Abstract
Dexmedetomidine (DEX), a common anesthetic, has significant effects on the biological features of cancer cells. Although numerous studies have been published on the impact of DEX on the biological characteristics of GC cells, the mechanism remains unknown. This study aimed to explore the effect of DEX on the biological properties of GC cells. DEX suppressed the viability and increased the apoptosis of GC cells in vitro and inhibited tumor growth in vivo. Besides, DEX raised the levels of reactive oxygen species (ROS) and iron, but decreased the levels of glutathione (GSH), glutathione peroxidase 4 (GPX4), and solute carrier family 7 member 11 (SLC7A11) in GC cells, which were abolished by Ferrostatin‐1 (the inhibitor of ferroptosis) treatment. In addition, the level of circ0008035 and E2F7 were downregulated, but miR‐302a level was upregulated in DEX‐treated GC cells. Circ0008035 increased the expression of E2F2 by acting as a sponge for miR‐302a. Circ0008035 inhibited DEX‐induced ferroptotic cell death in GC cells, which was reversed by miR‐302a overexpression or E2F7 reduction. Taken together, DEX mediated ferroptotic cell death in GC through regulating the circ0008035/miR‐302a/E2F7 axis, suggesting a feasible therapy option for GC.
Keywords: circular RNA, dexmedetomidine, ferroptosis, gastric cancer
1. INTRODUCTION
Gastric cancer is the third leading cause of cancer‐related death worldwide. 1 According to anatomical location and histological type, more than 95% of GCs are adenocarcinomas. Because it is frequently diagnosed at an advanced stage, most patients with gastric cancer have a dismal prognosis. Despite great progress has been made in increasing survival and quality of life in patients with locally advanced or metastatic gastric cancer, it remains the primary cause of death. 2 Previously, anesthetics have been proven to influence tumor cell metastasis and even patient long‐term survival. 3 Dexmedetomidine (DEX) is a potent and highly selective 2‐AR agonist that has been widely used as an anesthetic adjuvant and sedative during surgery. 4 Clinical investigations have shown that DEX can reduce inflammatory factor expression and improve postoperative cognitive function recovery in patients who underwent a radical GC resection. 5 Furthermore, via controlling T‐lymphocyte subgroup expression, DEX improves the immune function deficiency in gastric cancer patients following surgery. 6 Noteworthily, DEX regulates the malignant phenotype of breast cancer cells 7 and ovarian cancer cells 8 in vivo and in vitro. Importantly, it has been confirmed that DEX exhibits different roles in cell biological behavior depending on cancer cell types. 9 Previous studies on DEX in GC have mostly focused on clinical aspects. Studies on DEX on postoperative analgesia and cellular immune function, 10 and postoperative cognitive function 5 in patients undergoing radical gastric cancer surgery have confirmed the safety of DEX anesthesia in surgery. However, the impact of DEX on the biological characteristics of GC cells and the mechanism underlying its action remains unknown.
Circular RNAs (circRNAs) are noncoding RNAs that form covalently closed continuous loops without 5′ or 3′ tails. 11 The previous study has revealed that anesthetics can affect the expression of noncoding RNA (circRNAs and miRNAs) to exhibit its biological activities. 12 However, it is unknown if circRNAs are associated with DEX‐mediated biological features of GC cells.
Huang et al. 13 analyzed the GEO dataset (GSE78092) and discovered that the expression of five circRNAs [hsa_circ_0005529 (circ0005529), hsa_circ_0061274 (circ0061274), hsa_circ_0008035 (circ0008035), hsa_circ_0032821 (circ0032821), and hsa_circ_0061265 (circ0061265)] was considerably upregulated in GC. In the current study, we also discovered that the expression of circ0005529, circ0061274, circ0032821, and circ0008035 was significantly upregulated in SNU1 and AGS cells. More importantly, DEX suppressed the expression of circ0008035 in SNU1 and AGS cells. It is worth noting that circ0008035 was implicated in the regulation of ferroptosis in GC. 14 Therefore, we chose circ0008035 to evaluate the anti‐cancer efficacy of DEX in GC. We look forward to providing ideas for the clinical application of DEX in the treatment of GC.
2. MATERIALS AND METHODS
2.1. Cell lines
Human gastric adenocarcinoma cell lines (SNU1 and AGS) and human gastric epithelial cell lines (GES‐1) were obtained from the American Tissue Culture Collection (Rockville, MD, USA). All the cells were incubated in Dulbecco's Modified Eagle Medium (DMEM; Gibco BRL, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; Gibco), 100 U/ml streptomycin, and 100 μg/ml penicillin G in 5% CO2 atmosphere at 37°C. For drug treatment, SNU1 and AGS cells were treated with DEX (H20130027; Cisen Pharmaceutical Co., Ltd., Shanghai, China) at various concentrations (0.1–100 μM) and incubated for 24 h at 37°C. 15 To confirm the relationship between DEX and ferroptosis, the cells were pretreated for 1 h with 5 μM of Ferrostatin‐1 (Fer‐1; the inhibitor of ferroptosis), ZVAD‐FMK (the inhibitor of apoptosis), or necrosulfonamide (NEC, the inhibitor of necroptosis), and then treated with DEX.
2.2. Cell viability assay
SNU1 and AGS cells (2 × 103 cells/well) were seeded into 96‐well plates and cultured for 24 h until 70%–80% confluence. Then, the cells were incubated with 10 μl of Cell Counting Kit‐8 (CCK‐8) solution (Beyotime, China) for 2 h. The optical densities at 450 nm (OD450) were recorded using the microplate reader (Molecular Devices, CA, USA).
2.3. EdU assay
SNU1 and AGS cells (7 × 103 cells/well) were seeded into 96‐well plates and cultured for 24 h. After indicated treatment, the cells were treated with 20 μM of 5‐ethynyl‐20‐deoxyuridine (EdU; Beyotime) and incubated for 2 h. The cells were fixed with 4% paraformaldehyde for 20 min and then treated with 0.5% Triton‐X‐100 for 20 min. 100 μl of DAPI was added to stain the DNA of cells, and the proportion of EdU‐positive cells was visualized under a fluorescent microscope.
2.4. Determination of cell death
FACS analysis of propidium iodide (PI)‐stained cells were employed to examine cell death. as previously described. 16 Briefly, SNU1 and AGS cells (2 × 106 cells/well) were seeded onto 6‐well plates and cultivated for 24 h. After treatment, the cells were collected and stained with 2 μg/ml PI (Beyotime). A flow cytometer was used to count the number of dead cells.
2.5. Determination of ROS generation, GSH activity, and iron levels
Intracellular ROS levels were determined by oxidative conversion of cell‐permeable 2′,7′‐dichlorofluorescein diacetate (DCFH‐DA) according to the manufacturer's instructions. The GSH and GSSG assay kits were used to determine the relative levels of GSH (Beyotime). The iron concentration in cell lysates was determined using the iron assay kit (Abcam) according to the manufacturer's instructions.
2.6. Western blot
Total proteins were isolated from SNU1 and AGS cells and then lysed in a lysis solution. The protein concentration was determined using a BCA protein assay kit (Beyotime). After that, 40 mg of protein was separated using 10% SDS‐PAGE and transferred to PVDF membranes. Then, the membranes were blocked with 5% BSA in TBST before being incubated with primary antibodies overnight at 4°C. The primary antibodies were as follows: anti‐SLC7A11 (1:2000; cat. no. ab175186; Abcam), anti‐GPX4 (1:1000; cat. no. ab125066; Abcam), anti‐E2F7 (1:600; cat. no. 24489‐1‐AP; Proteintech), and GAPDH (1:1000; cat. no. ab8245; Abcam). Then, the membranes were incubated with the secondary antibodies [goat anti‐ rabbit IgG‐HRP (1:5000; cat. no. SE134; Beijing Solarbio Science & Technology Co., Ltd.) and goat anti‐mouse IgG‐HRP (cat. no. GB23301; Wuhan Servicebio Technology Co., Ltd.)] for 1 h at 37°C. Finally, the blots were visualized using the ECL substrate solution (Beyotime). Results were quantified using ImageJ software (v1.52; National Institutes of Health).
2.7. Microarray data and differential expression analysis
The microarray expression profiling dataset GSE78092 was downloaded from the Gene Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/). The dataset was created using the GPL21485 ArrayStar Human Circular RNA microarray V2.0. Three cases of gastric cancer were used in the experiment. The GEO annotation file for GPL21485 was obtained. Using the online analysis program GEO2R, the expression circRNA profiles of GC tissues and adjacent normal mucosa tissues were compared to identify the differentially expressed circRNAs. Each sample's circRNAs were preserved if they met the following criteria: a |log2 (fold‐change)| ≥ 2 and a p < 0.05.
2.8. qRT‐PCR
The PrimeScript RT reagent kit (ThermoFisher, Waltham, MA, USA) or mirVanaTM qRT‐PCR miRNA Detection Kit (ThermoFisher) were used to synthesize the cDNAs. AceQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) and ABI 7500PCR system (Applied Biosystems, Foster City, CA, USA) were used to measure the relative expression of mRNA. GAPDH and U6 were used as the endogenous reference, respectively. The relative expression of mRNAs was calculated using 2−ΔΔCT methods. The sequences of primers were shown in Table 1.
TABLE 1.
Sequence information of primers
| Primers | Sequences (5′‐3′) |
|---|---|
| Circ0005529 | F: ATC TTG GTG GTG TTC TTG GGT |
| R: AGC CGT ACC ACC TCA TCA AG | |
| Circ0061274 | F: TCA ACA GCC TTC TCA ATT TTC T |
| R: CGG AGT CTT CAG ATT CCC TGT | |
| Circ0008035 | F: CTA CCA GCC AAA CAC CGC T |
| R: TCC AGG AAT CTG AAG GAC CCA | |
| Circ0032821 | F: AGG AAT CTG AGT TGC AGT GTC T |
| R: CTG CAA TCT GGC CTC TTC CA | |
| Circ0061262 | F: GAG CCG ACA CCT TTC AGG AT |
| R: CAC AGC ATT TGA TAG TGG CTC C | |
| MiR‐302a | GTC GTA TCC AGT GCA GGG TCC GAG GTG CAC TGG ATA CGA CTC ACC AAA |
| E2F7 | F: CCG TTT ACG TGG GAC ATC CA |
| R: TCT ACA ACG GAA GCC AGG TG | |
| GAPDH | F: TAT GGA CAC GCT CCC CTG A |
| R: CCC ATT CCC CAG CTC TCA TAC | |
| U6 | F: ATT GGA ACG ATA CAG AG |
| R: GGA ACG CTT CAC GAA TTT G |
2.9. Transfection
The small interfering RNA against circ0008035 (si‐circ0008035#1: 5′‐AGGAGAGAGCAAGGTATGATT‐3′, si‐circ0008035#2: 5′‐AAGGAGAGAGCAAGGTATGAT‐3′) and negative control (si‐NC: 5′‐UUC UCC GAA CGU GUC ACG UTT‐3′), the overexpression vector of circ0008035 (circ0008035‐oe) and its control (NC‐oe), as well as the small interfering RNA against E2F7 (si‐E2F7: 5′‐GCU UGU UAU CAG AUA UCA ACU‐3′) and its control (si‐Ctrl: 5′‐UUC UCC GAA CGU GUC ACG UTT‐3′) were purchased from GenePharma Co., Ltd (Shanghai, China). miR‐302a mimic (MBS8298190), miR‐302a inhibitor (anti‐miR‐302a; MBS8292943), and corresponding controls (miR‐NC, or anti‐NC) were purchased from MyBioSource (California, USA). Cell transfection was performed using the Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol.
2.10. Luciferase reporter assay
According to the bioinformatics software StarBase (https://starbase.sysu.edu.cn/index.php), circinteractome (https://circinteractome.nia.nih.gov/index.html), and circBank (http://www.circbank.cn/index.html), we predicted the binding sites between circ0008035 and miR‐302a. According to the bioinformatics software StarBase (https://starbase.sysu.edu.cn/index.php) and TargetScan (https://www.targetscan.org/vert_80/), we predicted the potential target genes of miR‐302a. The wild type circ0008035 sequence (circ‐wt) or the wild type E2F7‐3′ untranslated region (3′UTR) sequence (E2F7‐wt) containing miR‐302a binding site was subcloned into the pmirGLO luciferase reporter vectors. Additionally, the mutant type circ0008035 sequence (circ‐mut) or the mutant type E2F7‐3′UTR (E2F7‐mut) were amplified with the primers containing the mutant binding site of miR‐302a, and then inserted into the pmirGLO luciferase reporter vector, respectively. The luciferase reporter vectors and miR‐302a mimic or miR‐NC were co‐transfected into SNU1 and AGS cells using Lipoferamine 2000 according to the manufacturer's protocol.
2.11. Xenograft study
Female BALB/c nude mice (4–6 weeks old) were obtained from Beijing Institute of Life Sciences (Beijing, China) and the mice were maintained under the standard conditions. AGS cells (2 × 106 cells/mL) were suspended in 100 μl of PBS and were subcutaneously injected in the right flank of mice. After 1 week, mice were divided into four groups (n = 5): Ctrl, 0.5 μg/kg, 1.0 μg/kg, and 2.0 μg/kg groups. 17 The mice were intraperitoneally injected with DEX once a day for 15 days. 17 Mice in the control group were injected with the same amount of normal saline. Tumor size was measured every 2 days and calculated with the formula: 0.5 × length × width. 2 After the last DEX injection was completed, mice were euthanized with sodium pentobarbital (100 mg/kg) and then sacrificed by decapitation. The tumor tissues were isolated and weighted. Immunohistochemistry for Ki67 and TUNEL assay were performed on paraffin‐embedded xenograft tumor tissue sections.
2.12. Immunohistochemistry assay and TUNEL staining
The tissue sections were incubated with specific primary antibody against Ki67 at 4°C before detection with a standard Biotin‐Streptavidin HRP detection system (Zsbio, Beijing, China). For TUNEL staining assay, the TUNEL reaction solution containing TdT enzyme was added dropwise to the sections, and the number of TUNEL‐positive cells (brown) were recorded by light microscopy with a digital camera (DP73, Olympus, Japan).
2.13. Statistical analysis
SPSS 17.0 software was used for statistical analysis. Our Data were represented as means ± SD. The in vitro experiments were performed with three independent repeats and the in vivo experiments were performed with five independent repeats. Unpaired Student's t‐test was used to analyze the differences between two groups, and a One‐way ANOVA analysis was performed for the comparison of multiple groups, followed by Tukey's test. A value of p < 0.05 was considered statistically significant.
3. RESULTS
3.1. DEX induced cell death in GC cells
The chemical structure of DEX was depicted in Figure 1A. The CCK‐8 assay was performed to examine the effects of DEX on the viability of GC cells (Figure 1B). As a result, DEX reduced the viability of SNU1 and AGS cells in a dose‐dependent manner. The 50% inhibitory concentration (IC50) of DEX in SNU1 and AGS were 1.679 and 1.554 μM, respectively. However, the viability of normal GES‐1 cells was unaffected when the DEX concentration was less than 10 μM (Figure 1C). As a result, the concentrations (0.5, 1.0, and 2.0 μM) of DEX were chosen for the further testing. As shown in Figure 1D, the EdU‐positive stained cells were marked red. The percentage of EdU‐positive cells in DEX‐treated cells was markedly decreased compared with non‐treated cells, indicating that DEX inhibited GC cell proliferation in vitro (Figure 1D). The results of flow cytometry showed that DEX caused SNU1 and AGS cell death (Figure 1E).
FIGURE 1.

DEX induced cell death in GC cells. (A) The chemical structure of DEX. CCK‐8 assay was used to analyze the effects of DEX on the cell viability of SNU1 and AGS cells (B), and normal GES‐1 cells (C). (D) The proliferation of SNU1 and AGS cells treated with DEX (0.5, 1.0, or 2.0 μM) were analyzed using an EdU staining assay (Bar = 50 μm). (E) The cell death of SNU1 and AGS cells treated with DEX (0.5, 1.0, or 2.0 μM) was analyzed using flow cytometry. *p < 0.05, **p < 0.01.
3.2. DEX induced oxidative stress and ferroptosis in GC cells
DEX administration clearly increased the content of ROS in SNU1 and AGS cells compared with the control group (Figure 2A). Moreover, DEX treatment reduced the activity of GSH in SNU1 and AGS cells (Figure 2B). Furthermore, DEX treatment increased the levels of iron (Figure 2C), while decreased the expression of SLC7A11 and GPX4 in GC cells (Figure 2D,E). Additionally, Fer‐1 management reversed the inhibitory role of DEX in cell growth (Figure 2F). Furthermore, Fer‐1 treatment reduced DEX‐mediated increase of GC cell death (Figure 2G). On the other hand, ZVAD‐FMK and NEC had no effect on DEX‐induced cell death (Figure 2F,G). Consistently, Fer‐1 administration alleviated DEX‐induced oxidative stress, accompanied with a reduction of ROS levels (Figure 2H) and an enhancement of GSH activity (Figure 2I). Further, Fer‐1 treatment prevented DEX‐induced increase of iron production, (Figure 2J) and decrease of SLC7A11 and GPX4 expression in GC cells (Figure 2K,L). Collectively, the anticancer effect of DEX in GC cells was found to be dependent on the induction of ferroptosis.
FIGURE 2.

DEX induced oxidative stress and ferroptosis in GC cells. The levels of ROS (A), GSH (B), and iron (C) in SNU1 and AGS cells. (D‐E) The expression of SLC7A11 and GPX4 in SNU1 and AGS cells. (F) The viability of SNU1 and AGS cells. (G) The cell death of SNU1 and AGS cells. The levels of ROS (H), GSH (I), and iron (J) in SNU1 and AGS cells. (K‐L) The expression of SLC7A11 and GPX4 in SNU1 and AGS cells. *p < 0.05, **p < 0.01 versus Ctrl group; ##p < 0.01 versus DEX group.
3.3. DEX downregulated the expression of circ0008035 in GC cells
To investigate differentially expressed circRNAs in GC, we searched the Gene Expression Comprehensive Database (GEO, GSE78092) and analyzed the data using the GEO2R tool. According to |log2FC| ≥ 2.0 and p‐value <0.05, seven circRNAs were found to be significantly upregulated, whereas 38 circRNAs were found to be significantly downregulated in GC (Figure 3A). Among the seven upregulated circRNAs, two antisense circRNAs (circ0023642 and circ0000144) were excluded. In the current study, qRT‐PCR assay revealed that the expression of circ0005529, circ0061274, circ0032821, circ0061262, and circ0008035 in SNU1 and AGS cells was markedly higher than that in GES‐1 cells (Figure 3B). Additionally, as GC cells without DEX treatment were used as controls, DEX treatment (2 μM) decreased the levels of circ0008035 in both SNU1 and AGS cells (Figure 3C,D). Further, the data confirmed that DEX decreased the levels of circ0008035 in a dose‐dependent manner in SNU1 and AGS cells (Figure 3E).
FIGURE 3.
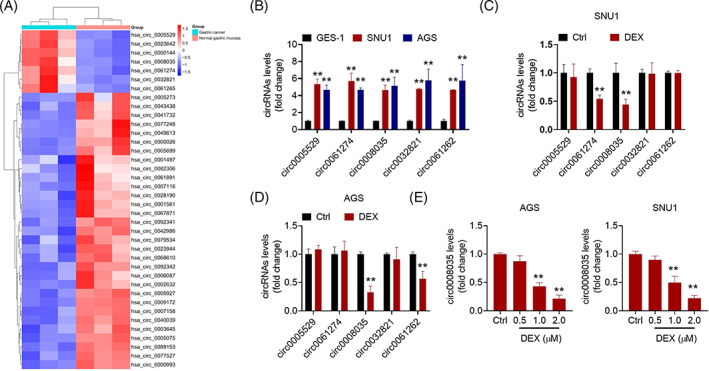
DEX downregulated the expression of circ0008035 in GC cells. (A) A heatmap of 48 differentially expressed circRNAs between GC tissues and adjacent normal tissues. (B) The expression of circ0005529, circ0061274, circ0008035, circ0032821, and circ0061262 in SNU1 and AGS cells. **p < 0.01 versus GES‐1 cells. The expression levels of circ0005529, circ0061274, circ0008035, circ0032821, and circ0061262 in SNU1 (C) and AGS (D) cells treated with DEX (2 μM). **p < 0.01 versus Ctrl group. (E) The expression of circ0008035 in SNU1 and AGS cells treated with DEX (0.5, 1.0 or 2.0 μM). **p < 0.01 versus Ctrl group.
3.4. circ0008035 suppressed DEX‐induced ferroptotic cell death in GC cells
The results of qRT‐PCR assay revealed that transfection of circ0008035‐oe dramatically boosted the levels of circ0008035 in SNU1 and AGS cells (Figure 4A). Furthermore, transfection of circ0008035‐oe alleviated the inhibitory effect of DEX on circ0008035 expression in SNU1 and AGS cells (Figure 4B). The CCK‐8 experiment revealed that circ0008035 overexpression abolished the inhibitory effect of DEX on GC cell survival (Figure 4C). Simultaneously, the addition of circ0008035 inhibited DEX‐induced cell death in SNU1 and AGS cells (Figure 4D). Additionally, circ0008035‐oe transfection prevented DEX‐induced increase of ROS levels (Figure 4E) and decrease of GSH contents (Figure 4F). DEX treatment resulted in elevated iron levels (Figure 4G) and decreased SLC7A11 and GPX4 expressions in GC cells (Figure 4H,I), which were reversed by circ0008035 overexpression. Taken together, DEX induced ferroptosis in GC cells by inhibiting circ0008035.
FIGURE 4.

circ0008035 suppressed DEX‐induced ferroptotic cell death in GC cells. (A) The expression of circ0008035 in SNU1 and AGS cells. (B) The effect of DEX (2 μM) on the expression of circ0008035 in SNU1 and AGS cells. The effect of DEX (2 μM) on the viability (C) and death (D) of SNU1 and AGS cells transfected with or without circ0008035‐oe. The effect of DEX (2 μM) on the levels of ROS (E), GSH (F), and iron (G) in SNU1 and AGS cells transfected with or without circ0008035‐oe. (H) The effect of DEX (2 μM) on the expression of SLC7A11 and GPX4 in SNU1 and AGS cells transfected with or without circ0008035‐oe. *p < 0.05, **p < 0.01.
3.5. circ0008035 positively regulated E2F7 expression by sponging miR‐302a
Bioinformatics studies (StarBase, circinteractome and circBank) revealed that there is a miR‐302a‐binding site in circ0008035 sequences (Figure 5A). In SNU1 and AGS cells, a dual‐luciferase reporter experiment revealed that miR‐302a overexpression reduced the luciferase activity of circ‐wt vectors but had no effect on the activity of circ‐mut luciferase reporter vectors (Figure 5B). Then, the cells were transfected with circ0008035‐oe or si‐circ0008035. si‐circ0008035 transfection, especially si‐circ0008035#1 transfection effectively suppressed the expression of circ0008035 in both SNU1 and AGS cells (Figure 5C). Thus, si‐circ0008035#1 was selected for future investigation. Moreover, circ0008035 overexpression decreased miR‐302a levels, but circ0008035 knockdown boosted the levels of miR‐302a in SNU1 and AGS cells (Figure 5D). E2F7 was predicted to have a potential miR‐302a‐binding site using the web applications (StarBase and TargetScan) (Figure 5E). Further, miR‐302a mimic transfection clearly suppressed the luciferase activity of E2F7‐wt vector in SNU1 and AGS cells, but did not influence the luciferase activity of E2F7‐mut vector in SNU1 and AGS cells (Figure 5F). GC cells were transfected with miR‐302a mimic or miR‐302a inhibitor (Figure 5G). As a result, miR‐302a overexpression restrained the mRNA and protein levels of E2F7, whereas inhibition of miR‐302a had the opposite effect (Figure 5H,I). Following that, transfection of circ0008035‐oe abrogated the inhibition effect of miR‐302a mimic on E2F7 expression in SNU1 cells (Figure 5J). Similarly, transfection of circ0008035 siRNA (si‐circ0008035) abolished the promotion of anti‐miR‐302a on E2F7 expression in AGS cells (Figure 5K). Collectively, circ0008035 acted as a miR‐302a sponge to positively regulate the expression of E2F7.
FIGURE 5.

circ0008035 positively regulated E2F7 expression by sponging miR‐302a. (A) Left: Venn diagram showed miRNAs targeted by circ0008035. Right: Prediction binding sites of circ0008035 and miR‐302a. (B) Luciferase activity in SNU1 and AGS cells co‐transfected with miR‐302a (or miR‐NC) and circ0008035 wild‐type reporter vector (circ‐wt) or mutant reporter vector (circ‐mut) was analyzed using a dual‐luciferase reporter assay. (C) The expression of circ0008035 in SNU1 and AGS cells. (D) The levels of miR‐302a in SNU1 and AGS cells. (E) Prediction binding sites of miR‐302a and E2F7 mRNA. (F) Luciferase activity in SNU1 and AGS cells co‐transfected with miR‐302a (or miR‐NC) and E2F7 wild‐type reporter vector (E2F7‐wt) or mutant reporter vector (E2F7‐mut). (G) The levels of miR‐302a in SNU1 and AGS cells transfected with miR‐302a mimic, or miR‐302a inhibitor (anti‐miR‐302a). The levels of E2F7 mRNA (H) and protein (I) in SNU1 and AGS cells. (J) The levels of E2F7 mRNA and protein in SNU1 cells transfected with miR‐302a mimic, and miR‐302a mimic+circ0008035‐oe. (K) The levels of E2F7 mRNA and protein in AGS cells transfected with anti‐miR‐302a, and anti‐miR‐302a + si‐circ0008035. *p < 0.05, **p < 0.01.
3.6. circ0008035 mediated DEX‐induced ferroptotic cell death in GC cells via miR‐302a/E2F7 axis
The miR‐302a levels were increased in SNU1 and AGS cells treated with DEX, which was reversed by circ0008035‐oe transfection (Figure 6A). Notably, the reduced expression of E2F7 in DEX‐treated SNU1 and AGS cells was abrogated by circ0008035‐oe transfection (Figure 6B,C). In DEX‐treated SNU1 and AGS cells, the effects of circ0008035‐oe on cell viability (Figure 6D) and cell death (Figure 6E) were abolished by the miR‐302a and si‐E2F7 transfections. Additionally, miR‐302a upregulation and E2F7 downregulation reversed the inhibition of circ0008035 overexpression on oxidative stress in DEX‐treated SNU1 and AGS cells, resulting in elevated levels of ROS and reduced activity of GSH (Figure 6F,G). Moreover, miR‐302a overexpression and E2F7 suppression abolished the effect of circ0008035 overexpression on iron production in DEX treated‐SNU1 and AGS cells (Figure 6H). Besides, the effect of circ0008035 overexpression on the expression of SLC7A11 and GPX4 in DEX‐treated SNU1 and AGS cells were recovered by miR‐302a mimic and si‐E2F7 transfection (Figure 6I,J). Therefore, circ0008035 modulated the DEX‐mediated ferroptotic cell death of GC cells via the miR‐302a/E2F7 axis.
FIGURE 6.

circ0008035 mediated DEX‐induced ferroptotic cell death in GC cells via miR‐302a/E2F7 axis. (A) The effects of DEX on miR‐302a expression. The effects of DEX on E2F7 mRNA (B) and protein (C) expression. (D) The viability of DEX‐treated SNU1 and AGS cells. (E) The cell death of DEX‐treated SNU1 and AGS cells. The levels of ROS (F), GSH (G), and iron (H) in DEX‐treated SNU1 and AGS cells. (I‐J) The expression of SLC7A11 and GPX4 in DEX‐treated SNU1 and AGS cells. *p < 0.05, **p < 0.01.
3.7. DEX inhibited tumorigenesis of GC cells in vivo
Tumor volume and weight were lower in the DEX groups than those in the control group (Figure 7A,B). DEX treatment reduced the number of Ki67‐positive cells compared to the control group (Figure 7C). Simultaneously, we validated that DEX treatment promoted GC cell death, as determined by the TUNEL staining assay (Figure 7C). Furthermore, DEX treatment increased the levels of ROS and iron but decreased the levels of GSH, SLC7A11, and GPX4 in xenograft tumors (Figure 7D,G). Moreover, compared to the control group, the levels of circ0008035 and E2F7 were markedly lower, but the levels of miR‐302a were significantly higher in tumors from DEX‐treated mice (Figure 7H,K).
FIGURE 7.
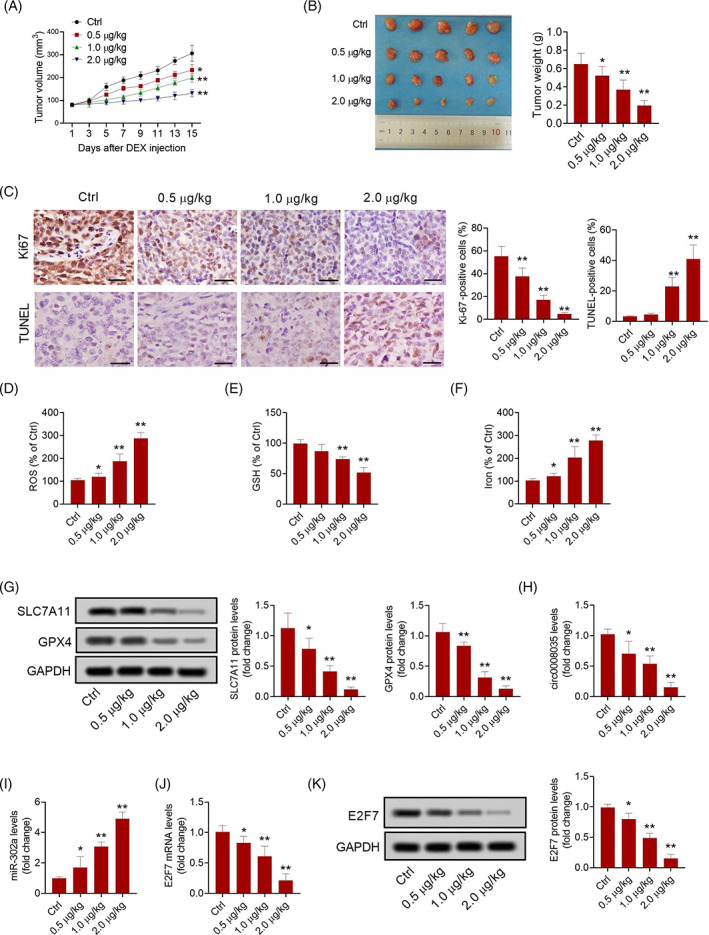
DEX inhibited tumorigenesis of GC cells in vivo. (A) The volume of xenograft tumors. (B) The weight of xenograft tumors. (C) Immunohistochemistry analysis of Ki67 and TUNEL staining in xenograft tumors (Bar = 50 μm). The levels of ROS (D), GSH (E), and iron (F) in xenograft tumors. (G) The expression of SLC7A11 and GPX4 in xenograft tumors. The expression of circ0008035 (H), and miR‐302a (I) in xenograft tumors. The expression of E2F7 mRNA (J) and protein (K) in xenograft tumors. N = 5. *p < 0.05, **p < 0.01.
4. DISCUSSION
DEX is a regularly used analgesic in clinical surgery that dramatically minimizes the requirement for opioids intraoperative and postoperative. 18 Several studies have shown that DEX protects immune function, reduces the inflammatory response in patients undergoing cancer surgery, and inhibits the growth of tumor cells, which may benefit cancer patients. 19 However, it has also been shown that DEX decreases overall survival after lung cancer surgery 20 and stimulates the growth of certain types of cancer cells. 21 Studies of DEX in clinical applications in GC patients have confirmed its safety. 22 However, studies on the effects of DEX on the biological properties of GC cells are relatively lacking. Exploring the molecular mechanism of the effect of DEX on GC cells may be beneficial to the improvement of intraoperative anesthetic delivery. In this investigation, we discovered that DEX caused GC cell death, which could be connected to ferroptosis. Mechanistically, DEX inhibited the development of GC by regulating the circ0008035/miR‐302a/E2F7 axis.
Ferroptosis is a unique type of programmed death. Unlike necrosis, autophagy, and apoptosis, ferroptosis is characterized by GPX4 inhibition and consequent intracellular buildup of ROS in an iron‐dependent manner. 23 Inducing ferroptosis in cancer cells to destroy them in reaction to certain substances could pave the way for new cancer treatments. 24 In the present study, DEX caused ferroptotic cell death by raising the amounts of ROS and iron in GC cells. SLC7A11 and GPX4 were downregulated in DEX‐treated GC cells, indicating that DEX‐mediated cell death was dependent on ferroptosis. Furthermore, we discovered that Fer‐1 prevented DEX‐caused ferroptotic cell death. As elucidated by the study of Qiu et al. 25 DEX modulated iron metabolism by modulating iron importers and exporters. Wang et al. 26 confirmed that DEX could prevent sepsis‐induced cardiac cellular damage by regulating ferroptosis. We hypothesized that the anti‐cancer impact of DEX in GC might be accompanied by ferroptosis, which may provide a novel sight for GC therapy.
Previous research has shown that anesthetics could exert their biological roles via modulating the expression of noncoding RNAs, such as circRNAs. 27 DEX, by modulating circ‐Shank3, enhanced hippocampal function in rats with postoperative cognitive impairment. 28 Through the lens of circRNAs, we aimed to study the anti‐cancer effects of DEX in GC. CircRNAs play an important role in GC cell proliferation, apoptosis, migration, and invasion. 29 Circ0008035 is derived from the EXT1 gene via splicing and has been demonstrated to be overexpressed in gastric cancer tissues and cells. 13 Previous studies discovered that silencing of circ0008035 inhibited the formation of GC cells. 14 We found that circ0008035 was consistently upregulated in GC cells and GC tissues. More notably, the current study discovered that DEX suppressed the expression of circ0008035 in both SNU1 and AGS cells. CircRNAs have garnered a lot of interest for their function in ferroptosis. By triggering ferroptosis, circRNA cIARS improved the susceptibility of hepatocellular carcinoma cells to sorafenib and erastin. 30 Previous study illustrated that circ0008035 accelerated the course of GC by reducing ferroptosis. 14 In this investigation, we discovered that overexpression of circ0008035 prevented DEX‐induced ferroptosis in GC cells, revealing that circ0008035 acts as a vital player in the anticancer effect of DEX in GC.
Given the capacity of circRNAs to bind to miRNAs, we used the bioinformatics website to find the potential miRNA that binds to circ0008035, miR‐302a. MiR‐302a was identified to be a tumor suppressor gene, which was overexpressed and regulated the expression of oncogenes (such as RELA, SOCS5, and FGF19) in several malignancies. 31 , 32 Ma et al. 33 found that GC patients with reduced miR‐302a expression may have aggressive cancer progression and unfavorable prognosis. We also identified E2F7 as a downstream target of miR‐302a, which was thought to be an oncogene in numerous malignancies. 34 , 35 The E2F is a family of transcription factor proteins with extensive functionality, including modulation of cell cycle, cell differentiation, DNA damage responses, and cell death. 36 E2F7 was confirmed to be overexpressed in GC cells. 37 Circ0008035 also prevented the inhibitory effect of miR‐302a on E2F7 expression. According to these findings, Circ0008035 increased E2F7 expression in GC cells by acting as a miR‐302a sponge. Importantly, DEX increased the expression of miR‐302a, but decreased the expression of E2F7 in GC cells, and these effects were reversed by overexpression of circ0008035. A previous study has demonstrated that DEX regulated the malignant phenotype of ovarian cancer cells via the miR‐155/HIF‐1 axis. 8 Furthermore, DEX inhibited the development of esophageal cancer via altering the miR‐143‐3p/epidermal growth factor receptor pathway substrate eight axis. 38 Therefore, we speculated that the pro‐ferroptosis effects of DEX on GC cells were at least partially mediated by the circ0008035/miR‐302a/E2F7 axis.
However, there are several limits in this study. The administration of DEX in the clinical setting is via inhalation and intravenous infusion, the study design of the animal model experiment (intraperitoneal injection) may not fully represent the real situation. Moreover, the current study lacks the relationship between the circ0008035/miR‐302a/E2F7 axis and the clinicopathological characteristics and prognosis of GC patients because there was not enough time to collect follow‐up data from patients. In addition, we were unable to determine the effect of DEX on primary gastric cancer cells due to the lack of tumor tissue samples from GC patients. In future studies, we will concentrate on this element.
In conclusion, the current work studied the role of DEX in GC cells and discovered that DEX suppressed GC growth by causing ferroptosis. Furthermore, the circ0008035/miR‐302a/E2F7 axis was involved in DEX‐induced ferroptotic cell death in GC. This novel regulatory mechanism may provide an experimental foundation for GC patients in selecting an appropriate anesthetic approach in the clinic.
CONFLICT OF INTEREST
All authors declare no conflict of interest.
ETHICS STATEMENT
There is no statement of Ethics to declare because we used the established human cancer cell lines (SNU1 and AGS) that are commonly used in Life Science field. All animal experiments performed in this study have been approved by Institutional Animal Care and Use Committee of the Affiliated People's Hospital of Ningbo [SYXK (Zhe) 2019–0005].
Gao X, Wang X‐L. Dexmedetomidine promotes ferroptotic cell death in gastric cancer via hsa_circ_0008035/miR‐302a/E2F7 axis. Kaohsiung J Med Sci. 2023;39(4):390–403. 10.1002/kjm2.12650
REFERENCES
- 1. Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(2):167–92. [DOI] [PubMed] [Google Scholar]
- 2. Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Malo‐Manso A, Raigon‐Ponferrada A, Diaz‐Crespo J, Escalona‐Belmonte JJ, Cruz‐Mañas J, Guerrero‐Orriach JL. Opioid free anaesthesia and cancer. Curr Pharm Des. 2019;25(28):3011–9. [DOI] [PubMed] [Google Scholar]
- 4. Qiu G, Wu Y, Yang Z, Li L, Zhu X, Wang Y, et al. Dexmedetomidine activation of dopamine neurons in the ventral tegmental area attenuates the depth of sedation in mice. Anesthesiology. 2020;133(2):377–92. [DOI] [PubMed] [Google Scholar]
- 5. Wang Z, Shen Z, Wang H, Zhang L, Dong R. Effect of dexmedetomidine on the cognitive function of patients undergoing gastric cancer surgery by regulating the PI3K/AKT signaling pathway. Oncol Lett. 2020;19(2):1151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dong W, Chen MH, Yang YH, Zhang X, Huang MJ, Yang XJ, et al. The effect of dexmedetomidine on expressions of inflammatory factors in patients with radical resection of gastric cancer. Eur Rev Med Pharmacol Sci. 2017;21(15):3510–5. [PubMed] [Google Scholar]
- 7. Xia M, Ji NN, Duan ML, Tong JH, Xu JG, Zhang YM, et al. Dexmedetomidine regulate the malignancy of breast cancer cells by activating α2‐adrenoceptor/ERK signaling pathway. Eur Rev Med Pharmacol Sci. 2016;20(16):3500–6. [PubMed] [Google Scholar]
- 8. Zheng L, Jia R, Zhao J. Dexmedetomidine regulates proliferation, apoptosis, migration, and invasion in ovarian cancer cells via MiR‐155‐HIF‐1α axis. Med Sci Monit. 2019;25:10164–72. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9. Cai Q, Liu G, Huang L, Guan Y, Wei H, Dou Z, et al. The role of dexmedetomidine in tumor‐progressive factors in the perioperative period and cancer recurrence: a narrative review. Drug Des Devel Ther. 2022;16:2161–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu R, Suo S, Wang Y, Wang M. Effects of dexmedetomidine and propofol on postoperative analgesia and the cellular immune function of patients undergoing radical gastrectomy for gastric cancer. Contrast Media Mol Imaging. 2022;2022:7440015. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11. Mahmoudi E, Green MJ, Cairns MJ. Dysregulation of circRNA expression in the peripheral blood of individuals with schizophrenia and bipolar disorder. J Mol Med. 2021;99(7):981–91. [DOI] [PubMed] [Google Scholar]
- 12. He J, Zhao H, Liu X, Wang D, Wang Y, Ai Y, et al. Sevoflurane suppresses cell viability and invasion and promotes cell apoptosis in colon cancer by modulating exosome‐mediated circ‐HMGCS1 via the miR‐34a‐5p/SGPP1 axis. Oncol Rep. 2020;44(6):2429–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang S, Zhang X, Guan B, Sun P, Hong CT, Peng J, et al. A novel circular RNA hsa_circ_0008035 contributes to gastric cancer tumorigenesis through targeting the miR‐375/YBX1 axis. Am J Transl Res. 2019;11(4):2455–62. [PMC free article] [PubMed] [Google Scholar]
- 14. Li C, Tian Y, Liang Y, Li Q. Circ_0008035 contributes to cell proliferation and inhibits apoptosis and ferroptosis in gastric cancer via miR‐599/EIF4A1 axis. Cancer Cell Int. 2020;20(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15. Chi M, Shi X, Huo X, Wu X, Zhang P, Wang G. Dexmedetomidine promotes breast cancer cell migration through Rab11‐mediated secretion of exosomal TMPRSS2. Ann Transl Med. 2020;8(8):531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tesfay L, Paul BT, Konstorum A, Deng Z, Cox AO, Lee J, et al. Stearoyl‐CoA desaturase 1 protects ovarian cancer cells from ferroptotic cell death. Cancer Res. 2019;79(20):5355–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tian H, Hou L, Xiong Y, Cheng Q, Huang J. Effect of dexmedetomidine‐mediated insulin‐like growth factor 2 (IGF2) signal pathway on immune function and invasion and migration of cancer cells in rats with ovarian cancer. Med Sci Monit. 2019;25:4655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tang C, Xia Z. Dexmedetomidine in perioperative acute pain management: a non‐opioid adjuvant analgesic. J Pain Res. 2017;10:1899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nair AS, Saifuddin MS, Naik V, Rayani BK. Dexmedetomidine in cancer surgeries: present status and consequences with its use. Indian J Cancer. 2020;57(3):234–8. [DOI] [PubMed] [Google Scholar]
- 20. Cata JP, Singh V, Lee BM, Villarreal J, Mehran JR, Yu J, et al. Intraoperative use of dexmedetomidine is associated with decreased overall survival after lung cancer surgery. J Anaesthesiol Clin Pharmacol. 2017;33(3):317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lavon H, Matzner P, Benbenishty A, Sorski L, Rossene E, Haldar R, et al. Dexmedetomidine promotes metastasis in rodent models of breast, lung, and colon cancers. Br J Anaesth. 2018;120(1):188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wan W, Hou Z, Qiu Q. Postoperative analgesic effect of dexmedetomidine combined with TPVB applied to open gastrectomy for gastric cancer. Immunopharmacol Immunotoxicol. 2022;1–6. [DOI] [PubMed] [Google Scholar]
- 23. Ma L, Zhang X, Yu K, Xu X, Chen T, Shi Y, et al. Targeting SLC3A2 subunit of system XC‐ is essential for m6A reader YTHDC2 to be an endogenous ferroptosis inducer in lung adenocarcinoma. Free Radic Biol Med. 2021;168:25–43. [DOI] [PubMed] [Google Scholar]
- 24. Wang Z, Diao J, Zhao X, Xu Z, Zhang X. Clinical and functional significance of a novel ferroptosis‐related prognosis signature in lung adenocarcinoma. Clin Transl Med. 2021;11(3):e364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qiu L, Ge L, Hu Q. Dexmedetomidine protects SK‐N‐SH nerve cells from oxidative injury by maintaining iron homeostasis. Biol Pharm Bull. 2020;43(3):424–31. [DOI] [PubMed] [Google Scholar]
- 26. Wang C, Yuan W, Hu A, Lin J, Xia Z, Yang CF, et al. Dexmedetomidine alleviated sepsis‐induced myocardial ferroptosis and septic heart injury. Mol Med Rep. 2020;22(1):175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu W, Xue R, Xia R, Liu WW, Zheng JW, Tang L, et al. Sevoflurane impedes the progression of glioma through modulating the circular RNA has_circ_0012129/miR‐761/TGIF2 axis. Eur Rev Med Pharmacol Sci. 2020;24(10):5534–48. [DOI] [PubMed] [Google Scholar]
- 28. Cao C, Deng F, Hu Y. Dexmedetomidine alleviates postoperative cognitive dysfunction through circular RNA in aged rats. 3 Biotech. 2020;10(4):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang D, Hu Z, Zhang Y, Zhang X, Xu J, Fu H, et al. CircHIPK3 promotes the tumorigenesis and development of gastric cancer through miR‐637/AKT1 pathway. Front Oncol. 2021;11:637761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu Z, Wang Q, Wang X, Xu Z, Wei X, Li J. Circular RNA cIARS regulates ferroptosis in HCC cells through interacting with RNA binding protein ALKBH5. Cell Death Discov. 2020;6:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luo Z, Yi ZJ, Ou ZL, Han T, Wan T, Tang YC, et al. RELA/NEAT1/miR‐302a‐3p/RELA feedback loop modulates pancreatic ductal adenocarcinoma cell proliferation and migration. J Cell Physiol. 2019;234(4):3583–97. [DOI] [PubMed] [Google Scholar]
- 32. Zhang Z, Li J, Guo H, Wang F, Ma L, Du C, et al. BRM transcriptionally regulates miR‐302a‐3p to target SOCS5/STAT3 signaling axis to potentiate pancreatic cancer metastasis. Cancer Lett. 2019;449:215–25. [DOI] [PubMed] [Google Scholar]
- 33. Ma G, Li Q, Dai W, Yang X, Sang A. Prognostic implications of miR‐302a/b/c/d in human gastric cancer. Pathol Oncol Res. 2017;23(4):899–905. [DOI] [PubMed] [Google Scholar]
- 34. Yuan Y, Zhou X, Kang Y, Kuang H, Peng Q, Zhang B, et al. Circ‐CCS is identified as a cancer‐promoting circRNA in lung cancer partly by regulating the miR‐383/E2F7 axis. Life Sci. 2021;267:118955. [DOI] [PubMed] [Google Scholar]
- 35. Wang Y, Pei X, Xu P, Tan Z, Zhu Z, Zhang G, et al. E2F7, regulated by miR‐30c, inhibits apoptosis and promotes cell cycle of prostate cancer cells. Oncol Rep. 2020;44(3):849–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Manicum T, Ni F, Ye Y, Fan X, Chen BC. Prognostic values of E2F mRNA expression in human gastric cancer. Biosci Rep. 2018;38(6):BSR20181264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang F, Guo C, Cao X, Yan Y, Zhang J, Lv S. Gastric cancer cell‐derived extracellular vesicles elevate E2F7 expression and activate the MAPK/ERK signaling to promote peritoneal metastasis through the delivery of SNHG12. Cell Death Discov. 2022;8(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang P, He H, Bai Y, Liu W, Huang L. Dexmedetomidine suppresses the progression of esophageal cancer via miR‐143‐3p/epidermal growth factor receptor pathway substrate 8 axis. Anticancer Drugs. 2020;31(7):693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]


