Abstract
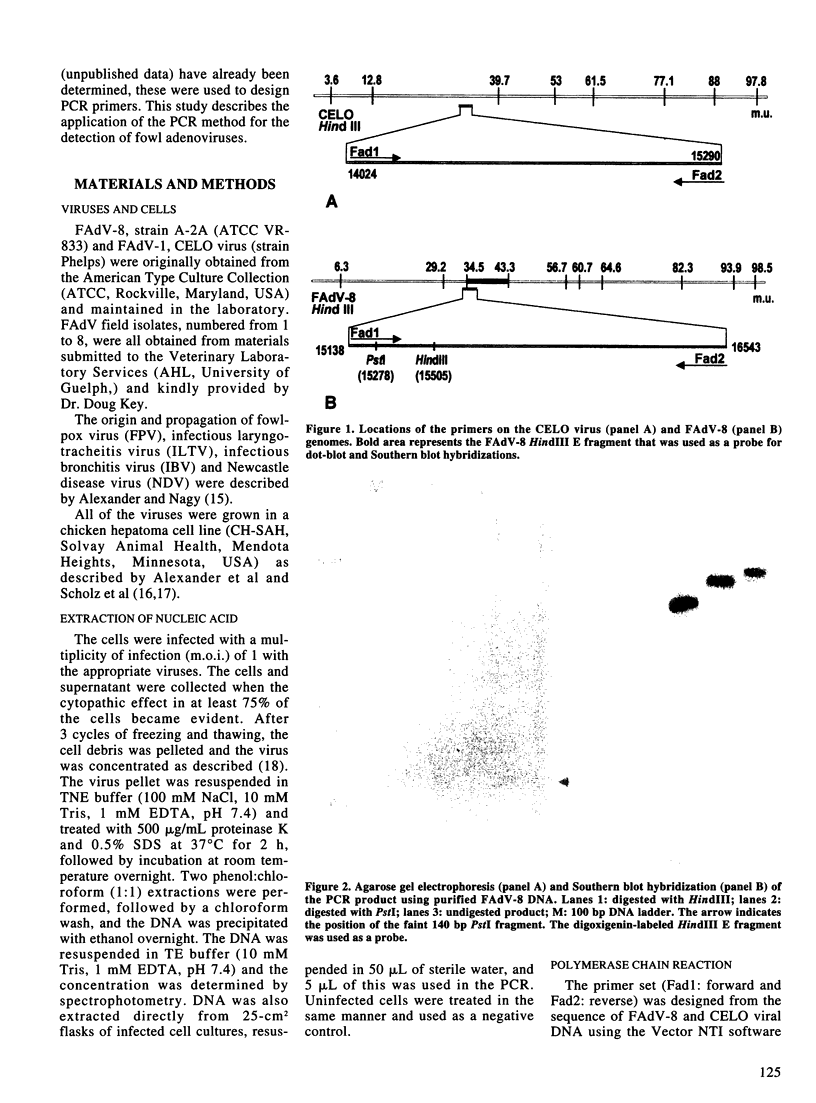
The possibility of using the polymerase chain reaction (PCR) for the detection of fowl adenoviruses (FAdV) was tested. The optimal reaction parameters were evaluated and defined for purified genomic DNA of type 8 fowl adenovirus (FAdV-8), and then the same conditions were applied for nucleic acid extracted from infected cells. One hundred picograms of purified viral DNA, or 250 FAdV-8-infected cells, were detected by ethidium bromide staining of the PCR products in agarose gels. The sensitivity was increased to 10 pg purified viral DNA, or 25 infected cells, when the PCR products were hybridized with a specific labeled probe. Several field isolates of FAdV and the CELO virus (FAdV serotype 1) could be amplified by the same primers and conditions, but the size of the amplicons was smaller than that for the FAdV-8 PCR product. Other avian viruses and uninfected cell cultures tested negative.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander H. S., Huber P., Cao J., Krell P. J., Nagy E. Growth characteristics of fowl adenovirus type 8 in a chicken hepatoma cell line. J Virol Methods. 1998 Sep;74(1):9–14. doi: 10.1016/s0166-0934(98)00062-7. [DOI] [PubMed] [Google Scholar]
- Alexander H. S., Nagy E. Polymerase chain reaction to detect infectious laryngotracheitis virus in conjunctival swabs from experimentally infected chickens. Avian Dis. 1997 Jul-Sep;41(3):646–653. [PubMed] [Google Scholar]
- Cao J. X., Krell P. J., Nagy E. Sequence and transcriptional analysis of terminal regions of the fowl adenovirus type 8 genome. J Gen Virol. 1998 Oct;79(Pt 10):2507–2516. doi: 10.1099/0022-1317-79-10-2507. [DOI] [PubMed] [Google Scholar]
- Chiocca S., Kurzbauer R., Schaffner G., Baker A., Mautner V., Cotten M. The complete DNA sequence and genomic organization of the avian adenovirus CELO. J Virol. 1996 May;70(5):2939–2949. doi: 10.1128/jvi.70.5.2939-2949.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavijo A., Krell P. J., Nagy E. Molecular cloning and restriction enzyme mapping of avian adenovirus type 8 DNA. Virus Res. 1996 Dec;45(2):93–99. doi: 10.1016/s0168-1702(96)01366-4. [DOI] [PubMed] [Google Scholar]
- Fitzgerald S. D., Reed W. M., Burnstein T. Detection of type II avian adenoviral antigen in tissue sections using immunohistochemical staining. Avian Dis. 1992 Apr-Jun;36(2):341–347. [PubMed] [Google Scholar]
- Fitzgerald S. D., Reed W. M., Langheinrich K. A., Porter A. S., Lumbert L. A. A retrospective immunohistochemical study of type II avian adenoviral infection in turkey, pheasant, and chicken tissues. Avian Dis. 1994 Jan-Mar;38(1):78–85. [PubMed] [Google Scholar]
- Fitzgerald S. D., Richard A. Comparison of four fixatives for routine splenic histology and immunohistochemical staining for group II avian adenovirus. Avian Dis. 1995 Apr-Jun;39(2):425–431. [PubMed] [Google Scholar]
- Hess M., Blöcker H., Brandt P. The complete nucleotide sequence of the egg drop syndrome virus: an intermediate between mastadenoviruses and aviadenoviruses. Virology. 1997 Nov 10;238(1):145–156. doi: 10.1006/viro.1997.8815. [DOI] [PubMed] [Google Scholar]
- Hussain I., Nagaraja K. V. A monoclonal antibody-based immunoperoxidase method for rapid detection of haemorrhagic enteritis virus of turkeys. Res Vet Sci. 1993 Jul;55(1):98–103. doi: 10.1016/0034-5288(93)90041-d. [DOI] [PubMed] [Google Scholar]
- Huw Lee L., Hwa Lee K. Application of the polymerase chain reaction for the diagnosis of fowl poxvirus infection. J Virol Methods. 1997 Jan;63(1-2):113–119. doi: 10.1016/s0166-0934(96)02119-2. [DOI] [PubMed] [Google Scholar]
- Iltis J. P., Daniels S. B., Wyand D. S. Demonstration of an avian adenovirus as the causative agent of marble spleen disease. Am J Vet Res. 1977 Jan;38(1):95–100. [PubMed] [Google Scholar]
- Latimer K. S., Niagro F. D., Williams O. C., Ramis A., Goodwin M. A., Ritchie B. W., Campagnoli R. P. Diagnosis of avian adenovirus infections using DNA in situ hybridization. Avian Dis. 1997 Oct-Dec;41(4):773–782. [PubMed] [Google Scholar]
- Lee L. H., Yu S. L., Shieh H. K. Detection of infectious bursal disease virus infection using the polymerase chain reaction. J Virol Methods. 1992 Dec 1;40(3):243–253. doi: 10.1016/0166-0934(92)90083-p. [DOI] [PubMed] [Google Scholar]
- Saifuddin M., Wilks C. R., Birtles M. J. Development of an immunocytochemical procedure to detect adenoviral antigens in chicken tissues. J Vet Diagn Invest. 1991 Oct;3(4):313–318. doi: 10.1177/104063879100300407. [DOI] [PubMed] [Google Scholar]
- Saifuddin M., Wilks C. R. Pathogenesis of an acute viral hepatitis: inclusion body hepatitis in the chicken. Arch Virol. 1991;116(1-4):33–43. doi: 10.1007/BF01319229. [DOI] [PubMed] [Google Scholar]
- Scholz E., Welniak E., Nyholm T., Guo P. An avian hepatoma cell line for the cultivation of infectious laryngotracheitis virus and for the expression of foreign genes with a mammalian promoter. J Virol Methods. 1993 Aug;43(3):273–286. doi: 10.1016/0166-0934(93)90146-i. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Smith L. M., Brown S. R., Howes K., McLeod S., Arshad S. S., Barron G. S., Venugopal K., McKay J. C., Payne L. N. Development and application of polymerase chain reaction (PCR) tests for the detection of subgroup J avian leukosis virus. Virus Res. 1998 Mar;54(1):87–98. doi: 10.1016/s0168-1702(98)00022-7. [DOI] [PubMed] [Google Scholar]
- Zsák L., Kisary J. Studies on egg drop syndrome (EDS) and chick embryo lethal orphan (CELO) avian adenovirus DNAs by restriction endonucleases. J Gen Virol. 1981 Sep;56(Pt 1):87–95. doi: 10.1099/0022-1317-56-1-87. [DOI] [PubMed] [Google Scholar]