Abstract
Sepsis‐associated acute lung injury (ALI) is a life‐threatening condition in intensive care units with high mortality. LncRNAs have been confirmed to participate in the underlying pathogenesis of septic ALI. This study investigated the biological functions of lncRNA CDKN2B‐AS1 in septic ALI and its potential mechanism.BEAS‐2B cells were challenged with lipopolysaccharide (LPS) and mice were subjected to caecal ligation and puncture (CLP) to induce septic ALI in vitro and in vivo. The expression levels of CDKN2B‐AS1, LIN28B, HIF‐1α, and pyroptosis‐related molecules were assessed by qRT–PCR or Western blotting. The production of IL‐1β and IL‐18 was detected by ELISA. BEAS‐2B cell pyroptosis was examined by flow cytometry. The interaction between LIN28B and CDKN2B‐AS1/HIF‐1α was validated by RIP and RNA pull‐down assays. Colocalization of CDKN2B‐AS1 and LIN28B was observed by FISH. ALI was determined by HE staining, the lung wet‐to‐dry (W/D) weight ratio, inflammatory cell numbers, and total protein concentration in bronchoalveolar lavage fluid (BALF). Caspase‐1 expression in the lung tissues was examined by immunohistochemical staining.CDKN2B‐AS1 was upregulated in BEAS‐2B cells after LPS stimulation. CDKN2B‐AS1 knockdown inhibited pyroptosis in LPS‐exposed BEAS‐2B cells in vitro and the lung tissues of septic mice in vivo. Mechanistically, CDKN2B‐AS1 interacted with LIN28B to enhance HIF‐1α stability. Rescue experiments showed that HIF‐1α overexpression counteracted the inhibitory effect of sh‐CDKN2B‐AS1 on LPS‐induced pyroptosis. CDKN2B‐AS1 bound to LIN28B to trigger NLRP3‐mediated pyroptosis by stabilizing HIF‐1α, which promoted sepsis‐induced ALI. CDKN2B‐AS1 might be a novel therapeutic target for this disease.
Keywords: CDKN2B‐AS1, HIF‐1α, LIN28B, pyroptosis, Sepsis‐induced acute lung injury
Abbreviations
- ALI
acute lung injury
- BALF
bronchoalveolar lavage fluid
- CDKN2B‐AS1
cyclin‐dependent kinase inhibitor 2B antisense RNA 1
- CLP
caecal ligation and puncture
- ELISA
enzyme‐linked immunosorbent assay
- FISH
fluorescence in situ hybridization
- HE
hematoxylin‐eosin
- HIF‐1α
hypoxia inducible factor 1α
- LIN28B
lin‐28 homologue B
- lncRNAs
long noncoding RNAs
- LPS
lipopolysaccharide
- NLRP3
nod‐like receptor protein 3
- PI
prodium iodide
- qRT–PCR
quantitative reverse transcription PCR
- RBPs
RNA binding proteins
- RIP
RNA immunoprecipitation
- shCDKN2B‐AS1
small hairpin RNA targeting CDKN2B‐AS1
- shNC
negative control shRNA
- SD
standard deviation
- W/D
wet‐to‐dry
1. INTRODUCTION
Sepsis is a systemic inflammatory response caused by bacterial infection, which has a high mortality. 1 Without prompt and proper treatments, sepsis quickly leads to tissue damage, multiple organ failure, and death. 2 As a critical complication of sepsis, acute lung injury (ALI) is a pivotal contributor to acute respiratory failure. 3 ALI has a high mortality rate of 35%–40%, and the physical functions of survivors may be irreversibly impaired. 4 Pyroptosis is an important type of programmed cell death, characterized by the production of proinflammatory cytokines including IL‐18 and IL‐1β. The activation of nod‐like receptor protein 3 (NLRP3) inflammasome and Caspase1 can trigger pyroptosis by enhancing the production of IL‐1β and IL‐18. 5 Accumulating evidence has indicated that pyroptosis plays a particularly important role in the progression of septic ALI and can be a potential therapeutic target for ALI. For example, dihydromyricetin treatment relieves septic ALI via inhibiting NLRP3‐mediated pyroptosis. 6 Thus, uncovering the modulatory mechanism of pyroptosis may be helpful for the development of effective interventions for septic ALI.
Long noncoding RNAs (lncRNAs) are a type of non‐coding RNAs with a length of over 200 nucleotides. Currently, the regulatory roles of lncRNAs in ALI have been reported. SNHG16 silencing protects against sepsis‐induced ALI via modulating the miR‐128‐3p/HMGB3 pathway. 7 Hong et al. documented that SNHG14 knockdown inhibited LPS‐induced ALI through regulating the miR‐223‐3p/Foxo3a axis to repress autophagy. 8 Cyclin‐dependent kinase inhibitor 2B antisense RNA 1 (CDKN2B‐AS1) is a novel lncRNA, was found to be highly expressed in patients with sepsis. 9 Wang et al. suggested that the upregulation of CDKN2B‐AS1 resulted in lung epithelial cell apoptosis and inflammation through the miR‐140‐5p/TGFBR2/Smad3 pathway. 10 Up‐to‐date, the biological function of CDKN2B‐AS1 in pyroptosis during septic ALI development, as well as the potential mechanisms have not been clarified.
LncRNAs can affect the stability of mRNAs via the interaction with RNA binding proteins (RBPs). For instance, lncRNA KCNQ1OT1 contributes to the development of acute myeloid leukemia by interacting with FUS to enhance the stability of MAP3K1 mRNA. 11 Lin‐28 homologue B (LIN28B) is an RBP, and aberrant high expression of LIN28B in LPS‐exposed cardiomyocytes can accelerate septic cardiomyopathy development via increasing PDCD4 mRNA stability. 12 Interestingly, the StarBase database predicted that CDKN2B‐AS1 could bind to LIN28B. However, whether LIN28B participates in CDKN2B‐AS1‐mediated septic ALI remains unclear. Hypoxia inducible factor 1α (HIF‐1α) is an important transcription factor, and its abnormal activation in sepsis‐induced ALI was responsible for inflammatory lung injury. 13 Previous studies have demonstrated that HIF‐1α inhibition protected against endotoxin‐induced ALI. 14 , 15 Notably, HIF‐1α was found to play pivotal roles in NLRP3 inflammasome‐mediated pyroptosis in traumatic brain injury. 16 The RNA‐Protein Interaction Prediction (RPISeq)predicted that, HIF‐1α was a target gene of LIN28B.
Therefore, we hypothesized that CDKN2B‐AS1 might enhance HIF‐1α stability via the interaction with LIN28B, which subsequently triggering NLRP3‐mediated pyroptosis to exacerbate septic ALI. This study aimed to verify this hypothesis, which elucidated the potential mechanism of CDKN2B‐AS1 in pyroptosis during septic ALI. Our findings provide theoretical basis for CDKN2B‐AS1 inhibition as a therapeutic strategy for septic ALI.
2. MATERIALS AND METHODS
2.1. Cell culture and treatment
The BEAS‐2B human lung epithelial cell line (American Type Culture Collection, USA) was maintained in DMEM (Gibco, USA) containing 10% FBS (Gibco) at 37°C with 5% CO2. BEAS‐2B cells were treated with various concentrations (2.5, 5, and 10 μg/mL) of lipopolysaccharide (LPS, Sigma–Aldrich, USA) for 24 h. To inhibit NLRP3‐mediated inflammasome activation, LPS‐induced BEAS‐2B cells were treated with 10 μM MCC950 (an inhibitor of NLRP3, Sigma–Aldrich) for another 48 h.
2.2. Cell transfection
Small hairpin RNA targeting CDKN2B‐AS1 (sh‐CDKN2B‐AS1), sh‐LIN28B, and negative control shRNA (sh‐NC) were provided by GenePharma (Shanghai, China). The full‐length sequences of HIF‐1α cDNA were inserted into the pcDNA3.1 vector to establish HIF‐1α expression plasmid. BEAS‐2B cells were transfected with the shRNAs or plasmids using the Lipofectamine 2000 (Thermo Fisher, USA).
2.3. Quantitative reverse transcription PCR (qRT–PCR)
Total RNA was extracted from BEAS‐2B cells or lung tissues using the TRIzol reagent (Thermo Fisher). cDNA was synthesized by reverse transcription using the PrimeScript™ RT Reagent kit (Thermo Fisher). Subsequently, real‐time quantitative PCR was performed using SYBR® Green Real‐time PCR Master Mix (Toyobo, Japan). The relative gene levels normalized to GAPDH and analyzed using the 2−ΔΔCT method. The primer sequences are listed in Table 1.
TABLE 1.
Oligonucleotide primer sets for qPCR.
| Name | Sequence (5′¬3′) | Length |
|---|---|---|
| CDKN2B‐AS1 F | GCCGCTCCGCTCCTCTTCTAG | 21 |
| CDKN2B‐AS1 R | CGTGTCCAGATGTCGCGTCAG | 21 |
| LIN28B F | GCTAGCAAAGGTGGTGGAGA | 20 |
| LIN28B R | CAAATCCCATGCGCACA | 17 |
| HIF‐1α F | GGACAAGTCACCACAGGACA | 20 |
| HIF‐1α R | GGGAGAAAATCAAGTCGTGC | 20 |
| GAPDH F | GCACCGTCAAGGCTGAGAAC | 20 |
| GAPDH R | TGGTGAAGACGCCAGTGGA | 19 |
2.4. Western blotting
The cells or lung tissue were treated with the RIPA lysis buffer (Beyotime, Haimen, China) to isolate total protein. The protein samples were separated by polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes. Then, the membranes were blocked in skim milk for 1 h. The membranes were incubated with the primary antibodies against NLRP3 (ab263899, 1:1000, Abcam, UK), cleaved Caspase1 (bs‐0169R, 1:500, Bioss, Beijing, China), ASC (bs‐6741R, 1:500, Bioss), IL‐1β (bs‐0812R, 1:500, Bioss), IL‐18 (bs‐55111R, 1:1000, Bioss), GSDMD‐N (A20197, 1:500, ABclonal, Wuhan, China), LIN28B (ab191881, 1:2000, Abcam), HIF‐1α (bs‐20399R, 1:1000, Bioss), β‐actin (bs‐0061R, 1:5000, Bioss) at 4°C overnight. After the blots were incubated with the secondary antibody, the protein bands were visualized by the ECL chromogenic agent (Millipore, USA).
2.5. CCK‐8 assay
BEAS‐2B cells were seeded into the 96‐well plates. The next day, the adherent cells were added with 10 μL of the CCK‐8 solution (Solarbio, Beijing, China). After being incubated at 37°C for 2 h, the absorbance at 450 nm was detected with a microplate reader (Bio Tek, USA).
2.6. Enzyme‐linked immunosorbent assay
The levels of IL‐1β and IL‐18 in the supernatant of BEAS‐2B cells or lung tissues were detected and the commercial ELISA kits (Solarbio) according to the manufacturer's instructions.
2.7. Flow cytometry
BEAS‐2B cell pyroptosis was evaluated using the FAM‐FLICA Caspase‐1 Kit (ImmunoChemistry Technologies, USA) by flow cytometry. Briefly, the BEAS‐2B cells were stained with the caspase‐1 probe at 37°C for 1 h, followed by staining with propidium iodide (PI) for 10 min. The stained cells were measured on a flow cytometer. The pyroptosis rate was calculated as the percentage of double positive staining of caspase‐1 and PI.
2.8. RNA immunoprecipitation assay
The direct interaction between LIN28B and CDKN2B‐AS1/HIF‐1α was evaluated by RIP assay using the EZ‐Magna RIP kit (Millipore, USA). Briefly, the lysates of BEAS‐2B cells were collected and incubated with the magnetic beads that were preconjugated with anti‐LIN28B (Abcam) or anti‐IgG (Abcam) at 4°C overnight. After treatment with Proteinase K, the RNA was extracted from the immunoprecipitations and detected by qRT–PCR.
2.9. RNA pull‐down assay
RNA pull‐down assay was performed using the RNA pull‐down kit (Bersinbio, Guangzhou, China). The specific biotinylated probes for CDKN2B‐AS1 and HIF‐1α were provided by GenePharma. The BEAS‐2B cell lysates were incubated with the magnetic beads coupled with biotinylated probe. After isolation of protein from the magnetic beads, the protein level of LIN28B was determined by Western blotting.
2.10. Fluorescence in situ hybridization
The co‐localization of CDKN2B‐AS1 and LIN28B in BEAS‐2B cells was observed by FISH using the Ribo™ FISH kit (RiboBio, Guangzhou, China). The specific probes for CDKN2B‐AS1 and LIN28B were purchased from GenePharma. In brief, BEAS‐2B cells were fixed with 4% paraformaldehyde and blocked with prehybridization buffer. Then, the cells were incubated with CDKN2B‐AS1 and LIN28B probes at 37°C overnight. The fluorescence was observed under a fluorescence microscope (Leica, Germany).
2.11. Detection of mRNA stability
BEAS‐2B cells were treated with 2 mg/mL actinomycin D (MCE, USA) for 24 h. Subsequently, total RNA was isolated from actinomycin D‐treated cells and determined by qRT–PCR as described above.
2.12. Animal model of septic ALI
Eight‐week‐old male C57BL/6 mice were purchased from Slac Jingda Laboratory Animal Co., Ltd. (Hunan, China). The mice were randomly divided into the following four groups: sham, caecal ligation and puncture (CLP), CLP+ sh‐NC, and CLP+ sh‐CDKN2B‐AS1 (n = 15 per group). The mice were anesthetized by intraperitoneal injection with 50 mg/kg pentobarbital sodium. Subsequently, a 2 cm‐incision was made along the midline in the lower abdomen to expose the caecum, followed by ligation with a 4–0 silk suture and puncture using a 21‐gauge sterilized needle. After the wound was sutured, the mice were rewarmed until they awoke. The sham mice were received the same operation procedure except for CLP. For mice in CLP+ sh‐NC and CLP+ sh‐CDKN2B‐AS1 groups, lentivirus expressing sh‐NC or sh‐CDKN2B‐AS1 (GenePharma, 5 × 107 pfu per mouse) were intravenously injected into mice 5 days before CLP. At 24 h after CLP, all mice were euthanized by intraperitoneal overdose of sodium pentobarbital. The lung tissues were collected for the following experiments.
2.13. Survival analysis
For survival analysis, the mice underwent the same experimental procedures as described above. The survival rate was calculated every 12 h until 72 h after surgery.
2.14. Lung wet‐to‐dry (W/D) weight ratio
Pulmonary oedema was assessed by determining the lung W/D weight ratio. The collected lungs were weighed to determine the wet weight. After being dried at 80°C for 1 day, the dry weight of lungs was detected. Finally, the W/D weight ratio was calculated.
2.15. Bronchoalveolar lavage fluid
Bronchoalveolar lavage fluid (BALF) was collected after washing with normal saline via tracheal catheter for three times. The collected BALF was centrifuged at 2000 rpm for 15 min and then resuspended in PBS. The number of total cells and neutrophils in BALF was counted. The total protein concentration was assessed using the BCA Protein Assay Kit (Abcam).
2.16. Hematoxylin–eosin staining
The lung tissues were fixed in 4% paraformaldehyde and embedded in paraffin. The sliced 4 μm‐sections were stained using a commercial HE staining kit (Boster, Wuhan, China) and observed under a light microscope (Leica).
2.17. Immunohistochemical staining
The paraffin sections of lung tissues were received dewaxing and rehydration, followed by incubation in 0.3% hydrogen peroxide and blocking in normal goat serum for 30 min. The sections were incubated with primary antibodies against caspase‐1 (1:100, Bioss) at 4°C overnight. Subsequently, the sections were incubated with goat anti‐rabbit IgG H&L/HRP (1:3000, Bioss) for 30 min. After development with diaminobenzidine, the photographs were taken using a light microscope. The percentage of caspase1 positive cells was quantified in five high‐magnification fields of view using the Image J software.
2.18. Statistical analysis
All values are expressed as the mean ± standard deviation (SD). Student's t test or one‐way ANOVA followed by Tukey's test was performed to analyze data between two groups or among multiple groups using GraphPad Prism 7. p values <0.05 were considered as statistically significant.
3. RESULTS
3.1. CDKN2B‐AS1 was up‐regulated in the in vitro model of septic ALI
BEAS‐2B cells were stimulated with various concentrations of LPS to mimic septic ALI in vitro. The levels of IL‐1β and IL‐18 in the supernatant of BEAS‐2B cells were dose dependently enhanced after LPS exposure (Figure 1A,B). In addition, the pyroptosis rate of BEAS‐2B cells was increased by LPS in a dose‐dependent manner (Figure 1C). Moreover, the protein levels of NLRP3, cleaved Caspase1, ASC, IL‐1β, IL‐18, and GSDMD‐N were elevated with the increasing of LPS concentration (Figure 1D). More importantly, we observed a dose‐dependent increase in CDKN2B‐AS1 expression in LPS‐treated BEAS‐2B cells (Figure 1E). To examine whether the cell death by LPS was caused by inflammasome activation, MCC950, an inhibitor of NLRP3, was added to LPS‐treated cells. We found that treatment with MCC950 remarkably reduced the pyroptotic rate of LPS‐exposed BEAS‐2B cells (Figure S2). Therefore, up‐regulation of CDKN2B‐AS1 might be responsible for the pathological development of septic ALI.
FIGURE 1.
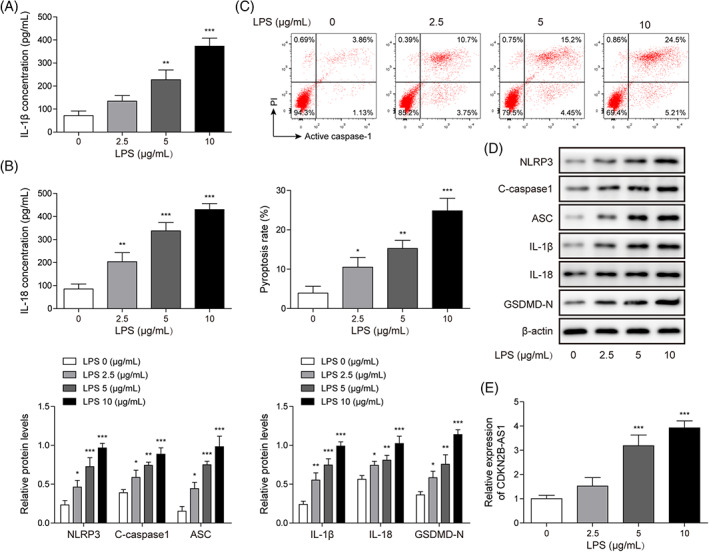
CDKN2B‐AS1 level was increased in LPS‐treated lung epithelial cells. BEAS‐2B cells were stimulated with LPS (2.5, 5, and 10 μg/mL) for 24 h. IL‐1β (A) and IL‐18 (B) levels were assessed by ELISA. (C) Pyroptosis was evaluated by flow cytometry. (D) Western blotting analysis of the protein levels of NLRP3, cleaved Caspase1, ASC, IL‐1β, IL‐18, and GSDMD‐N. (E) CDKN2B‐AS1 level was assessed by qRT–PCR. *p < 0.05, ** p < 0.01, *** p < 0.001 compared with the control group.
3.2. Knockdown of CDKN2B‐AS1 repressed LPS‐induced pyroptosis in BEAS‐2B cells
To investigate the influence of CDKN2B‐AS1 on LPS‐induced pyroptosis, BEAS‐2B cells were transfected with sh‐CDKN2B‐AS1 or sh‐NC. The knockdown efficiency of CDKN2B‐AS1 was validated by qRT–PCR (Figure 2A). Moreover, up‐regulation of CDKN2B‐AS1 in LPS‐treated BEAS‐2B cells was reversed by sh‐CDKN2B‐AS1 transfection (Figure 2B). CCK‐8 data indicated that CDKN2B‐AS1 deficiency remarkably increased BEAS‐2B cell viability in the presence of LPS (Figure 2C). The excessive production of IL‐1β and IL‐18 in LPS‐exposed BEAS‐2B cells was abolished by CDKN2B‐AS1 silencing (Figure 2D,E). Furthermore, CDKN2B‐AS1 knockdown significantly restrained pyroptosis of LPS‐challenged BEAS‐2B cells (Figure 2F). The increased protein levels of NLRP3, cleaved Caspase1, ASC, IL‐1β, IL‐18, and GSDMD‐N were strikingly reversed by CDKN2B‐AS1 depletion (Figure 2G). The above results indicated that CDKN2B‐AS1 silencing restrained LPS‐induced pyroptosis in BEAS‐2B cells.
FIGURE 2.
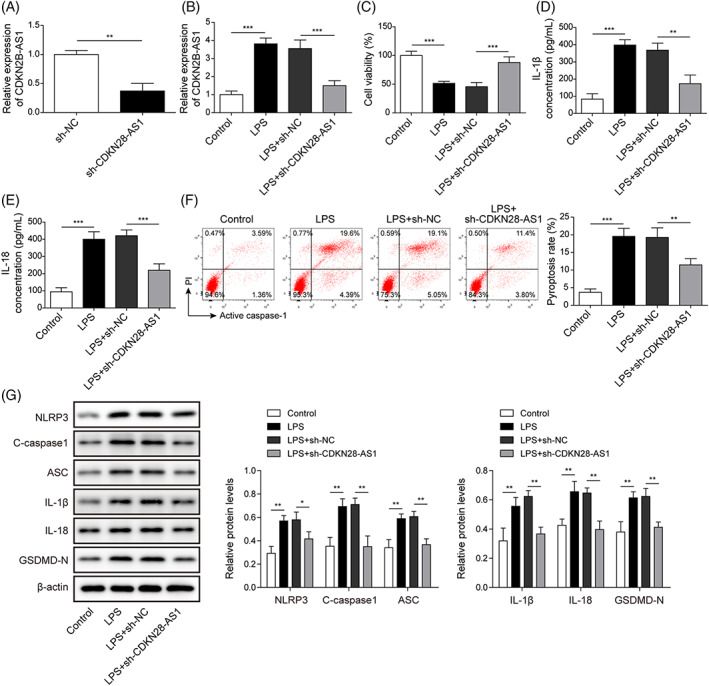
CDKN2B‐AS1 depletion restrained LPS‐induced pyroptosis in BEAS‐2B cells. Sh‐CDKN2B‐AS1 or sh‐NC was transfected into BEAS‐2B cells. (A) CDKN2B‐AS1 expression was detected by qRT–PCR. (B) qRT–PCR analysis of CDKN2B‐AS1 level upon LPS stimulation. (C) BEAS‐2B cell viability was assessed by CCK‐8 assay. ELISA was carried out to determine IL‐1β (D) and IL‐18 (E) levels. (F) Pyroptosis of BEAS‐2B cells with various treatments was detected by flow cytometry. (G) Protein levels of pyroptosis‐related proteins were assessed by Western blotting. *p < 0.05, ** p < 0.01, *** p < 0.001 compared with the specific group.
3.3. Depletion of CDKN2B‐AS1 mitigated septic ALI in mice
We further evaluated the effect of CDKN2B‐AS1 on septic ALI in mice in vivo. The survival rate of septic mice was declined along with the time prolonging, which could be strikingly improved by down‐regulation of CDKN2B‐AS1 (Figure 3A). qRT–PCR analysis showed that the enhanced level of CDKN2B‐AS1 in the lung tissues of septic mice was reversed by shCDKN2B‐AS1 (Figure 3B). The CLP evidently raised the lung W/D weight ratio, which was abolished by CDKN2B‐AS1 depletion (Figure 3C). HE staining showed that CLP led to obvious pathological changes including hyperemia, hemorrhage, and inflammatory cell infiltration were observed in the lung tissues, whereas CDKN2B‐AS1 deficiency could alleviate these pathological changes (Figure 3D). Moreover, CDKN2B‐AS1 silencing significantly reduced the number of total cells and neutrophils, and total protein concentration in the BALF of septic mice (Figure 3E–G). Thus, sepsis‐induced ALI in mice was ameliorated by CDKN2B‐AS1 silencing.
FIGURE 3.

CDKN2B‐AS1 knockdown alleviated sepsis‐induced ALI in mice. Sepsis was induced by CLP in mice. Lentiviruses containing sh‐CDKN2B‐AS1 were intravenously injected 5 days before CLP to silence CDKN2B‐AS1. (A) The survival of mice was monitored. (C) qRT–PCR analysis of CDKN2B‐AS1 expression in the lung tissues of mice. (D) Lung W/D weight ratio was measured. (E) HE staining was performed to observe pathological changes in the lung tissues. Total cell number (F), number of neutrophils (F), and total protein concentration (G) in the BALF were determined. *p < 0.05, ** p < 0.01, *** p < 0.001 compared with the specific group.
3.4. CDKN2B‐AS1 deficiency repressed pyroptosis in mice with septic ALI
ELISA analysis suggested that the enhanced IL‐1β and IL‐18 levels in the lung tissues of CLP mice were reversed by CDKN2B‐AS1 deficiency (Figure 4A,B). The abnormal high expression of caspase‐1 in the lungs of septic mice was counteracted when CDKN2B‐AS1 was depleted (Figure 4C). Consistently, CDKN2B‐AS1 silencing neutralized CLP‐induced up‐regulation of HIF‐1α, NLRP3, cleaved Caspase1, ASC, IL‐1β, IL‐18, and GSDMD‐N protein levels in the lung tissues (Figure 4D). To sum up, depletion of CDKN2B‐AS1 relieved pyroptosis in septic ALI in vivo.
FIGURE 4.

CDKN2B‐AS1 silencing restrained pyroptosis in the mouse model of septic ALI. The levels of IL‐1β (A) and IL‐18 (B) in the lung tissues were assessed by ELISA. (C) Caspase‐1 expression in the lungs was evaluated by immunohistochemical staining. (D) The protein levels of HIF‐1α, NLRP3, cleaved Caspase1, ASC, IL‐1β, IL‐18, and GSDMD‐N in the lung tissues were measured by Western blotting. *p < 0.05, ** p < 0.01, *** p < 0.001 compared with the specific group.
3.5. CDKN2B‐AS1 depletion restrained HIF‐1α expression through reducing its stability
We further investigated the molecular mechanism of CDKN2B‐AS1 in the regulation of septic ALI. As assessed by qRT–PCR and Western blotting, LPS stimulation dose dependently enhanced HIF‐1α mRNA and protein levels in BEAS‐2B cells (Figure 5A,B). Notably, CDKN2B‐AS1 silencing strikingly abolished LPS‐mediated up‐regulation of HIF‐1α (Figure 5C,D). Besides, the mRNA and protein levels of HIF‐1α were down‐regulated in CDKN2B‐AS1‐silenced BEAS‐2B cells (Figure 5E,F). Knockdown of CDKN2B‐AS1 remarkably reduced the stability of HIF‐1α mRNA (Figure 5G). These findings suggested that CDKN2B‐AS1 contributed to HIF‐1α expression by raising HIF‐1α mRNA stability.
FIGURE 5.
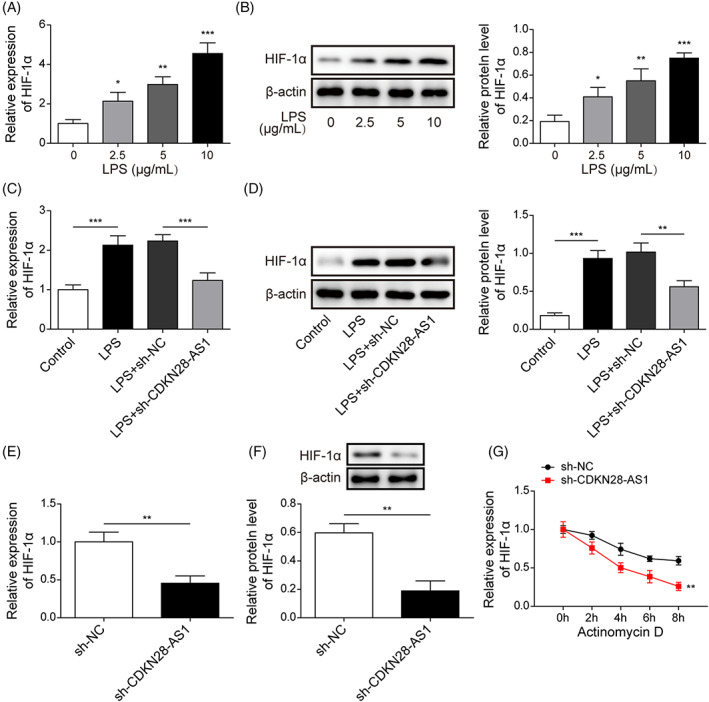
CDKN2B‐AS1 modulated HIF‐1α expression in LPS‐stimulated BEAS‐2B cells. After treatment with LPS (2.5, 5, and 10 μg/mL) for 24 h, the mRNA and protein levels of HIF‐1α were evaluated by qRT–PCR (A) and Western blotting (B). LPS‐stimulated BEAS‐2B cells were transfected with sh‐NC or sh‐CDKN2B‐AS1. The mRNA and protein expression of HIF‐1α in BEAS‐2B cells was determined by qRT–PCR (C) and Western blotting (D). The mRNA and protein levels of HIF‐1α in sh‐CDKN2B‐AS1‐transfected BEAS‐2B cells were measured by qRT–PCR (E) and Western blotting (F). (G) The stability of HIF‐1α mRNA in response to actinomycin D was detected by qRT–PCR. *p < 0.05, ** p < 0.01, *** p < 0.001 compared with the specific group.
3.6. CDKN2B‐AS1 recruited LIN28B to enhance HIF‐1α stability
Next, we explored the detailed regulatory mechanism of CDKN2B‐AS1 in HIF‐1α expression. As predicted by StarBase database, CDKN2B‐AS1 possessed binding sites in LIN28B (Figure 6A). The mRNA and protein levels of LIN28B in BEAS‐2B cells were dose dependently increased by LPS (Figure 6B,C). Consistently, LIN28B mRNA and protein levels in lung tissues were higher in CLP group as compared with sham group (Figure S1A,B). Additionally, the enrichment of CDKN2B‐AS1 was enhanced by immunoprecipitation with anti‐LIN28B antibody (Figure 6D). Moreover, the direct binding of CDKN2B‐AS1 to LIN28B was verified by RNA pull‐down assay (Figure 6E). Besides, FISH confirmed the co‐localization of CDKN2B‐AS1 and LIN28B in the cytoplasm of BEAS‐2B cells (Figure 6F). In addition, the mRNA and protein expression of LIN28B in BEAS‐2B cells was reduced by sh‐LIN28B transfection (Figure 6G,H). Given that there was a direct interaction between CDKN2B‐AS1 and LIN28B, we speculated that CDKN2B‐AS1 might affect the stability of HIF‐1α via recruitment of LIN28B (Figure 6I). Notably, the interaction between LIN28B and HIF‐1α mRNA was predicted by RNA‐Protein Interaction Prediction (Figure S1C). We found that LIN28B silencing reduced HIF‐1α expression in BEAS‐2B cells (Figure 6J,K). Besides, depletion of LIN28B significantly decreased the mRNA stability of HIF‐1α (Figure 6L). Taken together, CDKN2B‐AS1 directly interacted with LIN28B to enhance the stability of HIF‐1α mRNA in BEAS‐2B cells.
FIGURE 6.
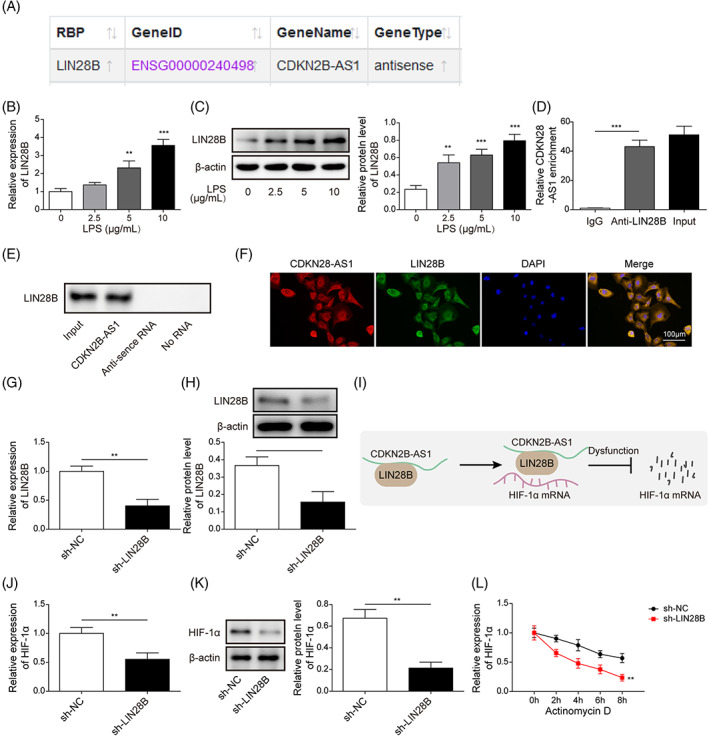
CDKN2B‐AS1 recruited LIN28B to enhance HIF‐1α mRNA stability and expression. (A) StarBase database predicted the direct interaction between CDKN2B‐AS1 and LIN28B. After stimulation with LPS (2.5, 5, and 10 μg/mL) for 24 h, the mRNA and protein expression of LIN28B was determined by qRT–PCR (B) and Western blotting (C). The direct binding between CDKN2B‐AS1 and LIN28B was validated by RIP (D) and RNA pull‐downassays (E). (F) Co‐localization of CDKN2B‐AS1 and LIN28B in the cytoplasm of BEAS‐2B cells was observed by FISH. The mRNA and protein levels of LIN28B after transfection with sh‐LIN28B were assessed by qRT–PCR (G) and Western blotting (H). (I) A schematic map illustrating that CDKN2B‐AS1 recruited LIN28B to directly bind to HIF‐1α and promote its mRNA stability. The mRNA and protein expression of HIF‐1α after down‐regulation of LIN28B was determined by qRT–PCR (J) and Western blotting (K). (L) The stability of HIF‐1α mRNA in response to actinomycin D was assessed by qRT–PCR. *p < 0.05, ** p < 0.01, *** p < 0.001 compared with the specific group.
3.7. CDKN2B‐AS1 contributed to LPS‐induced pyroptosis via regulating HIF‐1α
To further verify the involvement of HIF‐1α in CDKN2B‐AS1‐mediated regulation in pyrotosis, BEAS‐2B cells were transfected with HIF‐1α overexpression plasmid. The overexpression efficiency of HIF‐1α was confirmed by qRT–PCR (Figure 7A). As illustrated in Figure 5B, silencing of CDKN2B‐AS1 reduced the expression of HIF‐1α in LPS‐stimulated BEAS‐2B cells, whereas HIF‐1α expression was restored by transfection with HIF‐1α overexpression plasmid (Figure 7B). Additionally, CDKN2B‐AS1 knockdown‐mediated reduction in IL‐1β and IL‐18 levels could be reversed by HIF‐1α overexpression (Figure 7C,D). Consistently, LPS‐triggered pyroptosis and up‐regulation of HIF‐1α, NLRP3, cleaved Caspase1, ASC, IL‐1β, IL‐18, and GSDMD‐N were weakened by sh‐CDKN2B‐AS1, however, CDKN2B‐AS1 depletion‐mediated these changes were counteracted by enforced expression of HIF‐1α (Figure 7E,F). Thus, CDKN2B‐AS1 contributed to LPS‐triggered pyroptosis via regulating HIF‐1α expression.
FIGURE 7.
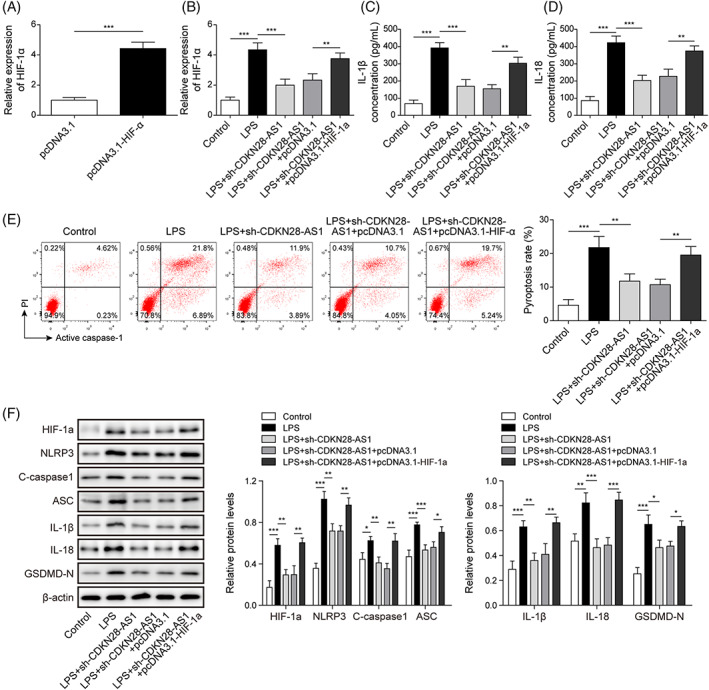
HIF‐1α was involved in CDKN2B‐AS1‐induced pyroptosis in LPS‐stimulated BEAS‐2B cells. LPS‐stimulated BEAS‐2B cells were transfected with sh‐CDKN2B‐AS1 together with or without HIF‐1α overexpression plasmid. (A) and (B) CDKN2B‐AS1 expression in various groups was detected by qRT–PCR. The production of IL‐1β (C) and IL‐18 (D) was evaluated by ELISA. (E) Flow cytometry was adopted to measure pyroptosis. (F) The protein levels of HIF‐1α, NLRP3, cleaved Caspase1, ASC, IL‐1β, IL‐18, and GSDMD‐N were determined by Western blotting. *p < 0.05, ** P < 0.01, *** p < 0.001 compared with the specific group.
4. DISCUSSION
Sepsis remains a life‐threatening organ dysfunction with high morbidity and mortality. ALI, a common complication of most septic patients, is the dominant cause of death for patients in intensive care. LncRNAs are implicated in the pathogenesis of septic ALI, which have been identified as potential therapeutic targets for ALI. In this study, we aimed to explore the biological function of lncRNA CDKN2B‐AS1 in pyroptosis in sepsis‐induced ALI models. Our results demonstrated that CDKN2B‐AS1 promoted pyroptosis to exacerbate septic ALI. Mechanically, CDKN2B‐AS1 directly bound to LIN28B to cause the activation of HIF‐1α/NLRP3 pathway. Our observations revealed that CDKN2B‐AS1 functioned as a promoter of septic ALI through modulating LIN28B/HIF‐1α axis, suggesting that CDKN2B‐AS1 might be an effective therapeutic target for sepsis‐associated ALI.
The biological functions of lncRNA CDKN2B‐AS1 have been reported in multiple malignancies, including endometrial cancer, 17 glioma, 18 colorectal carcinoma, 19 and so on. A recent study revealed that CDKN2B‐AS1 served as a promotor of inflammation response during diabetic nephropathy development. 20 NLRP3 inflammasome activation is responsible for inflammation of ALI, and inactivation of NLRP3 can effectively attenuate ALI. 21 LPS challenge promoted ASC expression to activate NLRP3 inflammasome, which subsequently promoted inflammatory cascade activation, resulting in over production of IL‐1β and IL‐18. 22 Caspase‐1‐GSDMD is a classical pathway that regulates apoptosis and pyroptosis. The activation of Caspase‐1 can cleave BID, a member of Bcl‐2 family proteins in the absence of GSDMD, resulting in the release of Cyt‐c, and downstream apoptotic signaling. 23 In addition, during apoptosis, the activated Caspase‐3 can cleave GSDME into a GSDME‐N fragment that mediates pyroptosis progression. 24 More importantly, down‐regulation of CDKN2B‐AS1 protected against LPS‐induced inflammation and apoptosis in BEAS‐2B cells. 10 In the present study, we provided first evidence that CDKN2B‐AS1 knockdown repressed pyroptosis in the in vitro and in vivo models of septic ALI. Therefore, there was a potential crosstalk of CDKN2B‐AS1‐regulated pyroptosis and apoptosis in LPS‐induced ALI. Our data elucidated a novel regulatory mechanism of CDKN2B‐AS1 in septic ALI.
Another important finding of our study is that LIN28B was highly expressed in septic ALI models. As an RNA‐binding protein, LIN28B participates in multiple pathophysiological processes, such as tumorigenesis and immunoregulation. 25 Dysregulation of LIN28B plays pivotal roles in different human cancers. 26 , 27 Except for this, previous report has showed that LIN28B promoted inflammatory cytokine production and resulted in impaired T‐cell development. 28 Yao et al found that negative regulation of LIN28B could inhibit vascular endothelial cell pyroptosis in atherosclerosis. 29 Our results for the first time revealed that up‐regulation of LIN28B facilitated LPS‐induced pyroptosis in BEAS‐2B cells. It is well known that lncRNAs can regulate protein functions via direct binding to them. 30 For instance, lncRNA OIP5‐AS1 directly interacted with HuR to restrain HuR‐mediated proliferation of HeLa cells. 31 In this study, the direct interplay between CDKN2B‐AS1 and LIN28B was verified during septic ALI.
HIF‐1α is a transcription factor that induces multiple responses upon hypoxia condition, which is involved in the pathological progression of multiple inflammatory diseases, including sepsis. High expression of HIF‐1α was found in the lungs of septic mice. 32 Inactivation of HIF‐1α pathway has been proved to alleviate LPS‐induced inflammatory response in lung epithelial cells. 32 Notably, HIF‐1α is an activator of NLRP3, which promoted the release of IL‐1β in bleomycin‐induced ALI. 33 In line with the previous studies, our results indicated that HIF‐1α expression was enhanced by LPS stimulation in lung epithelial cells. The RBPs have been confirmed to modulate the stability of mRNAs. For instance, LIN28B has found to affect the stability of UBAP2L, 34 EWS‐FLI1, 35 and BCL‐2. 36 In this study, LIN28B could raise the stability of HIF‐1α mRNA in lung epithelial cells. Moreover, CDKN2B‐AS1‐mediated HIF‐1α mRNA stability further promoted NLRP3‐triggerred pyroptosis, thus intensifying septic ALI.
Taken together, our findings showed that lncRNA CDKN2B‐AS1/LIN28B/HIF‐1α pathway was abnormally activated in septic ALI models. Knockdown of CDKN2B‐AS1 attenuated septic ALI via suppressing NLRP3‐mediated pyroptosis through recruitment of LIN28B to enhance HIF‐1α mRNA stability. CDKN2B‐AS1 inhibition may serve as a promising treatment strategy for septic ALI.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
Supporting information
Figure S1: Expression of LIN28B in the mouse model of septic ALI. (A) and (B) qRT–PCR and Western blotting analysis of LIN28B expression in the lung tissues of mice from sham and CLP groups. (C) RNA‐Protein Interaction Prediction predicted the direct interaction between LIN28B and HIF‐1α. ** p < 0.01, *** p < 0.001 compared with the sham group.
Figure S2: BEAS‐2B cells were exposed to LPS, followed by treatment with 10 μM MCC950 (an inhibitor of NLRP3) for 48 h. Pyroptosis of BEAS‐2B cells was assessed by flow cytometry. *p < 0.05, ** p < 0.01, *** p < 0.001 compared with the specific group.
Miao R‐F, Tu J. LncRNA CDKN2B‐AS1 interacts with LIN28B to exacerbate sepsis‐induced acute lung injury by inducing HIF‐1α/NLRP3‐mediated pyroptosis. Kaohsiung J Med Sci. 2023;39(9):883–895. 10.1002/kjm2.12697
REFERENCES
- 1. Iskander KN, Osuchowski MF, Stearns‐Kurosawa DJ, Kurosawa S, Stepien D, Valentine C, et al. Sepsis: multiple abnormalities, heterogeneous responses, and evolving understanding. Physiol Rev. 2013;93(3):1247–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Caraballo C, Jaimes F. Organ Dysfunction in Sepsis: An Ominous Trajectory From Infection To Death. Yale J Biol Med. 2019;92(4):629–40. [PMC free article] [PubMed] [Google Scholar]
- 3. Guo R, Li Y, Han M, Liu J, Sun Y. Emodin attenuates acute lung injury in Cecal‐ligation and puncture rats. Int Immunopharmacol. 2020;85:106626. [DOI] [PubMed] [Google Scholar]
- 4. Ali FEM, Ahmed SF, Eltrawy AH, Yousef RS, Ali HS, Mahmoud AR, et al. Pretreatment with Coenzyme Q10 Combined with Aescin Protects against Sepsis‐Induced Acute Lung Injury. Cells Tissues Organs. 2021;210(3):195–217. [DOI] [PubMed] [Google Scholar]
- 5. Zhang Y, Yang W, Li W, Zhao Y. NLRP3 Inflammasome: Checkpoint Connecting Innate and Adaptive Immunity in Autoimmune Diseases. Front Immunol. 2021;12:732933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang YC, Liu QX, Zheng Q, Liu T, Xu XE, Liu XH, et al. Dihydromyricetin alleviates sepsis‐induced acute lung injury through inhibiting NLRP3 inflammasome‐dependent pyroptosis in mice model. Inflammation. 2019;42(4):1301–10. [DOI] [PubMed] [Google Scholar]
- 7. Sun J, Xin K, Leng C, Ge J. Down‐regulation of SNHG16 alleviates the acute lung injury in sepsis rats through miR‐128‐3p/HMGB3 axis. BMC Pulm Med. 2021;21(1):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hong J, Mo S, Gong F, Lin Z, Cai H, Shao Z, et al. lncRNA‐SNHG14 Plays a Role in Acute Lung Injury Induced by Lipopolysaccharide through Regulating Autophagy via miR‐223‐3p/Foxo3a. Mediators Inflamm. 2021;2021:7890288–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gui F, Peng H, Liu Y. Elevated circulating lnc‐ANRIL/miR‐125a axis level predicts higher risk, more severe disease condition, and worse prognosis of sepsis. J Clin Lab Anal. 2019;33(6):e22917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.1Wang B, Sun Q, Ye W, Li L, Jin P. Long non‐coding RNA CDKN2B‐AS1 enhances LPS‐induced apoptotic and inflammatory damages in human lung epithelial cells via regulating the miR‐140‐5p/TGFBR2/Smad3 signal network. BMC Pulm Med. 2021;21:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tang D, Luo Y, Jiang Y, Hu P, Peng H, Wu S, et al. LncRNA KCNQ1OT1 activated by c‐Myc promotes cell proliferation via interacting with FUS to stabilize MAP3K1 in acute promyelocytic leukemia. Cell Death Dis. 2021;12(9):795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ni SY, Xu WT, Liao GY, Wang YL, Li J. LncRNA HOTAIR Promotes LPS‐Induced Inflammation and Apoptosis of Cardiomyocytes via Lin28‐Mediated PDCD4 Stability. Inflammation. 2021;44(4):1452–63. [DOI] [PubMed] [Google Scholar]
- 13. Sun HD, Liu YJ, Chen J, Chen MY, Ouyang B, Guan XD. The pivotal role of HIF‐1α in lung inflammatory injury induced by septic mesenteric lymph. Biomed Pharmacother. 2017;91:476–84. [DOI] [PubMed] [Google Scholar]
- 14. Sun HL, Peng ML, Lee SS, Chen CJ, Chen WY, Yang ML, et al. Endotoxin‐induced acute lung injury in mice is protected by 5,7‐dihydroxy‐8‐methoxyflavone via inhibition of oxidative stress and HIF‐1α. Environ Toxicol. 2016;31(12):1700–9. [DOI] [PubMed] [Google Scholar]
- 15. Yeh CH, Cho W, So EC, Chu CC, Lin MC, Wang JJ, et al. Propofol inhibits lipopolysaccharide‐induced lung epithelial cell injury by reducing hypoxia‐inducible factor‐1alpha expression. Br J Anaesth. 2011;106(4):590–9. [DOI] [PubMed] [Google Scholar]
- 16. Yuan D, Guan S, Wang Z, Ni H, Ding D, Xu W, et al. HIF‐1α aggravated traumatic brain injury by NLRP3 inflammasome‐mediated pyroptosis and activation of microglia. J Chem Neuroanat. 2021;116:101994. [DOI] [PubMed] [Google Scholar]
- 17. Yang D, Ma J, Ma XX. CDKN2B‐AS1 Promotes Malignancy as a Novel Prognosis‐Related Molecular Marker in the Endometrial Cancer Immune Microenvironment. Front Cell Dev Biol. 2021;9:721676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lu J, Chen Y, Wen L, Zhou Q, Yan S. LncRNA CDKN2B‐AS1 contributes to glioma development by regulating the miR‐199a‐5p/DDR1 axis. J Gene Med. 2022;24(1):e3389. [DOI] [PubMed] [Google Scholar]
- 19. Zheng Y, Zeng J, Xia H, Wang X, Chen H, Huang L, et al. Upregulated lncRNA Cyclin‐dependent kinase inhibitor 2B antisense RNA 1 induces the proliferation and migration of colorectal cancer by miR‐378b/CAPRIN2 axis. Bioengineered. 2021;12(1):5476–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chang J, Yu Y, Fang Z, He H, Wang D, Teng J, et al. Long non‐coding RNA CDKN2B‐AS1 regulates high glucose‐induced human mesangial cell injury via regulating the miR‐15b‐5p/WNT2B axis. Diabetol Metab Syndr. 2020;12(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ying Y, Mao Y, Yao M. NLRP3 Inflammasome Activation by MicroRNA‐495 Promoter Methylation May Contribute to the Progression of Acute Lung Injury. Mol Ther Nucleic Acids. 2019;18:801–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ding XQ, Wu WY, Jiao RQ, Gu TT, Xu Q, Pan Y, et al. Curcumin and allopurinol ameliorate fructose‐induced hepatic inflammation in rats via miR‐200a‐mediated TXNIP/NLRP3 inflammasome inhibition. Pharmacol Res. 2018;137:64–75. [DOI] [PubMed] [Google Scholar]
- 23. Tsuchiya K, Nakajima S, Hosojima S, Thi Nguyen D, Hattori T, Manh Le T, et al. Caspase‐1 initiates apoptosis in the absence of gasdermin D. Nat Commun. 2019;10(1):2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rogers C, Fernandes‐Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES. Cleavage of DFNA5 by caspase‐3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. 2017;8:14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jiang S, Baltimore D. RNA‐binding protein Lin28 in cancer and immunity. Cancer Lett. 2016;375(1):108–13. [DOI] [PubMed] [Google Scholar]
- 26. Murray MJ, Saini HK, Siegler CA, Hanning JE, Barker EM, van Dongen S, et al. LIN28 Expression in malignant germ cell tumors downregulates let‐7 and increases oncogene levels. Cancer Res. 2013;73(15):4872–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lovnicki J, Gan Y, Feng T, Li Y, Xie N, Ho CH, et al. LIN28B promotes the development of neuroendocrine prostate cancer. J Clin Invest. 2020;130(10):5338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beachy SH, Onozawa M, Chung YJ, Slape C, Bilke S, Francis P, et al. Enforced expression of Lin28b leads to impaired T‐cell development, release of inflammatory cytokines, and peripheral T‐cell lymphoma. Blood. 2012;120(5):1048–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yao F, Lv X, Jin Z, Chen D, Zheng Z, Yang J, et al. Sirt6 inhibits vascular endothelial cell pyroptosis by regulation of the Lin28b/let‐7 pathway in atherosclerosis. Int Immunopharmacol. 2022;110:109056. [DOI] [PubMed] [Google Scholar]
- 30. Kapusta A, Feschotte C. Volatile evolution of long noncoding RNA repertoires: mechanisms and biological implications. Trends Genet. 2014;30(10):439–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim J, Abdelmohsen K, Yang X, De S, Grammatikakis I, Noh JH, et al. LncRNA OIP5‐AS1/cyrano sponges RNA‐binding protein HuR. Nucleic Acids Res. 2016;44(5):2378–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhao S, Gao J, Li J, Wang S, Yuan C, Liu Q. PD‐L1 Regulates Inflammation in LPS‐Induced Lung Epithelial Cells and Vascular Endothelial Cells by Interacting with the HIF‐1α Signaling Pathway. Inflammation. 2021;44(5):1969–81. [DOI] [PubMed] [Google Scholar]
- 33. Huang JJ, Xia J, Huang LL, Li YC. HIF‐1α promotes NLRP3 inflammasome activation in bleomycin‐induced acute lung injury. Mol Med Rep. 2019;20(4):3424–32. [DOI] [PubMed] [Google Scholar]
- 34. Huang R, Yang Q, Wang T. Norcantharidin‐blocked ANXA2P2 inhibits fibroblast proliferation by increasing UBAP2L mRNA stability through LIN28B. Life Sci. 2021;279:119645. [DOI] [PubMed] [Google Scholar]
- 35. Keskin T, Bakaric A, Waszyk P, Boulay G, Torsello M, Cornaz‐Buros S, et al. LIN28B underlies the pathogenesis of a subclass of Ewing Sarcoma LIN28B control of EWS‐FLI1 stability. Cell Rep. 2020;30(13):4567–83.e5. [DOI] [PubMed] [Google Scholar]
- 36. Yuan L, Tian J. LIN28B promotes the progression of colon cancer by increasing B‐cell lymphoma 2 expression. Biomed Pharmacother. 2018;103:355–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Expression of LIN28B in the mouse model of septic ALI. (A) and (B) qRT–PCR and Western blotting analysis of LIN28B expression in the lung tissues of mice from sham and CLP groups. (C) RNA‐Protein Interaction Prediction predicted the direct interaction between LIN28B and HIF‐1α. ** p < 0.01, *** p < 0.001 compared with the sham group.
Figure S2: BEAS‐2B cells were exposed to LPS, followed by treatment with 10 μM MCC950 (an inhibitor of NLRP3) for 48 h. Pyroptosis of BEAS‐2B cells was assessed by flow cytometry. *p < 0.05, ** p < 0.01, *** p < 0.001 compared with the specific group.


