhea virus with multiple viral infections in bovine respiratory disease outbreaks. Can Vet J. 1988 Sep;29(9):713–717. [PMC free article] [PubMed] [Google Scholar]
- Rosenquist B. D., Dobson A. W. Multiple viral infection in calves with acute bovine respiratory tract disease. Am J Vet Res. 1974 Mar;35(3):363–365. [PubMed] [Google Scholar]
- Roth J. A., Bolin S. R., Frank D. E. Lymphocyte blastogenesis and neutrophil function in cattle persistently infected with bovine viral diarrhea virus. Am J Vet Res. 1986 May;47(5):1139–1141. [PubMed] [Google Scholar]
- Samuelsson B., Goldyne M., Granström E., Hamberg M., Hammarström S., Malmsten C. Prostaglandins and thromboxanes. Annu Rev Biochem. 1978;47:997–1029. doi: 10.1146/annurev.bi.47.070178.005025. [DOI] [PubMed] [Google Scholar]
- Schnyder J., Dewald B., Baggiolini M. Effects of cyclooxygenase inhibitors and prostaglandin E2 on macrophage activation in vitro. Prostaglandins. 1981 Sep;22(3):411–421. doi: 10.1016/0090-6980(81)90102-7. [DOI] [PubMed] [Google Scholar]
- Schrijver R. S., Kramps J. A., Middel W. G., Langedijk J. P., van Oirschot J. T. Bovine respiratory syncytial virus replicates minimally in bovine alveolar macrophages. Arch Virol. 1995;140(11):1905–1917. doi: 10.1007/BF01322681. [DOI] [PubMed] [Google Scholar]
- Toth T. E., Hesse R. A. Elimination of contaminating bovine viral diarrhea virus from bovine respiratory syncytial virus stock. J Virol Methods. 1983 Apr;6(4):241–244. doi: 10.1016/0166-0934(83)90051-4. [DOI] [PubMed] [Google Scholar]
- Toth T., Hesse R. A. Replication of five bovine respiratory viruses in cultured bovine alveolar macrophages. Arch Virol. 1983;75(3):219–224. doi: 10.1007/BF01315276. [DOI] [PubMed] [Google Scholar]
- Trigo E., Liggitt H. D., Evermann J. F., Breeze R. G., Huston L. Y., Silflow R. Effect of in vitro inoculation of bovine respiratory syncytial virus on bovine pulmonary alveolar macrophage function. Am J Vet Res. 1985 May;46(5):1098–1103. [PubMed] [Google Scholar]
- Warr G. A., Jakab G. J., Hearst J. E. Alterations in lung macrophage immune receptor(s) activity associated with viral pneumonia. J Reticuloendothel Soc. 1979 Oct;26(4):357–366. [PubMed] [Google Scholar]
- Welsh M. D., Adair B. M., Foster J. C. Effect of BVD virus infection on alveolar macrophage functions. Vet Immunol Immunopathol. 1995 Jun;46(3-4):195–210. doi: 10.1016/0165-2427(94)05366-z. [DOI] [PubMed] [Google Scholar]


