Abstract
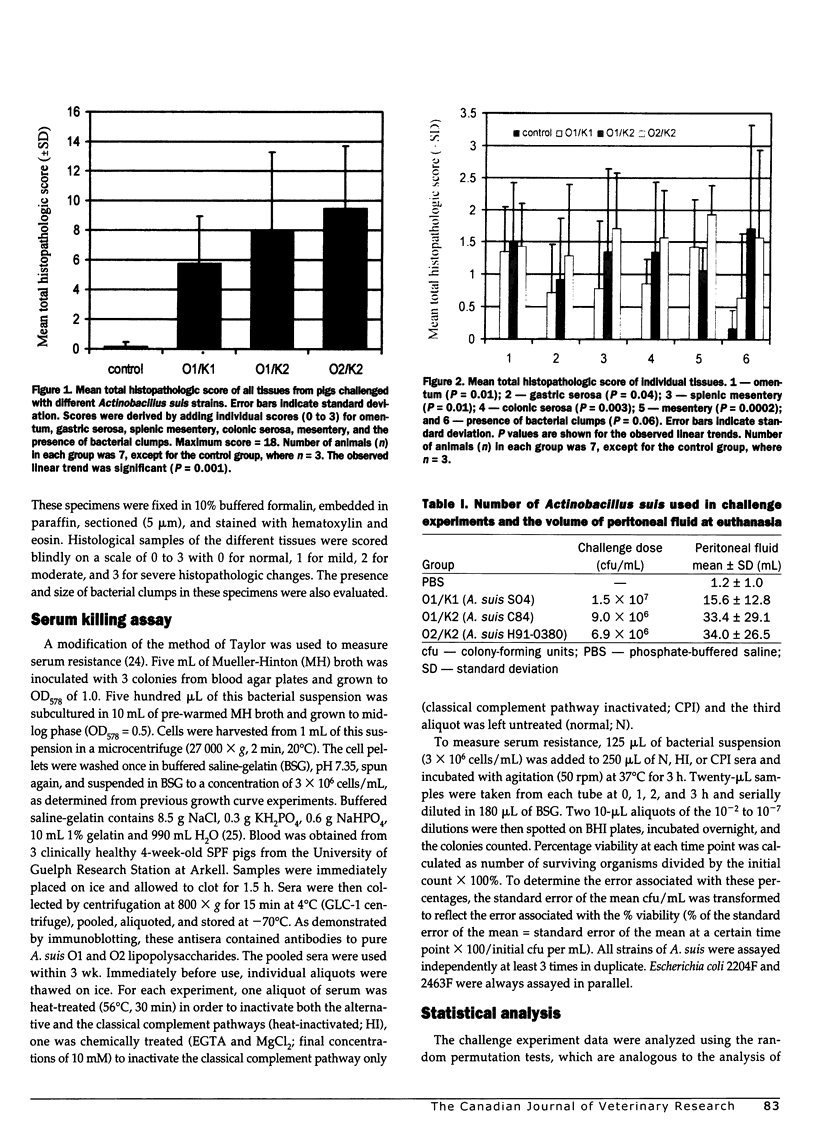
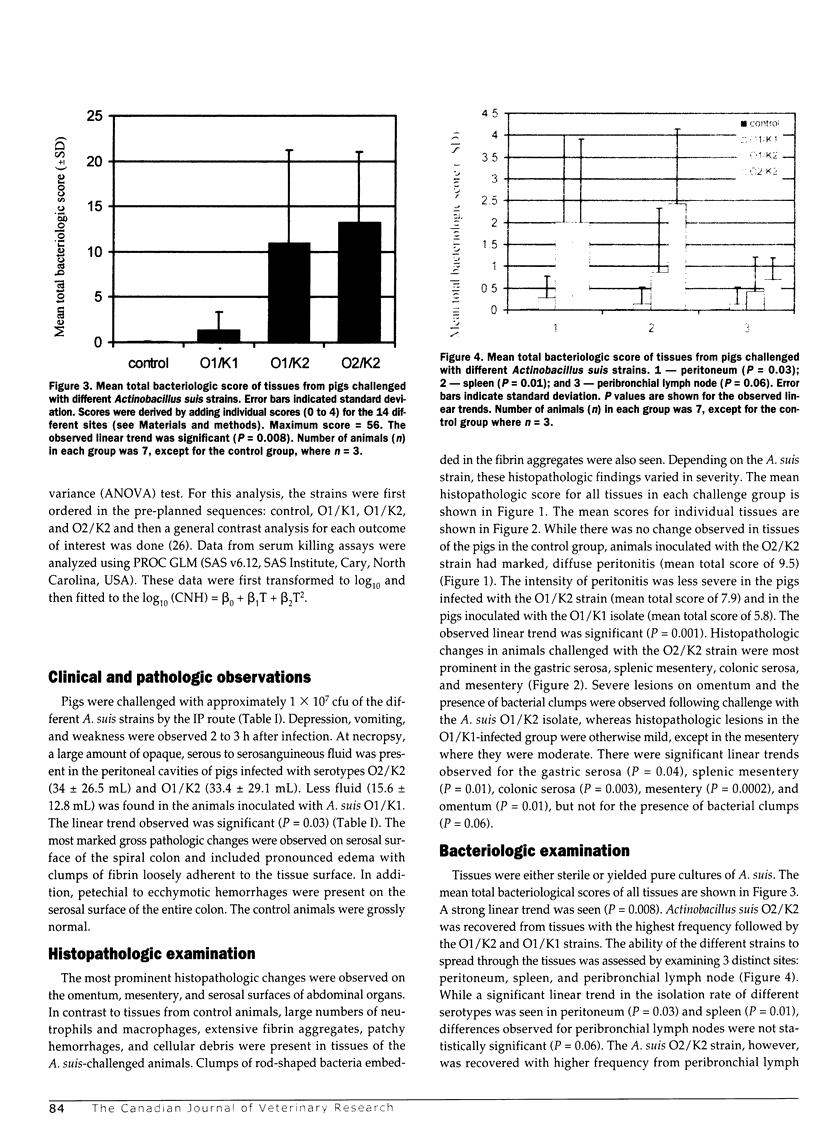
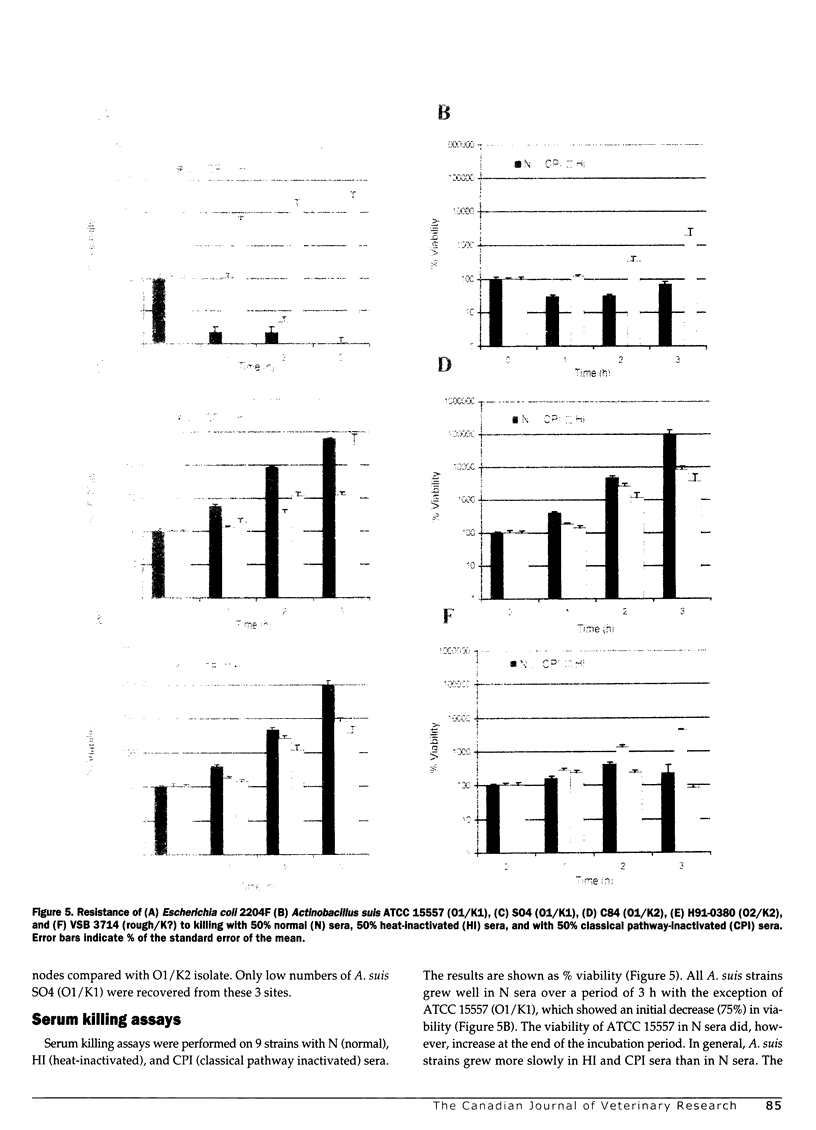
The pathogenicity of Actinobacillus suis serotypes O1/K1 (strain SO4), O1/K2 (strain C84), and O2/K2 (strain H91-0380) was evaluated in specific-pathogen-free (SPF) piglets challenged by intraperitoneal inoculation with approximately 1 x 10(7) colony-forming units per mL. All 3 strains produced peritonitis, but differences were observed in the composite histopathologic scores (P = 0.001) and in their ability to spread (P = 0.008) at 7 h post challenge. The O2/K2 strain caused the most severe peritonitis and disseminated most widely to other tissues. Moderate lesions were seen with the O1/K2 strain while the O1/K1 strain caused mild lesions and remained largely localized to the peritoneum. In an attempt to explain the basis of observed differences, the serum sensitivity of 9 A. suis strains with different O and K types was assessed. Regardless of the O/K type, all of the isolates tested were serum resistant. Moreover, most A. suis isolates grew as well or better in complement-replete sera as they did in complement-depleted sera. These observations indicate that although 02 and K2 strains had a greater propensity to cause a disseminating septic inflammatory response in pigs, they were no more resistant to complement-mediated killing than O1 strains.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bada R., Mittal K. R., Higgins R. Biochemical and antigenic relationships between porcine and equine isolates of Actinobacillus suis. Vet Microbiol. 1996 Aug;51(3-4):393–396. doi: 10.1016/0378-1135(96)00040-5. [DOI] [PubMed] [Google Scholar]
- Bossé J. T., Johnson R. P., Nemec M., Rosendal S. Protective local and systemic antibody responses of swine exposed to an aerosol of Actinobacillus pleuropneumoniae serotype 1. Infect Immun. 1992 Feb;60(2):479–484. doi: 10.1128/iai.60.2.479-484.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns S. M., Hull S. I. Comparison of loss of serum resistance by defined lipopolysaccharide mutants and an acapsular mutant of uropathogenic Escherichia coli O75:K5. Infect Immun. 1998 Sep;66(9):4244–4253. doi: 10.1128/iai.66.9.4244-4253.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Abraham E. Microbiologic findings and correlations with serum tumor necrosis factor-alpha in patients with severe sepsis and septic shock. J Infect Dis. 1999 Jul;180(1):116–121. doi: 10.1086/314839. [DOI] [PubMed] [Google Scholar]
- Cross-Canada disease report. Can Vet J. 1987 Oct;28(10):654–654. [PMC free article] [PubMed] [Google Scholar]
- Furesz S. E., Mallard B. A., Bossé J. T., Rosendal S., Wilkie B. N., MacInnes J. I. Antibody- and cell-mediated immune responses of Actinobacillus pleuropneumoniae-infected and bacterin-vaccinated pigs. Infect Immun. 1997 Feb;65(2):358–365. doi: 10.1128/iai.65.2.358-365.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman N., Leive L. Complement activation via the alternative pathway by purified Salmonella lipopolysaccharide is affected by its structure but not its O-antigen length. J Immunol. 1984 Jan;132(1):376–385. [PubMed] [Google Scholar]
- Hansen D. S., Mestre F., Alberti S., Hernández-Allés S., Alvarez D., Doménech-Sánchez A., Gil J., Merino S., Tomás J. M., Benedí V. J. Klebsiella pneumoniae lipopolysaccharide O typing: revision of prototype strains and O-group distribution among clinical isolates from different sources and countries. J Clin Microbiol. 1999 Jan;37(1):56–62. doi: 10.1128/jcm.37.1.56-62.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leying H., Suerbaum S., Kroll H. P., Stahl D., Opferkuch W. The capsular polysaccharide is a major determinant of serum resistance in K-1-positive blood culture isolates of Escherichia coli. Infect Immun. 1990 Jan;58(1):222–227. doi: 10.1128/iai.58.1.222-227.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier M., Sdicu A. M., Shahinian S., Bussey H. The Candida albicans KRE9 gene is required for cell wall beta-1, 6-glucan synthesis and is essential for growth on glucose. Proc Natl Acad Sci U S A. 1998 Aug 18;95(17):9825–9830. doi: 10.1073/pnas.95.17.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacInnes J. I., Rosendal S. Analysis of major antigens of Haemophilus (Actinobacillus) pleuropneumoniae and related organisms. Infect Immun. 1987 Jul;55(7):1626–1634. doi: 10.1128/iai.55.7.1626-1634.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair N. S., Randall C. J., Thomas G. W., Harbourne J. F., McCrea C. T., Cowl K. P. Actinobacillus suis infection in pigs. A report of four outbreaks and two sporadic cases. J Comp Pathol. 1974 Jan;84(1):113–119. doi: 10.1016/0021-9975(74)90033-4. [DOI] [PubMed] [Google Scholar]
- Miniats O. P., Spinato M. T., Sanford S. E. Actinobacillus suis septicemia in mature swine: two outbreaks resembling erysipelas. Can Vet J. 1989 Dec;30(12):943–947. [PMC free article] [PubMed] [Google Scholar]
- Mio T., Yamada-Okabe T., Yabe T., Nakajima T., Arisawa M., Yamada-Okabe H. Isolation of the Candida albicans homologs of Saccharomyces cerevisiae KRE6 and SKN1: expression and physiological function. J Bacteriol. 1997 Apr;179(7):2363–2372. doi: 10.1128/jb.179.7.2363-2372.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutharia L. M., Amor P. A. Monoclonal antibodies against Vibrio anguillarum O2 and Vibrio ordalii identify antigenic differences in lipopolysaccharide O-antigens. FEMS Microbiol Lett. 1994 Nov 1;123(3):289–298. doi: 10.1111/j.1574-6968.1994.tb07238.x. [DOI] [PubMed] [Google Scholar]
- Russo T. A., Moffitt M. C., Hammer C. H., Frank M. M. TnphoA-mediated disruption of K54 capsular polysaccharide genes in Escherichia coli confers serum sensitivity. Infect Immun. 1993 Aug;61(8):3578–3582. doi: 10.1128/iai.61.8.3578-3582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rycroft A. N., Cullen J. M. Complement resistance in Actinobacillus (Haemophilus) pleuropneumoniae infection of swine. Am J Vet Res. 1990 Sep;51(9):1449–1453. [PubMed] [Google Scholar]
- Sanford S. E., Josephson G. K., Rehmtulla A. J., Tilker A. M. Actinobacillus suis infection in pigs in southwestern Ontario. Can Vet J. 1990 Jun;31(6):443–447. [PMC free article] [PubMed] [Google Scholar]
- Sanford S. E., Miniats O. P. Ontario. Actinobacillus suis septicemia mimicking erysipelas in sows. Can Vet J. 1988 Jul;29(7):595–595. [PMC free article] [PubMed] [Google Scholar]
- Staats J. J., Feder I., Okwumabua O., Chengappa M. M. Streptococcus suis: past and present. Vet Res Commun. 1997 Aug;21(6):381–407. doi: 10.1023/a:1005870317757. [DOI] [PubMed] [Google Scholar]
- Van Ostaaijen J., Frey J., Rosendal S., MacInnes J. I. Actinobacillus suis strains isolated from healthy and diseased swine are clonal and carry apxICABDvar. suis and apxIICAvar. suis toxin genes. J Clin Microbiol. 1997 May;35(5):1131–1137. doi: 10.1128/jcm.35.5.1131-1137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C. K., Inzana T. J. Resistance of Actinobacillus pleuropneumoniae to bactericidal antibody and complement is mediated by capsular polysaccharide and blocking antibody specific for lipopolysaccharide. J Immunol. 1994 Sep 1;153(5):2110–2121. [PubMed] [Google Scholar]
- Yaeger M. J. An outbreak of Actinobacillus suis septicemia in grow/finish pigs. J Vet Diagn Invest. 1996 Jul;8(3):381–383. doi: 10.1177/104063879600800318. [DOI] [PubMed] [Google Scholar]