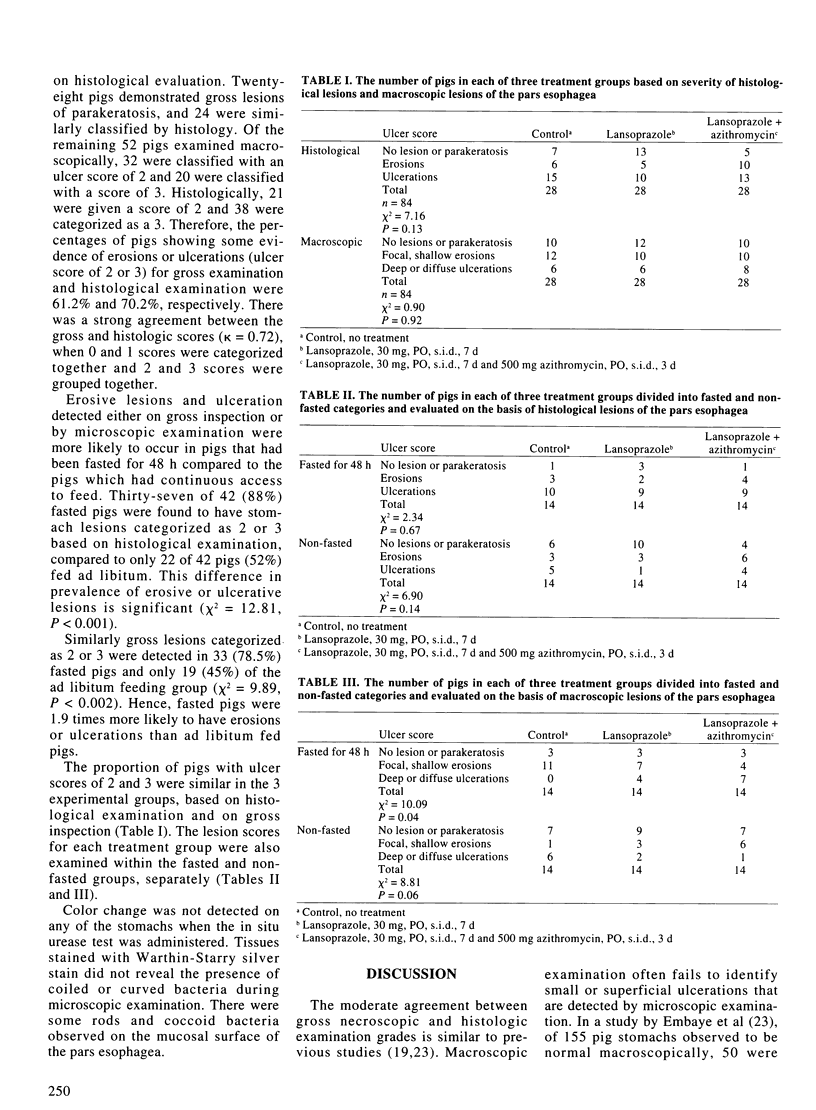
Abstract
Helicobacter-like organisms as well as fermentative bacteria have been implicated in gastric ulcer production in swine. Irregular feeding schedules are also considered a major risk factor. A research trial was conducted to determine whether medication with an acid secretion inhibitor (lansoprazole), either alone or in combination with an antibiotic (azithromycin), would protect pigs from gastric ulceration if the animals were subjected to a 48 h period of fasting. In a 2 x 3 factorial design, 48 pigs were fasted, while an equal number were fed ad libitum. Within these 2 study groups, pigs were randomly assigned to 1 of 3 treatments: control, 30 mg lansoprazole s.i.d. for 7 d, or lansoprazole (30 mg s.i.d. for 7 d) and azithromycin (500 mg s.i.d. for 3 d). Overall, fasted pigs were 1.9 times more likely to develop erosive or ulcerative lesions of the pars esophagea (chi2 = 9.89, P < 0.002). Treatment with an acid secretion inhibitor alone or in combination with an antibiotic did not protect pigs from developing gastric lesions. Helicobacter-like organisms were not detected in any of the stomachs. Possibly, the lansoprazole dose of 30 mg given once per day was insufficient to prevent pH levels from becoming low enough to cause damage to epithelial tissue. Alternatively other substances such as bile acids may have caused the ulcerative lesions, even though stomach acid production was suppressed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argenzio R. A., Eisemann J. Mechanisms of acid injury in porcine gastroesophageal mucosa. Am J Vet Res. 1996 Apr;57(4):564–573. [PubMed] [Google Scholar]
- Barbosa A. J., Silva J. C., Nogueira A. M., Paulino Júnior E., Miranda C. R. Higher incidence of Gastrospirillum sp. in swine with gastric ulcer of the pars oesophagea. Vet Pathol. 1995 Mar;32(2):134–139. doi: 10.1177/030098589503200206. [DOI] [PubMed] [Google Scholar]
- Blaser M. J. The bacteria behind ulcers. Sci Am. 1996 Feb;274(2):104–107. doi: 10.1038/scientificamerican0296-104. [DOI] [PubMed] [Google Scholar]
- Caselli M., Ruina M., Fabbri P., Balhous W., Alvisi V. A comparative trial of short term therapy with omeprazole plus either amoxycillin or azithromycin for Helicobacter pylori eradication. J Antimicrob Chemother. 1996 Apr;37(4):849–850. doi: 10.1093/jac/37.4.849. [DOI] [PubMed] [Google Scholar]
- Eaton K. A., Krakowka S. Effect of gastric pH on urease-dependent colonization of gnotobiotic piglets by Helicobacter pylori. Infect Immun. 1994 Sep;62(9):3604–3607. doi: 10.1128/iai.62.9.3604-3607.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embaye H., Thomlinson J. R., Lawrence T. L. Histopathology of oesophagogastric lesions in pigs. J Comp Pathol. 1990 Oct;103(3):253–264. doi: 10.1016/s0021-9975(08)80046-1. [DOI] [PubMed] [Google Scholar]
- Fiese E. F., Steffen S. H. Comparison of the acid stability of azithromycin and erythromycin A. J Antimicrob Chemother. 1990 Jan;25 (Suppl A):39–47. doi: 10.1093/jac/25.suppl_a.39. [DOI] [PubMed] [Google Scholar]
- Freston J. W. Overview of medical therapy of peptic ulcer disease. Gastroenterol Clin North Am. 1990 Mar;19(1):121–140. [PubMed] [Google Scholar]
- Girard A. E., Girard D., English A. R., Gootz T. D., Cimochowski C. R., Faiella J. A., Haskell S. L., Retsema J. A. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob Agents Chemother. 1987 Dec;31(12):1948–1954. doi: 10.1128/aac.31.12.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso G. M., Ripabelli G., Sammarco M. L., Ruberto A., Iannitto G. Prevalence of Helicobacter-like organisms in porcine gastric mucosa: a study of swine slaughtered in Italy. Comp Immunol Microbiol Infect Dis. 1996 Jun;19(3):213–217. doi: 10.1016/0147-9571(96)00007-0. [DOI] [PubMed] [Google Scholar]
- Grasso G. M., Sammarco M. L., Ripabelli G., Ruberto A., Iannitto G. In situ mapping of urease-positive areas in porcine gastric mucosa. Microbios. 1995;82(333):245–249. [PubMed] [Google Scholar]
- Hopkins R. J., Girardi L. S., Turney E. A. Relationship between Helicobacter pylori eradication and reduced duodenal and gastric ulcer recurrence: a review. Gastroenterology. 1996 Apr;110(4):1244–1252. doi: 10.1053/gast.1996.v110.pm8613015. [DOI] [PubMed] [Google Scholar]
- Krakowka S., Eaton K. A., Rings D. M., Argenzio R. A. Production of gastroesophageal erosions and ulcers (GEU) in gnotobiotic swine monoinfected with fermentative commensal bacteria and fed high-carbohydrate diet. Vet Pathol. 1998 Jul;35(4):274–282. doi: 10.1177/030098589803500406. [DOI] [PubMed] [Google Scholar]
- Lang J., Blikslager A., Regina D., Eisemann J., Argenzio R. Synergistic effect of hydrochloric acid and bile acids on the pars esophageal mucosa of the porcine stomach. Am J Vet Res. 1998 Sep;59(9):1170–1176. [PubMed] [Google Scholar]
- Lawrence B. V., Anderson D. B., Adeola O., Cline T. R. Changes in pars esophageal tissue appearance of the porcine stomach in response to transportation, feed deprivation, and diet composition. J Anim Sci. 1998 Mar;76(3):788–795. doi: 10.2527/1998.763788x. [DOI] [PubMed] [Google Scholar]
- Mackin A. J., Friendship R. M., Wilcock B. P., Ball R. O., Ayles H. L. Development and evaluation of an endoscopic technique permitting rapid visualization of the cardiac region of the porcine stomach. Can J Vet Res. 1997 Apr;61(2):121–127. [PMC free article] [PubMed] [Google Scholar]
- Meining A., Kroher G., Stolte M. Animal reservoirs in the transmission of Helicobacter heilmannii. Results of a questionnaire-based study. Scand J Gastroenterol. 1998 Aug;33(8):795–798. doi: 10.1080/00365529850171422. [DOI] [PubMed] [Google Scholar]
- Mendes E. N., Queiroz D. M., Rocha G. A., Nogueira A. M., Carvalho A. C., Lage A. P., Barbosa A. J. Histopathological study of porcine gastric mucosa with and without a spiral bacterium ("Gastrospirillum suis"). J Med Microbiol. 1991 Dec;35(6):345–348. doi: 10.1099/00222615-35-6-345. [DOI] [PubMed] [Google Scholar]
- Queiroz D. M., Rocha G. A., Mendes E. N., De Moura S. B., De Oliveira A. M., Miranda D. Association between Helicobacter and gastric ulcer disease of the pars esophagea in swine. Gastroenterology. 1996 Jul;111(1):19–27. doi: 10.1053/gast.1996.v111.pm8698198. [DOI] [PubMed] [Google Scholar]
- Unge P. Review of Helicobacter pylori eradication regimens. Scand J Gastroenterol Suppl. 1996;215:74–81. [PubMed] [Google Scholar]
- Verdú E. F., Fraser R., Armstrong D., Blum A. L. Effects of omeprazole and lansoprazole on 24-hour intragastric pH in Helicobacter pylori-positive volunteers. Scand J Gastroenterol. 1994 Dec;29(12):1065–1069. doi: 10.3109/00365529409094889. [DOI] [PubMed] [Google Scholar]
- Yeomans N. D., Kolt S. D. Helicobacter heilmannii (formerly Gastrospirillum): association with pig and human gastric pathology. Gastroenterology. 1996 Jul;111(1):244–247. doi: 10.1053/gast.1996.v111.agast961110244. [DOI] [PubMed] [Google Scholar]


