Abstract
A nested reverse transcription (RT) polymerase chain reaction (PCR) assay was evaluated for differentiating reference bovine viral diarrhea virus (BVDV) strains, BVDV from diagnostic accessions, modified-live virus (MLV) BVDV strains in bovine viral vaccines, and a reference border disease virus (BDV). The detection level of this assay was compared to viral infection in cell culture. The PCR assay was used to distinguish 3 ruminant pestiviruses, types 1 and 2 BVDV, and type 3 BDV. The consensus (first) PCR assay detected all 3 ruminant pestiviruses, a result of the shared sequence homology. The consensus PCR product was subjected to a second (nested) PCR which used type-specific primers. The nested PCR was able to differentiate the 3 ruminant pestiviruses. Viral stocks of BVDV were diluted 10-fold and processed for the 2-step PCR assay. The sensitivity of this 2-step PCR assay was compared to viral infectivity in cell culture based on identical volumes of the system tested (cell culture assay and processing for RNA). The RT-PCR type-specific assay differentiated BVDV laboratory reference strains (12), diagnostic laboratory isolates (15), 2 MLV BVDV vaccine strains, and a BDV strain. The 30 ruminant pestiviruses typed included: (1) 27 reference strains and diagnostic laboratory isolates; 18 cytopathic (CP) type 1 strains, 3 CP type 2 strains, 3 noncytopathic (NCP) type 1 strains, and 3 NCP type 2 strains; (2) 2 MLV strains, type 1; and (3) 1 CP BDV type 3. The PCR assay had a detection limit of 10 TCID50/0.025 mL of virus when 3 separate BVDV were tested. This 2 step RT-PCR assay would be useful for the typing of ruminant pestiviruses, particularly BVDV isolates from the diagnostic laboratory.
Full text
PDF




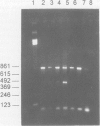
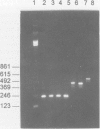
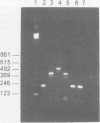
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alansari H., Brock K. V., Potgieter L. N. Single and double polymerase chain reaction for detection of bovine viral diarrhea virus in tissue culture and sera. J Vet Diagn Invest. 1993 Apr;5(2):148–153. doi: 10.1177/104063879300500201. [DOI] [PubMed] [Google Scholar]
- Baker J. C. The clinical manifestations of bovine viral diarrhea infection. Vet Clin North Am Food Anim Pract. 1995 Nov;11(3):425–445. doi: 10.1016/s0749-0720(15)30460-6. [DOI] [PubMed] [Google Scholar]
- Bolin S. R., Ridpath J. F. Prevalence of bovine viral diarrhea virus genotypes and antibody against those viral genotypes in fetal bovine serum. J Vet Diagn Invest. 1998 Apr;10(2):135–139. doi: 10.1177/104063879801000203. [DOI] [PubMed] [Google Scholar]
- Brock K. V. Detection of persistent bovine viral diarrhea virus infections by DNA hybridization and polymerase chain reaction assay. Arch Virol Suppl. 1991;3:199–208. doi: 10.1007/978-3-7091-9153-8_24. [DOI] [PubMed] [Google Scholar]
- Brock K. V. Diagnosis of bovine viral diarrhea virus infections. Vet Clin North Am Food Anim Pract. 1995 Nov;11(3):549–561. doi: 10.1016/s0749-0720(15)30466-7. [DOI] [PubMed] [Google Scholar]
- Fulton R. W., Confer A. W., Burge L. J., Perino L. J., d'Offay J. M., Payton M. E., Mock R. E. Antibody responses by cattle after vaccination with commercial viral vaccines containing bovine herpesvirus-1, bovine viral diarrhea virus, parainfluenza-3 virus, and bovine respiratory syncytial virus immunogens and subsequent revaccination at day 140. Vaccine. 1995;13(8):725–733. doi: 10.1016/0264-410x(94)00072-u. [DOI] [PubMed] [Google Scholar]
- Fulton R. W., Saliki J. T., Burge L. J., d'Offay J. M., Bolin S. R., Maes R. K., Baker J. C., Frey M. L. Neutralizing antibodies to type 1 and 2 bovine viral diarrhea viruses: detection by inhibition of viral cytopathology and infectivity by immunoperoxidase assay. Clin Diagn Lab Immunol. 1997 May;4(3):380–383. doi: 10.1128/cdli.4.3.380-383.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel A. L., Wasylyshen M. D., Nayar G. P. Rapid detection of bovine viral diarrhea virus by using RNA extracted directly from assorted specimens and a one-tube reverse transcription PCR assay. J Clin Microbiol. 1995 Feb;33(2):287–291. doi: 10.1128/jcm.33.2.287-291.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooft van Iddekinge B. J., van Wamel J. L., van Gennip H. G., Moormann R. J. Application of the polymerase chain reaction to the detection of bovine viral diarrhea virus infections in cattle. Vet Microbiol. 1992 Jan;30(1):21–34. doi: 10.1016/0378-1135(92)90091-7. [DOI] [PubMed] [Google Scholar]
- Hyndman L., Vilcek S., Conner J., Nettleton P. F. A novel nested reverse transcription PCR detects bovine viral diarrhoea virus in fluids from aborted bovine fetuses. J Virol Methods. 1998 Mar;71(1):69–76. doi: 10.1016/s0166-0934(97)00206-1. [DOI] [PubMed] [Google Scholar]
- Laamanen U. I., Neuvonen E. P., Yliviuhkola E. M., Veijalainen P. M. Comparison of RT-PCR assay and virus isolation in cell cultures for the detection of bovine viral diarrhoea virus (BVDV) in field samples. Res Vet Sci. 1997 Nov-Dec;63(3):199–203. doi: 10.1016/s0034-5288(97)90020-5. [DOI] [PubMed] [Google Scholar]
- Lopez O. J., Osorio F. A., Donis R. O. Rapid detection of bovine viral diarrhea virus by polymerase chain reaction. J Clin Microbiol. 1991 Mar;29(3):578–582. doi: 10.1128/jcm.29.3.578-582.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClurkin A. W., Littledike E. T., Cutlip R. C., Frank G. H., Coria M. F., Bolin S. R. Production of cattle immunotolerant to bovine viral diarrhea virus. Can J Comp Med. 1984 Apr;48(2):156–161. [PMC free article] [PubMed] [Google Scholar]
- Pellerin C., van den Hurk J., Lecomte J., Tijssen P. Identification of a new group of bovine viral diarrhea virus strains associated with severe outbreaks and high mortalities. Virology. 1994 Sep;203(2):260–268. doi: 10.1006/viro.1994.1483. [DOI] [PubMed] [Google Scholar]
- Radwan G. S., Brock K. V., Hogan J. S., Smith K. L. Development of a PCR amplification assay as a screening test using bulk milk samples for identifying dairy herds infected with bovine viral diarrhea virus. Vet Microbiol. 1995 Apr;44(1):77–91. doi: 10.1016/0378-1135(94)00121-c. [DOI] [PubMed] [Google Scholar]
- Ridpath J. F., Bolin S. R. Differentiation of types 1a, 1b and 2 bovine viral diarrhoea virus (BVDV) by PCR. Mol Cell Probes. 1998 Apr;12(2):101–106. doi: 10.1006/mcpr.1998.0158. [DOI] [PubMed] [Google Scholar]
- Ridpath J. F., Bolin S. R., Dubovi E. J. Segregation of bovine viral diarrhea virus into genotypes. Virology. 1994 Nov 15;205(1):66–74. doi: 10.1006/viro.1994.1620. [DOI] [PubMed] [Google Scholar]
- Ridpath J. F., Bolin S. R., Katz J. Comparison of nucleic acid hybridization and nucleic acid amplification using conserved sequences from the 5' noncoding region for detection of bovine viral diarrhea virus. J Clin Microbiol. 1993 Apr;31(4):986–989. doi: 10.1128/jcm.31.4.986-989.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliki J. T., Fulton R. W., Hull S. R., Dubovi E. J. Microtiter virus isolation and enzyme immunoassays for detection of bovine viral diarrhea virus in cattle serum. J Clin Microbiol. 1997 Apr;35(4):803–807. doi: 10.1128/jcm.35.4.803-807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan D. G., Akkina R. K. A nested polymerase chain reaction assay to differentiate pestiviruses. Virus Res. 1995 Oct;38(2-3):231–239. doi: 10.1016/0168-1702(95)00065-x. [DOI] [PubMed] [Google Scholar]