Abstract
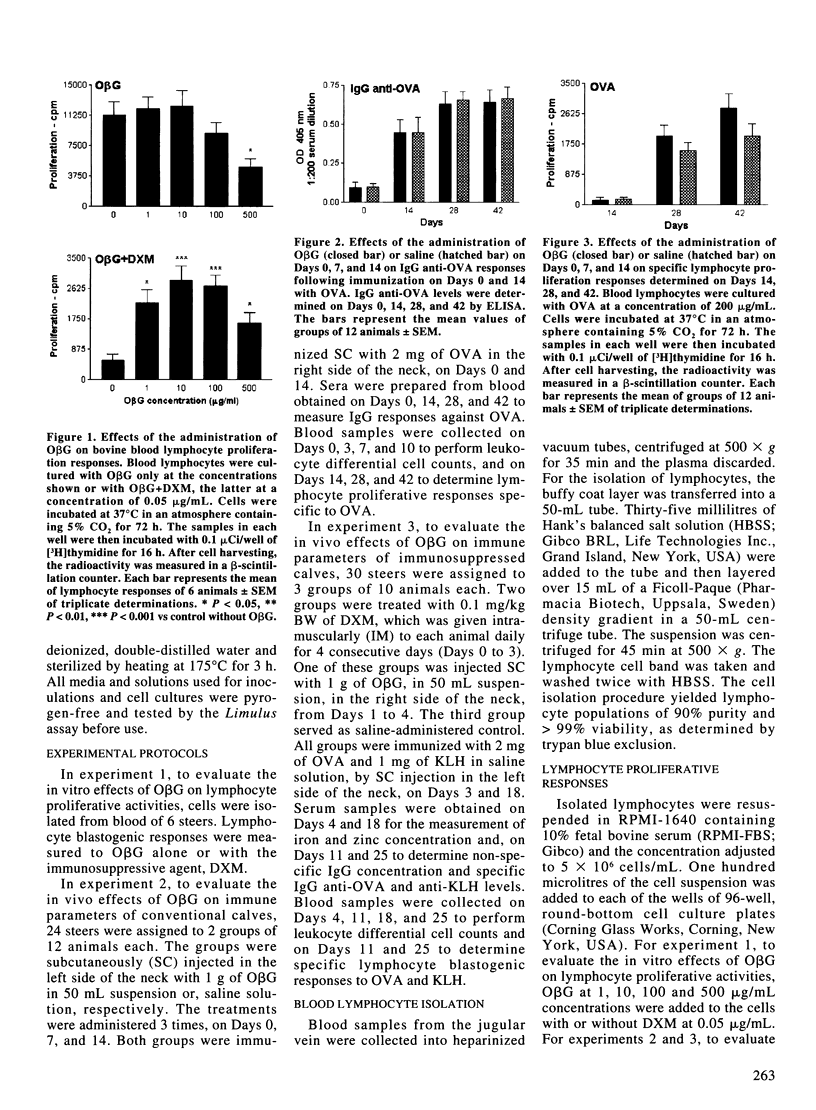
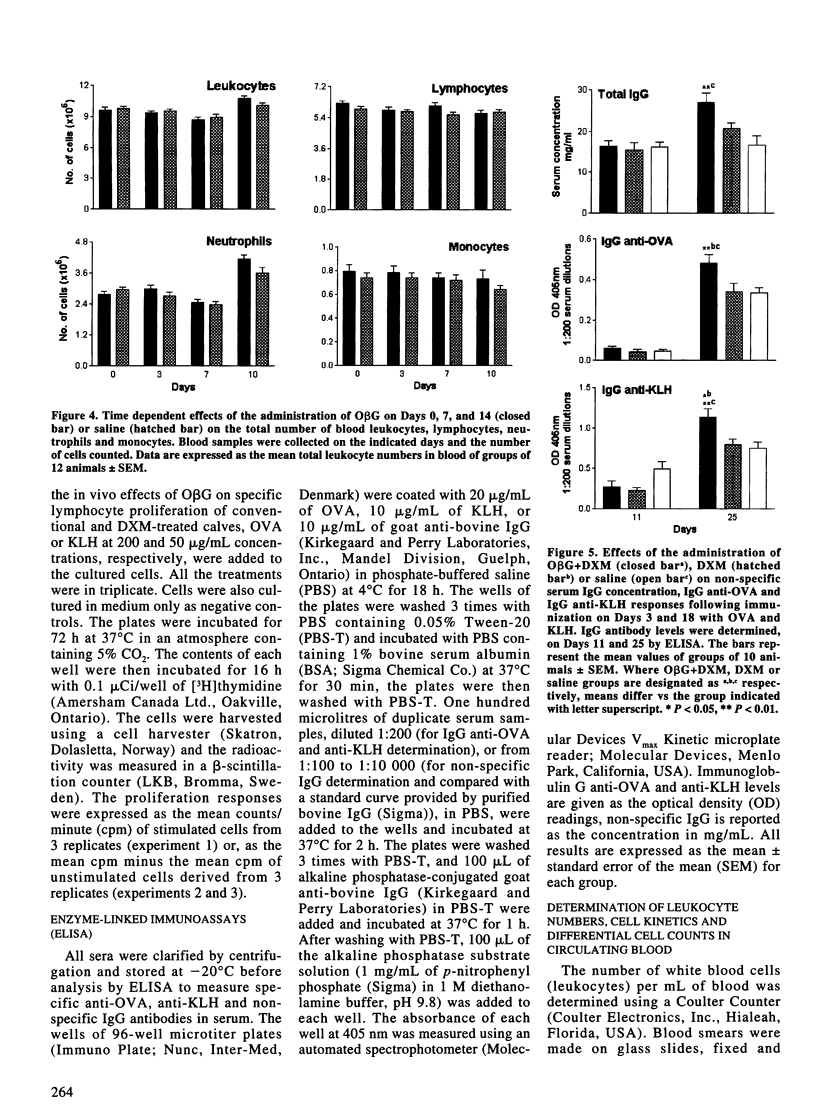
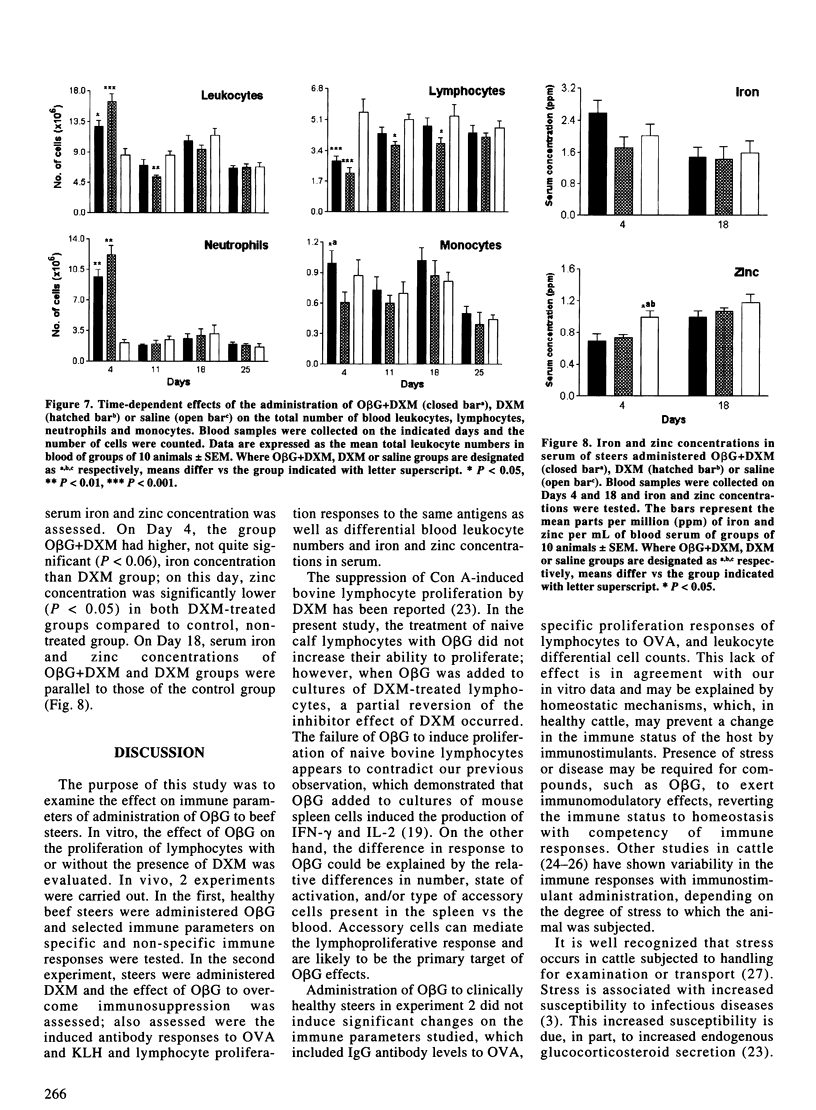
In order to assess the effect of oat beta-glucan (ObetaG) administration on immune parameters of beef steers, 3 experiments were carried out. In experiment 1, the in vitro effect of ObetaG on the proliferation of blood lymphocytes, with or without the presence of dexamethasone (DXM), was evaluated. In experiment 2, groups of 12 healthy steers were administered ObetaG or saline solution and immunized with ovalbumin (OVA). Immune parameters studied included IgG antibody levels to OVA, proliferation responses of blood lymphocytes to OVA, and blood leukocyte differential cell counts. For experiment 3, groups of 10 steers were treated with ObetaG and DXM, DXM only, or saline solution, and immunized with OVA and keyhole limpet hemocyanin (KLH). Serum antibody responses to OVA and KLH, serum IgG concentration levels, blastogenic responses of blood lymphocytes to OVA and KLH, differential blood leukocyte numbers, and iron and zinc concentration in serum were tested to evaluate the effect of ObetaG to overcome immunosuppression. The in vitro treatment of naive blood lymphocytes with ObetaG did not increase their ability to proliferate; however, when ObetaG was added to cultures of DXM-treated lymphocytes, a significant (P < 0.05 to P < 0.001) reversion of the immunosuppressive effect of DXM occurred. Administration of ObetaG to clinically healthy steers did not induce significant changes on any of the immune parameters studied. The administration of ObetaG to DXM-treated steers provoked, on Day 25, a significant increase in IgG anti-OVA (P < 0.01) and anti-KLH (P < 0.05) responses vs the DXM only group. On Day 25, the specific proliferation responses of lymphocytes, to both OVA and KLH, were significantly increased (P < 0.05) in ObetaG+DXM group compared to DXM group. On Day 4, a significant increase in the number of leukocytes (P < 0.01) and neutrophils (P < 0.001), and a significant decrease in the number of monocytes (P < 0.05) were observed in the group treated with DXM only compared to ObetaG+DXM group. No significant differences were observed in iron and zinc concentration between ObetaG+DXM and DXM groups. These results indicated that ObetaG did not influence immune responses of naive cells in vitro or of healthy steers in vivo; however, when cells or animals were treated with DXM, ObetaG significantly restored some of the specific and non-specific immune parameters studied.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. V., Youanes Y. D., Vestweber J. G., King C. A., Klemm R. D., Kennedy G. A. The effects of stressful exercise on leukocytes in cattle with experimental pneumonic pasteurellosis. Vet Res Commun. 1991;15(3):189–204. doi: 10.1007/BF00343224. [DOI] [PubMed] [Google Scholar]
- Blecha F., Anderson G. A., Osorio F., Chapes S. K., Baker P. E. Influence of isoprinosine on bovine herpesvirus type-1 infection in cattle. Vet Immunol Immunopathol. 1987 Jun;15(3):253–265. doi: 10.1016/0165-2427(87)90087-0. [DOI] [PubMed] [Google Scholar]
- Blecha F., Baker P. E. Effect of cortisol in vitro and in vivo on production of bovine interleukin 2. Am J Vet Res. 1986 Apr;47(4):841–845. [PubMed] [Google Scholar]
- Blecha F. Immunomodulation: a means of disease prevention in stressed livestock. J Anim Sci. 1988 Aug;66(8):2084–2090. doi: 10.2527/jas1988.6682084x. [DOI] [PubMed] [Google Scholar]
- Browder I. W., Williams D. L., Kitahama A., Di Luzio N. R. Modification of post-operative C. albicans sepsis by glucan immunostimulation. Int J Immunopharmacol. 1984;6(1):19–26. doi: 10.1016/0192-0561(84)90030-4. [DOI] [PubMed] [Google Scholar]
- Bøgwald J., Johnson E., Hoffman J., Seljelid R. Lysosomal glycosidases in mouse peritoneal macrophages stimulated in vitro with soluble and insoluble glycans. J Leukoc Biol. 1984 Apr;35(4):357–371. doi: 10.1002/jlb.35.4.357. [DOI] [PubMed] [Google Scholar]
- Chiang Y. W., Roth J. A., Andrews J. J. Influence of recombinant bovine interferon gamma and dexamethasone on pneumonia attributable to Haemophilus somnus in calves. Am J Vet Res. 1990 May;51(5):759–762. [PubMed] [Google Scholar]
- Cohn L. A. The influence of corticosteroids on host defense mechanisms. J Vet Intern Med. 1991 Mar-Apr;5(2):95–104. doi: 10.1111/j.1939-1676.1991.tb00939.x. [DOI] [PubMed] [Google Scholar]
- Czop J. K., Austen K. F. A beta-glucan inhibitable receptor on human monocytes: its identity with the phagocytic receptor for particulate activators of the alternative complement pathway. J Immunol. 1985 Apr;134(4):2588–2593. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and the pathogenesis of the acute-phase response. N Engl J Med. 1984 Nov 29;311(22):1413–1418. doi: 10.1056/NEJM198411293112205. [DOI] [PubMed] [Google Scholar]
- Estrada A., Yun C. H., Van Kessel A., Li B., Hauta S., Laarveld B. Immunomodulatory activities of oat beta-glucan in vitro and in vivo. Microbiol Immunol. 1997;41(12):991–998. doi: 10.1111/j.1348-0421.1997.tb01959.x. [DOI] [PubMed] [Google Scholar]
- Filion L. G., Bielefeldt Ohmann H., Owen P. W., Babiuk L. A. Characterization of the bovine secondary in vitro antibody response. Vet Immunol Immunopathol. 1984 Aug;7(1):19–32. doi: 10.1016/0165-2427(84)90024-2. [DOI] [PubMed] [Google Scholar]
- Goldman R., Jaffe C. L. Administration of beta-glucan following Leishmania major infection suppresses disease progression in mice. Parasite Immunol. 1991 Mar;13(2):137–145. doi: 10.1111/j.1365-3024.1991.tb00270.x. [DOI] [PubMed] [Google Scholar]
- González J., Araguth M. F., Yoshida N. Resistance to acute Trypanosoma cruzi infection resulting from immunization of mice with a 90-kilodalton antigen from metacyclic trypomastigotes. Infect Immun. 1991 Mar;59(3):863–867. doi: 10.1128/iai.59.3.863-867.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman O. A., Standing J. E., Limper A. H. Pneumocystis carinii stimulates tumor necrosis factor-alpha release from alveolar macrophages through a beta-glucan-mediated mechanism. J Immunol. 1993 May 1;150(9):3932–3940. [PubMed] [Google Scholar]
- Konopski Z., Rasmussen L. T., Seljelid R., Eskeland T. Phagocytosis of beta-1,3-D-glucan-derivatized microbeads by mouse peritoneal macrophages involves three different receptors. Scand J Immunol. 1991 Mar;33(3):297–306. doi: 10.1111/j.1365-3083.1991.tb01775.x. [DOI] [PubMed] [Google Scholar]
- Kruse D., Cole G. T. A seroreactive 120-kilodalton beta-1,3-glucanase of Coccidioides immitis which may participate in spherule morphogenesis. Infect Immun. 1992 Oct;60(10):4350–4363. doi: 10.1128/iai.60.10.4350-4363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manners D. J., Masson A. J., Patterson J. C. The structure of a beta-(1 leads to 3)-D-glucan from yeast cell walls. Biochem J. 1973 Sep;135(1):19–30. doi: 10.1042/bj1350019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata H., Takahashi H., Matsumoto H. The effects of road transportation on peripheral blood lymphocyte subpopulations, lymphocyte blastogenesis and neutrophil function in calves. Br Vet J. 1987 Mar-Apr;143(2):166–174. doi: 10.1016/0007-1935(87)90008-X. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Cisneros R. L., Hinkson P., Ostroff G. Anti-infective effect of poly-beta 1-6-glucotriosyl-beta 1-3-glucopyranose glucan in vivo. Infect Immun. 1992 Apr;60(4):1642–1647. doi: 10.1128/iai.60.4.1642-1647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pighetti G. M., Sordillo L. M. Specific immune responses of dairy cattle after primary inoculation with recombinant bovine interferon-gamma as an adjuvant when vaccinating against mastitis. Am J Vet Res. 1996 Jun;57(6):819–824. [PubMed] [Google Scholar]
- Pruett J. H., Fisher W. F., DeLoach J. R. Effects of dexamethasone on selected parameters of the bovine immune system. Vet Res Commun. 1987;11(4):305–323. doi: 10.1007/BF00346190. [DOI] [PubMed] [Google Scholar]
- Quinn P. J. Mechanisms of action of some immunomodulators used in veterinary medicine. Adv Vet Sci Comp Med. 1990;35:43–99. doi: 10.1016/b978-0-12-039235-3.50009-5. [DOI] [PubMed] [Google Scholar]
- Rasmussen L. T., Lipsky P. E., Seljelid R. Production of prostaglandin E2 and interleukin 1 by mouse peritoneal macrophages stimulated with beta-1,3-D-glucan derivatized plastic beads. Scand J Immunol. 1987 Dec;26(6):731–736. doi: 10.1111/j.1365-3083.1987.tb02310.x. [DOI] [PubMed] [Google Scholar]
- Roth J. A., Abruzzini A. F., Frank D. E. Influence of recombinant human interleukin-2 administration on lymphocyte and neutrophil function in clinically normal and dexamethasone-treated cattle. Am J Vet Res. 1990 Apr;51(4):546–549. [PubMed] [Google Scholar]
- Roth J. A., Kaeberle M. L. Effect of levamisole on lymphocyte blastogenesis and neutrophil function in dexamethasone-treated cattle. Am J Vet Res. 1984 Sep;45(9):1781–1784. [PubMed] [Google Scholar]
- Roth J. A., Kaeberle M. L. Enhancement of lymphocyte blastogenesis and neutrophil function by avridine in dexamethasone-treated and nontreated cattle. Am J Vet Res. 1985 Jan;46(1):53–57. [PubMed] [Google Scholar]
- Seljelid R., Rasmussen L. T., Larm O., Hoffman J. The protective effect of beta 1-3D-glucan-derivatized plastic beads against Escherichia coli infection in mice. Scand J Immunol. 1987 Jan;25(1):55–60. doi: 10.1111/j.1365-3083.1987.tb01046.x. [DOI] [PubMed] [Google Scholar]
- Suda M., Ohno N., Adachi Y., Yadomae T. Tissue distribution of intraperitoneally administered (1-->3)-beta-D-glucan (SSG), a highly branched antitumor glucan, in mice. J Pharmacobiodyn. 1992 Aug;15(8):417–426. doi: 10.1248/bpb1978.15.417. [DOI] [PubMed] [Google Scholar]
- Vácha J., Znojil V., Pospísil M., Holá J., Pipalová I. Microcytic anemia and changes in ferrokinetics as late after-effects of glucan administration in murine hepatitis virus-infected C57BL/10ScSnPh mice. Int J Immunopharmacol. 1994 Jan;16(1):51–60. doi: 10.1016/0192-0561(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Weeks B. R., Smith J. E., DeBowes R. M., Smith J. M. Decreased serum iron and zinc concentrations in cattle receiving intravenous dexamethasone. Vet Pathol. 1989 Jul;26(4):345–346. doi: 10.1177/030098588902600412. [DOI] [PubMed] [Google Scholar]
- Williams D. L., Di Luzio N. R. Glucan-induced modification of murine viral hepatitis. Science. 1980 Apr 4;208(4439):67–69. doi: 10.1126/science.7361108. [DOI] [PubMed] [Google Scholar]
- Yun C. H., Estrada A., Van Kessel A., Gajadhar A. A., Redmond M. J., Laarveld B. beta-(1-->3, 1-->4) oat glucan enhances resistance to Eimeria vermiformis infection in immunosuppressed mice. Int J Parasitol. 1997 Mar;27(3):329–337. doi: 10.1016/s0020-7519(96)00178-6. [DOI] [PubMed] [Google Scholar]
- Yun C. H., Estrada A., Van Kessel A., Gajadhar A., Redmond M., Laarveld B. Immunomodulatory effects of oat beta-glucan administered intragastrically or parenterally on mice infected with Eimeria vermiformis. Microbiol Immunol. 1998;42(6):457–465. doi: 10.1111/j.1348-0421.1998.tb02309.x. [DOI] [PubMed] [Google Scholar]


