Abstract
Nonalcoholic fatty liver disease (NAFLD) is a hepatic metabolic syndrome with a rapidly increasing prevalence globally. Plantamajoside (PMS), a phenylethanoid glycoside component extracted from Plantago asiatica, has various biological properties. However, its effect on NAFLD remains unknown. The study aimed to explore the effect and mechanism of PMS on NAFLD in the high‐fat diet (HFD)‐feeding rats. PMS induced a decrease in body and liver weight, and the amelioration in the blood lipid parameters and pathological symptoms in HFD‐feeding rats. The increase in the serum concentrations and the relative protein expressions of proinflammatory factors was decreased by the PMS treatment in HFD‐induced NAFLD rats. Additionally, PMS reduced the excessive lipid vacuoles, and modified the relative expressions of proteins involved in the fatty acid synthesis and uptake in HFD‐feeding rats. Mechanically, the downregulation of AMPK/Nrf2 pathway in HFD‐feeding rats was restored by the PMS treatment. Inhibition of AMPK pathway reversed the PMS‐induced the increase in the level of inflammatory factors, pathological symptoms, excessive lipid vacuoles, and the relative expression of proteins involved in the fatty acid synthesis and uptake. Collectively, PMS ameliorated immune dysregulation and abnormal hepatic lipid metabolism by activating AMPK/Nrf2 pathway in rats with NAFLD.
Keywords: AMPK/Nrf2, hepatic lipid metabolism, immune deregulation, nonalcoholic fatty liver disease, plantamajoside
1. INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common hepatic disorder worldwide with the characteristics of hepatic fat accumulation and hepatocyte steatosis, which can further develop steatohepatitis, cirrhosis, fibrosis, and even hepatocellular carcinoma. 1 It is reported that the overall prevalence of NAFLD is 32.4% globally, which is prominently higher than the previous estimation with a prevalence of 25%. 2 Several factors contribute to the development of NAFLD, such as obesity, diabetes mellitus, hyperlipidemia, and insulin resistance. 1 The prevalence of NAFLD predictably increases at a striking rate, especially with the rapidly increasing of obesity cases. NAFLD is demonstrated to be the leading cause of liver‐associated incidence and mortality. Several approaches, such as weight loss, amelioration of insulin resistance, as well as healthy lifestyle are still critical strategies for the prevention and treatment of NAFLD. 3 In addition, multiple drugs are in advanced stages of development, however, no pharmaceutical therapy has been approved for NAFLD. 3 Therefore, continued research to develop viable drugs remains urgent for the clinical treatment of NAFLD.
Inflammation is a crucial pathogenesis for the development of NAFLD that contributes to the transformation from NAFLD to nonalcoholic steatohepatitis (NASH). 4 Also, inflammation can boost liver fibrosis, which eventually results in cirrhosis. It is proposed that the existence of histological inflammation can act as independent predictor of the development of NASH to advanced fibrosis. 5 Inflammation can serve as the straw for the break of NAFLD liver. In addition, abnormal lipids accumulation is widely found within the liver of NAFLD. 6 Thus, alleviation of hepatic lipid metabolism is a potential strategy for the treatment of NAFLD.
Plantamajoside (PMS), with a formula of C29H36O16, is an unique phenylethanoid glycoside component extracted from Plantago asiatica. 7 PMS has been revealed to has multitudinous biological capacities, such as anti‐inflammation, antioxidant, anti‐fibrosis, anticancer, antiviral abilities, and improvement of immunity. 8 PMS inhibits the level of pro‐inflammatory factors in osteoarthritis via repressing the NF‐κB and MAPK signaling. 9 The inhibitory effect of PMS on inflammatory response through suppressing PI3K/AKT pathway is also reported in lipopolysaccharide‐induced human gingival fibroblasts. 10 PMS also shows the anti‐fibrotic activities in the liver and heart. 11 , 12 Additionally, PMS attenuates the progression, epithelial–mesenchymal transition, and drug resistance of hepatocellular carcinoma. 13 , 14 However, the effect of PMS on NAFLD is still uncharted.
Therefore, a NAFLD rat model was constructed by the feed of high‐fat diet (HFD). The effect of PMS on NAFLD was investigated, and then the function of PMS on immune dysregulation and hepatic lipid metabolism was further addressed in HFD‐feeding rats. Moreover, the related mechanism was also explored in HFD‐induced NAFLD rats.
2. MATERIALS AND METHODS
2.1. Animal
Male Sprague Dawley rats (180–220 g) were provided by Junke biological Co., LTD. (Nanjing, China) and fed in the laboratory room with 40%–60% relative humidity and 12 h/12 h light–dark cycle at 22°C. All the procedures were strictly conducted based on the Board and Ethics Committee of Inner Mongolia Minzu University.
2.2. Animal treatment
Following the acclimatization for 1 week, rats were divided into six groups randomly (n = 6), including sham, HFD, HFD + PMS (20, 40, and 80 mg/kg), and HFD + 80 mg/kg PMS + CC. HFD rats were fed with 60% fat diet (TP23400, Trophic Animal Feed High‐Tech Co., Ltd., Nanjing, China) for 4 weeks to induce a NAFLD model as the previous study, 15 while rats in sham group were supplied with 10% fat diet (LAD 0024, Trophic Animal Feed High‐Tech Co., Ltd.) for the same time. Rats in HFD + PMS (20, 40, and 80 mg/kg) groups were intraperitoneally injected with PMS (20, 40, and 80 mg/kg), 16 , 17 while rats in sham and HFD groups were intraperitoneally administrated with the same dose of saline (ST341, Beyotime, Shanghai, China). PMS was bought from Sigma (PHL83304, St. Louis, MO, USA) with a purity of ≥95.0% (HPLC). Rats in HFD + 80 mg/kg PMS + CC group were intraperitoneally injected with 80 mg/kg PMS and intragastrically administered with the inhibitor of AMPK pathway compound C (CC, 0.2 mg/kg) (P5499, purity≥98% [HPLC], Sigma) 18 once a day for 4 weeks. At the end of experiment, rats were weighed, and then intraperitoneally injected with sodium pentobarbital (100 mg/kg) for sacrifice. Blood was collected from the carotid artery and then centrifuged to isolate the serum. In addition, live tissues were quickly removed, weighed, and fixed into 4% formaldehyde (P0099, Beyotime) or − 80°C for the following assays.
2.3. Biochemical examination
The serum concentrations of biochemical makers, including total cholesterol (TC), triglycerine (TG), high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol (LDL‐C), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were measured by commercial kits based on the operation instruction.
2.4. Hematoxylin and eosin and Oil red O staining
Fixed liver tissues were subjected to embeddedness, and then cut into pieces (5 μm) for hematoxylin and eosin (H&E) staining by Hematoxylin and Eosin Staining Kit (C0105S, Beyotime). The histopathology was scored according to the previous study. 19 Additionally, the accumulation of lipid droplets in liver tissues was examined by Oil red O staining (C0157S, Beyotime), and the percent of Oil red O staining was determined by ImageJ software (National Institutes of Health, USA). Pictures were captured using a light microscope (Olympus, Tokyo, Japan).
2.5. Enzyme linked‐immunosorbent assay
The serum concentrations of interleukin (IL)‐1β, IL‐6 and tumor necrosis factor (TNF)‐α were measured by commercial enzyme linked‐immunosorbent assay (ELISA) kits, including Rat IL‐1β ELISA Kit (PI303, Beyotime), Rat IL‐6 ELISA Kit (PI328, Beyotime), and Rat TNF‐α ELISA Kit (PT516, Beyotime) according to the operating manual. The absorbance at 450 nm was read using a microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).
2.6. Western blot
Liver tissues were lysed with RIPA lysis buffer (P0013B, Beyotime) and quantified with BCA Protein Assay Kit (P0012S, Beyotime). Protein samples (20 μg) were separated using 10% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and then transferred onto PVDF membranes (IPVH00010, EMD Millipore, Billerica, MA, USA). The membranes were blocked in 5% skimmed milk powder (P0216, Beyotime) at room temperature for 1 h, and then treated with primary antibodies at 4°C overnight, followed by the incubation with the Goat Anti‐Rabbit IgG H&L (HRP) (1:20000, ab6721, Abcam, Cambridge, UK) at room temperature for 1 h. The bands were visualized using a BeyoECL Plus kit (P0018S, Beyotime). The gray value was measured using Image‐ProPlus software (Media Cybernetics, Inc., Rockville, MD, USA). Protein expression levels were calculated after being normalized with GAPDH. The primary antibodies included anti‐IL‐1β (1:1000, ab254360, Abcam), anti‐IL‐6 (1:1000, ab259341, Abcam), anti‐TNF‐α (1:1000, ab286149, Abcam), anti‐SREBF1 (1:2000, AF8055, Beyotime), anti‐PPARγ (1:1000, ab209350, Abcam), anti‐FABP1 (1:3000, ab153924, Abcam), anti‐CPT1α (1:1000, ab234111, Abcam), anti‐AMPK (1:1000, ab207442, Abcam), anti‐phosphorylated AMPK (p‐AMPK) (1:5000, ab133448, Abcam), anti‐HO‐1 (1:2000, ab13243, Abcam), anti‐Nrf2 (1:1000, 20,733, Cell Signaling Technology, Inc., Danvers, MA, USA), anti‐Histone H3 (:5000, ab1791, Abcam), and anti‐GAPDH (1:10000, ab181602, Abcam).
2.7. Statistical analysis
Statistical analysis was performed using SPSS 20.0 software (IBM Corp., Armonk, NY, USA). All data were shown as mean ± standard deviation (SD). The difference among multiple groups was determined by one‐way analysis of variance followed by post hoc Bonferroni test. The difference was defined as statistically significant when p < 0.05.
3. RESULTS
3.1. PMS relieved liver damage in HFD‐induced NAFLD rats
To investigate the effect of PMS on NAFLD, rats were fed with HFD for 4 weeks and simultaneously administrated with PMS. As shown in Figure 1A, a significant difference in body weight between the HFD group and the sham group can be observed at week one. HFD induced a prominent increase in body weight, which was observably decreased with 40 and 80 mg/kg PMS treatment, but not 20 mg/kg PMS treatment (Figure 1A). The same results were also indicated in live weight (Figure 1B). Besides, administration of 40 and 80 mg/kg PMS significantly reduced HFD‐induced the serum concentrations of ALT, AST, LDL‐C, TG, and TC, while reverse outcomes were found in the serum concentrations of HDL‐C (Figure 1C–H). The irregular hepatocyte arrangement and inflammatory cell infiltration were shown in HFD‐feeding rats, which were obviously ameliorated by the treatment of 40 and 80 mg/kg PMS (Figure 1I). Correspondingly, 40 and 80 mg/kg PMS treatment, but not 20 mg/kg PMS treatment significantly reduced the HFD‐induced pathology scores (Figure 1I). Taken together, PMS alleviated liver injury in rats with NAFLD.
FIGURE 1.

PMS alleviated liver injury in rats with NAFLD. (A) The weight change of the rat from the beginning to the end of the experiment (a total of 4 weeks) was monitored. (B) The liver weight. (C–H) The serum concentrations of ALT, AST, HDL‐C, LDL‐C, TG, and TC were measured by commercial kits. (I) The histopathological examination by HE staining and score. The black arrows indicated the immune cell infiltration. Scale bar = 50 μm. ALT, alanine aminotransferase; AST, aspartate aminotransferase; HDL‐C, high‐density lipoprotein cholesterol; HFD, high‐fat diet; LDL‐C, low‐density lipoprotein cholesterol; NAFLD, Nonalcoholic fatty liver disease; PMS, Plantamajoside; TC, total cholesterol; TG, triglycerine. **p < 0.01 and ***p < 0.001 versus sham; # p < 0.05 and ## p < 0.01 versus HFD.
3.2. PMS improved immune dysregulation in HFD‐feeding rats
To evaluate the effect of PMS on inflammation in rats with NAFLD, the release of inflammatory factors, including IL‐1β, IL‐6, and TNF‐α was determined. Results from Figure 2A revealed that the serum concentrations of IL‐1β, IL‐6, and TNF‐α were prominently increased in HFD‐feeding rats, which were markedly counteracted with 40 and 80 mg/kg PMS treatment, but not 20 mg/kg PMS treatment. Additionally, treatment of 40 and 80 mg/kg PMS significantly decreased the HFD‐induced the relative protein expressions of IL‐1β, IL‐6, and TNF‐α in liver tissues (Figure 2B). Thus, PMS inhibited the release of inflammatory factors in rats with NAFLD.
FIGURE 2.

PMS inhibited the release of inflammatory factors in rats with NAFLD. (A) The serum concentrations of IL‐1β, IL‐6 and TNF‐α were measured by ELISA kits. (B) The relative protein expressions of IL‐1β, IL‐6 and TNF‐α in liver tissues were examined by western blot. Data were expressed after being normalized with GAPDH. HFD, high‐fat diet; NAFLD, Nonalcoholic fatty liver disease; PMS, Plantamajoside. **p < 0.01 versus sham; # p < 0.05 and ## p < 0.01 versus HFD.
3.3. PMS ameliorated abnormal liver lipid metabolism in HFD‐feeding rats
In addition, the effect of PMS on liver lipid metabolism was evaluated in rats with NAFLD. Oil red O staining exhibited that excessive lipid vacuoles were found in HFD‐feeding rats, which were markedly reduced with 40 and 80 mg/kg PMS treatment, but not 20 mg/kg PMS treatment (Figure 3A). Consistently, 40 and 80 mg/kg PMS treatment notably decreased the percent area of Oil red O staining (Figure 3A). Moreover, the relative protein expressions of SREBF1, PPARγ, and FABP1 were observably increased in HFD‐feeding rats, which were markedly neutralized with 40 and 80 mg/kg PMS treatment, but not 20 mg/kg PMS treatment (Figure 3B). On the other hand, the relative protein expression of CPT1α was prominently reduced in HFD‐feeding rats, which was significantly recovered with 40 and 80 mg/kg PMS treatment, but not 20 mg/kg PMS treatment (Figure 3B). Thus, PMS improved liver lipid metabolism in rats with NAFLD.
FIGURE 3.

PMS ameliorated liver lipid metabolism in rats with NAFLD. (A) The accumulation of lipid droplets in liver tissues was determined by Oil red O staining. Scale bar = 50 μm. (B) The relative protein expressions of SREBF1, PPARγ, FABP1, and CPT1α were quantified by western blot. Data were expressed after being normalized with GAPDH. HFD, high‐fat diet; NAFLD, Nonalcoholic fatty liver disease; PMS, Plantamajoside. **p < 0.01 and ***p < 0.001 versus sham; # p < 0.05 and ## p < 0.01 versus HFD.
3.4. PMS activated the AMPK/Nrf2 pathway in HFD‐induced NAFLD rats
As demonstrated in Figure 4A, the relative protein level of p‐AMPK/AMPK was notably decreased in HFD‐feeding rats. Also, the relative protein expression of nuclear Nrf2 and HO‐1 was markedly reduced in HFD‐feeding rats. These results suggested that the expression of AMPK/Nrf2 pathway downregulated in HFD‐induced NAFLD rats. However, injection of 40 and 80 mg/kg PMS treatment, but not 20 mg/kg PMS markedly restored the HFD‐induced the relative protein level of p‐AMPK/AMPK, nuclear Nrf2, and HO‐1. Hence, PMS induced the activation of the AMPK/Nrf2 pathway in rats with NAFLD.
FIGURE 4.

PMS induced the activation of the AMPK/Nrf2 pathway in rats with NAFLD. (A and B) The relative protein expressions of AMPK, p‐AMPK, Nrf2, and HO‐1 were detected by western blot. Data were expressed after being normalized with GAPDH or Histone H3. HFD, high‐fat diet; NAFLD, Nonalcoholic fatty liver disease; PMS, Plantamajoside. **p < 0.01 versus sham; # p < 0.05 and ## p < 0.01 versus HFD.
3.5. PMS ameliorated immune dysregulation by activating AMPK/Nrf2 pathway in HFD‐induced NAFLD rats
To further confirm the direct role of AMPK/Nrf2 pathway in alleviative effect of PMS on immune disorders, mice were administrated with CC. Injection of CC markedly counteracted PMS‐increased the relative protein level of p‐AMPK/AMPK, Nrf2, and HO‐1 in HFD‐feeding rats (Figure 5A), which suggested that CC effectively inhibited AMPK/Nrf2 pathway in rats with NAFLD. Application of CC prominently rescued PMS‐reduced the serum concentrations of IL‐1β, IL‐6, and TNF‐α (Figure 5B) and the relative protein expressions of IL‐1β, IL‐6, and TNF‐α (Figure 5C,D) in HFD‐feeding rats. Altogether, PMS mitigated immune disorders in rats with NAFLD via activating AMPK/Nrf2 pathway.
FIGURE 5.

PMS relieved immune disorders via activating the AMPK/Nrf2 pathway in rats with NAFLD. (A) The relative protein expressions of AMPK, p‐AMPK, Nrf2, and HO‐1 were detected by western blot. Data were expressed after being normalized with GAPDH or Histone H3. (B) The serum concentrations of IL‐1β, IL‐6, and TNF‐α were measured by ELISA kits. (C and D) The relative protein expressions of IL‐1β, IL‐6, and TNF‐α in liver tissues were examined by western blot. Data were expressed after being normalized with GAPDH. HFD, high‐fat diet; NAFLD, Nonalcoholic fatty liver disease; PMS, Plantamajoside. **p < 0.01 versus sham; ## p < 0.01 versus HFD; & p < 0.05 versus HFD + 80 mg/kg PMS.
3.6. PMS improved abnormal liver lipid metabolism through the activation of the AMPK/Nrf2 pathway in HFD‐feeding rats
Additionally, the pathological symptoms, such as irregular hepatocyte arrangement and inflammatory cell infiltration, and the excessive lipid vacuoles were overtly alleviated with PMS treatment in HFD‐feeding rats, which were obviously aggravated by the supply of CC (Figure 6A,B). The corresponding histology score and the percent area of Oil red O staining also showed the same tendency (Figure 6A, B). Besides, administration of PMS significantly decreased the HFD‐induced the relative protein expressions of SREBF1, PPARγ, and FABP1, which were significantly reversed with the treatment of CC in rats (Figure 6C). However, injection of CC prominently antagonized PMS‐increased the relative protein expression of CPT1α in HFD‐feeding rats (Figure 6C). Totally, PMS ameliorated liver lipid metabolism through the activation of AMPK/Nrf2 pathway in rats with NAFLD.
FIGURE 6.
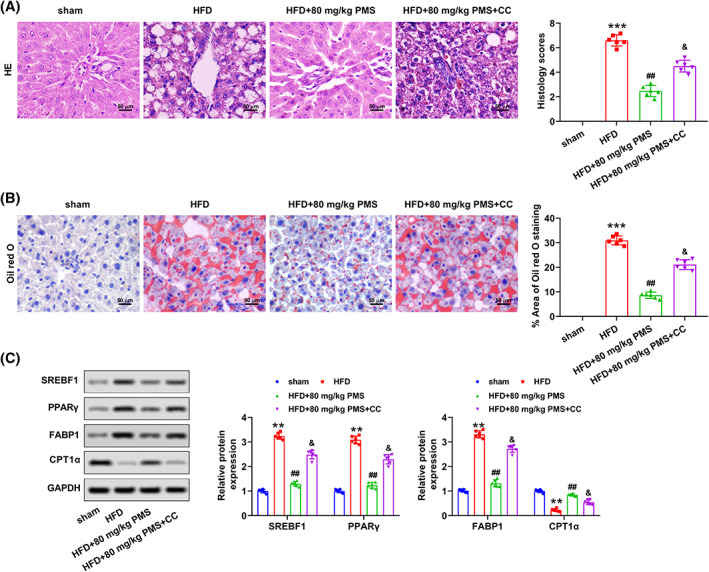
PMS improved liver lipid metabolism through the activation of the AMPK/Nrf2 pathway in rats with NAFLD. (A) The histopathological examination by HE staining and score. Scale bar = 50 μm. (B) The accumulation of lipid droplets in liver tissues was evaluated by Oil red O staining. Scale bar = 50 μm. (C) The relative protein expressions of SREBF1, PPARγ, FABP1, and CPT1α were examined by western blot. Data were expressed after being normalized with GAPDH. HFD, high‐fat diet; NAFLD, Nonalcoholic fatty liver disease; PMS, Plantamajoside. **p < 0.01 and ***p < 0.001 versus sham; ## p < 0.01 versus HFD; & p < 0.05 versus HFD + 80 mg/kg PMS.
4. DISCUSSION
In the present study, the effect and mechanism of PMS on NAFLD were explored in HFD‐feeding rats. The results showed that PMS alleviated liver damage, and improved immune dysregulation and abnormal hepatic lipid metabolism in HFD‐induced NAFLD rats. Mechanically, the downregulation in the relative protein level of p‐AMPK/AMPK, Nrf2, and HO‐1 in HFD‐feeding rats was restored by the treatment of PMS (Figure 7). Inhibition of AMPK pathway via CC reversed the PMS‐induced the increase in the level of inflammatory factors, pathological symptoms, excessive lipid vacuoles, and the relative expression of proteins involved in fatty acid synthesis and uptake. Taken together, PMS improved immune dysregulation and abnormal hepatic lipid metabolism in rats with NAFLD via activating AMPK/Nrf2.
FIGURE 7.
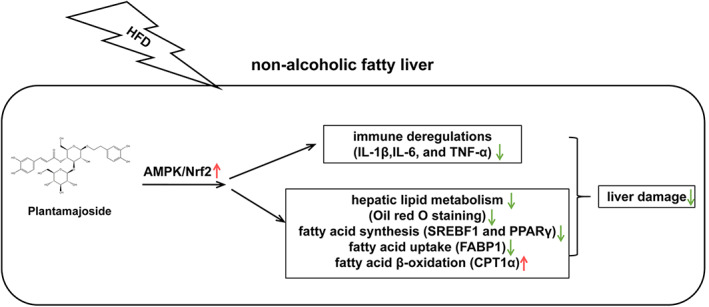
The overview of the present study. PMS mitigated liver damage in HFD‐induced NAFLD rats. At the molecular level, PMS improved immune dysregulation and abnormal hepatic lipid metabolism in HFD‐induced NAFLD rats. Mechanically, the downregulation in the relative protein level of p‐AMPK/AMPK, Nrf2, and HO‐1 in HFD‐feeding rats was restored by the treatment of PMS. Collectively, PMS improved immune dysregulation and abnormal hepatic lipid metabolism in rats with NAFLD via activating AMPK/Nrf2 pathway. HFD, high‐fat diet; NAFLD, Nonalcoholic fatty liver disease; PMS, Plantamajoside.
A crowd of plant extracts are demonstrated to regulate the progression and development of NAFLD in vitro and in vivo. For instance, Liu et al. 20 reported that PCP1, a homogeneous polysaccharide isolated from Polygonatum cyrtonema Hua rhizomes, relieves liver pathological damage, ameliorates oxidative stress, and abnormal lipid metabolism, accelerates the short‐chain fatty acids generation, and equilibrates the intestinal microbiota constitution in NAFLD mice. The natural flavonoid isoquercetin improves liver damage, inflammation, and glucose intolerance accompanied with the amelioration of metabolic disorders and the regulation of gut microbiota composition in NAFLD mice. 21 Carotenoids in orange carrots alleviate liver pathological injury and abnormal lipid metabolism in NAFLD mice. 22 PMS, a phenylethanoid glycoside component extracted from P. asiatica, has been shown to inhibit hepatic fibrosis via inactivating hepatic stellate cell, 11 suppress proliferation, epithelial–mesenchymal transition, and drug resistance of liver cancer, 13 , 14 as well as induce proliferation arrest, apoptosis and autophagy of hepatocellular carcinoma. 23 In the present study, PMS reduced HFD‐induced body, live weight, and the serum concentrations of ALT, AST, LDL‐C, TG, and TC, increased HFD‐induced the serum concentrations of HDL‐C, and ameliorated irregular hepatocyte arrangement and inflammatory cell infiltration in HFD‐feeding rats, in along with the previous studies. 24 , 25 Thus, PMS mitigated liver pathological damage in HFD‐induced NAFLD rats.
Chronic inflammation is revealed to drive the progression of NAFLD. 26 The expression and secretion of pro‐inflammatory factors, including IL‐1β, IL‐6, and TNF‐α were upregulated in mice with NAFLD in the current study, in accordance with the results from Wikan et al. 27 The increase in the serum concentrations of IL‐1β, IL‐6, and TNF‐α and the relative protein expressions of IL‐1β, IL‐6, and TNF‐α in liver tissues was also reduced by the treatment of PMS in HFD‐feeding rats. PMS has been revealed to reduce the levels of IL‐1β, IL‐6, and TNF‐α in myocardial ischemia/reperfusion injury, 28 diabetic nephropathy, 29 and acute lung injury. 30 Besides, PMS also suppresses inflammation in models of endothelial dysfunction, 31 periodontitis, 10 and osteoarthritis. 32 Totally, PMS improved immune dysregulation in HFD‐treated rats.
Dysregulated hepatic lipid homeostasis is acknowledged as one of the crucial cause of NAFLD, which can be resulted from increased uptake of liver TG and the synthesis of fatty acid, as well as reduced β‐oxidation. 33 Here, an elevated serum concentrations of TG and TC with excessive lipid vacuoles in the liver tissue indicated the fat deposition in HFD‐feeding rats. Moreover, the relative protein expressions of SREBF1, PPARγ, and FABP1 were increased, but that of CPT1α was reduced in HFD‐feeding rats. SREBF1, PPARγ, and FABP1 are identified to import fatty acids, or modulate the genes expression of proteins or enzymes related in lipid metabolism. 34 CPT1 is a rate limiting enzyme in catabolism, which takes charge of the TG hydrolysis and transports esters of fatty acids into mitochondria for β‐oxidation. 35 Thus, the results from the present study suggested that an unbalanced liver lipid metabolism was presented in HFD‐feeding rats. However, PMS reversed these changes in HFD‐feeding rats, which indicated that PMS improved liver lipid metabolism in rats with NAFLD.
AMPK widely distributes in the liver and skeletal muscle that modulates the lipid metabolism. The phosphorylated level of AMPK is the major kinase modulator of the downstream lipogenic enzymes, such as SREBP1, acetyl‐CoA carboxylase, and fatty acid synthase, which are tightly involved in the fatty acid synthesis. 36 Nrf2 plays an important role in neutralizing oxidative stress and conserving redox homeostasis in the cell through the generation of cytoprotective and antioxidant enzymes, such as HO‐1 and NQO1. 37 The role of Nrf2 in liver steatosis, inflammation, fibrosis, as well as related therapeutic strategies has been recently summarized by Bukke et al. 38 Besides, the studies that the natural plants components alleviate NAFLD via AMPK/Nrf2 pathway have been reported extensively. 36 , 39 , 40 In this study, the downregulation in the relative protein level of p‐AMPK/AMPK, Nrf2, and HO‐1 in HFD‐feeding rats was restored by the treatment of PMS, suggesting that PMS activated the AMPK/Nrf2 pathway in HFD‐induced NAFLD rats. Zeng et al 28 have reported that PMS promotes the activation of Nrf2/HO‐1 pathway in hypoxia/reoxygenation‐induced injury. Functionally, inhibition of AMPK pathway via CC reversed the PMS‐induced the increase in the level of inflammatory factors, pathological symptoms, excessive lipid vacuoles, and the relative expression of proteins involved in fatty acid synthesis and uptake. Taken together, PMS improved immune dysregulation and abnormal hepatic lipid metabolism via activating AMPK/Nrf2 in rats with NAFLD.
In summary, PMS alleviated liver pathological damage in HFD‐induced NAFLD rats. At the molecular level, PMS improved immune dysregulation and abnormal hepatic lipid metabolism in HFD‐induced NAFLD rats. Mechanically, PMS activated the AMPK/Nrf2 pathway in HFD‐induced NAFLD rats. Altogether, PMS ameliorated immune dysregulation and abnormal hepatic lipid metabolism via activating AMPK/Nrf2 pathway in rats with NAFLD. However, several limitations remain to be addressed in the future. Due to the pivotal role of Nrf2 in oxidative stress, combined with the fact that oxidative stress is also significant pathogenesis of NAFLD, the effect of PMS on oxidative stress can be discussed in HFD‐feeding rats in the following study. Additionally, the further preclinical and clinical trials are needed in the future. Briefly, the results expound that PMS may be a potential agent for the treatment of NAFLD.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflict of interest.
Wu J‐M, Zhaori G, Mei L, Ren X‐M, Laga AT, Deligen B. Plantamajoside modulates immune dysregulation and hepatic lipid metabolism in rats with nonalcoholic fatty liver disease via AMPK/Nrf2 elevation. Kaohsiung J Med Sci. 2023;39(8):801–810. 10.1002/kjm2.12712
REFERENCES
- 1. Powell EE, Wong VW, Rinella M. Non‐alcoholic fatty liver disease. Lancet. 2021;397(10290):2212–24. [DOI] [PubMed] [Google Scholar]
- 2. Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol. 2022;7(9):851–61. [DOI] [PubMed] [Google Scholar]
- 3. Mundi MS, Velapati S, Patel J, Kellogg TA, Abu Dayyeh BK, Hurt RT. Evolution of NAFLD and its management. Nutr Clin Pract. 2020;35(1):72–84. [DOI] [PubMed] [Google Scholar]
- 4. Albhaisi S, Noureddin M. Current and potential therapies targeting inflammation in NASH. Front Endocrinol. 2021;12:767314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Argo CK, Northup PG, Al‐Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non‐alcoholic steatohepatitis. J Hepatol. 2009;51(2):371–9. [DOI] [PubMed] [Google Scholar]
- 6. Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23(47):8263–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Samuelsen AB. The traditional uses, chemical constituents and biological activities of Plantago major L. A review. J Ethnopharmacol. 2000;71(1–2):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li Y, Gan L, Li GQ, Deng L, Zhang X, Deng Y. Pharmacokinetics of plantamajoside and acteoside from Plantago asiatica in rats by liquid chromatography‐mass spectrometry. J Pharm Biomed Anal. 2014;89:251–6. [DOI] [PubMed] [Google Scholar]
- 9. Wen Y, Zhan Y, Tang S, Kang J, Wu R, Tang X. Mechanistic prediction of Chinese herb compound (Zhi Zhu Ma Ren Pill) in the treatment of constipation using network pharmacology and molecular docking. Nat Prod Commun. 2022;17:1934578X2211247. [Google Scholar]
- 10. Liu F, Huang X, He JJ, Song C, Peng L, Chen T, et al. Plantamajoside attenuates inflammatory response in LPS‐stimulated human gingival fibroblasts by inhibiting PI3K/AKT signaling pathway. Microb Pathog. 2019;127:208–11. [DOI] [PubMed] [Google Scholar]
- 11. Wang Y, Yan D. Plantamajoside exerts antifibrosis effects in the liver by inhibiting hepatic stellate cell activation. Exp Ther Med. 2019;18(4):2421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang L, Guo YN, Liu J, Wang LH, Wu HQ, Wang T, et al. Plantamajoside attenuates cardiac fibrosis via inhibiting AGEs activated‐RAGE/autophagy/EndMT pathway. Phytother Res. 2023;37(3):834–47. [DOI] [PubMed] [Google Scholar]
- 13. Yin W, Xu J, Li C, Dai X, Wu T, Wen J. Plantamajoside inhibits the proliferation and epithelial‐to‐mesenchymal transition in hepatocellular carcinoma cells via modulating hypoxia‐inducible factor‐1α‐dependent gene expression. Cell Biol Int. 2020;44(8):1616–27. [DOI] [PubMed] [Google Scholar]
- 14. Zan Y, Dai Z, Liang L, Deng Y, Dong L. Co‐delivery of plantamajoside and sorafenib by a multi‐functional nanoparticle to combat the drug resistance of hepatocellular carcinoma through reprograming the tumor hypoxic microenvironment. Drug Deliv. 2019;26(1):1080–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu N, Luo H, Li M, Wu J, Wu X, Chen L, et al. β‐patchoulene improves lipid metabolism to alleviate non‐alcoholic fatty liver disease via activating AMPK signaling pathway. Biomed Pharmacother. 2021;134:111104. [DOI] [PubMed] [Google Scholar]
- 16. Lim L, Ki YJ, Kim H, Chu B, Choi IY, Choi DH, et al. Plantamajoside attenuates neointima formation via upregulation of tissue inhibitor of metalloproteinases in balloon‐injured rats. J Med Food. 2022;25(5):503–12. [DOI] [PubMed] [Google Scholar]
- 17. Hu H, Jian X. The protective mechanism of action of plantamajoside on a rat model of acute spinal cord injury. Exp Ther Med. 2021;21(4):378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mohammed HM. Zingerone ameliorates non‐alcoholic fatty liver disease in rats by activating AMPK. J Food Biochem. 2022;46(7):e14149. [DOI] [PubMed] [Google Scholar]
- 19. El‐Sherbiny M, Eldosoky M, El‐Shafey M, Othman G, Elkattawy HA, Bedir T, et al. Vitamin D nanoemulsion enhances hepatoprotective effect of conventional vitamin D in rats fed with a high‐fat diet. Chem Biol Interact. 2018;288:65–75. [DOI] [PubMed] [Google Scholar]
- 20. Liu W, Shao T, Tian L, Ren Z, Gao L, Tang Z, et al. Structural elucidation and anti‐nonalcoholic fatty liver disease activity of Polygonatum cyrtonema Hua polysaccharide. Food Funct. 2022;13(24):12883–95. [DOI] [PubMed] [Google Scholar]
- 21. Shi Z, Zhang C, Lei H, Chen C, Cao Z, Song Y, et al. Structural insights into amelioration effects of quercetin and its glycoside derivatives on NAFLD in mice by modulating the gut microbiota and host metabolism. J Agric Food Chem. 2022;70(46):14732–43. [DOI] [PubMed] [Google Scholar]
- 22. Balbuena E, Cheng J, Eroglu A. Carotenoids in orange carrots mitigate non‐alcoholic fatty liver disease progression. Front Nutr. 2022;9:987103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Z, Zuo J, Zhang L, Zhang Z, Wei Y. Plantamajoside promotes metformin‐induced apoptosis, autophagy and proliferation arrest of liver cancer cells via suppressing Akt/GSK3β signaling. Hum Exp Toxicol. 2022;41:9603271221078868. [DOI] [PubMed] [Google Scholar]
- 24. El‐Shial EM, Kabbash A, El‐Aasr M, El‐Feky OA, El‐Sherbeni SA. Elucidation of natural components of Gardenia thunbergia thunb. Leaves: effect of methanol extract and rutin on non‐alcoholic fatty liver disease. Molecules. 2023;28(2):879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Y, Geng C, Liu X, Li M, Gao M, Liu X, et al. Celastrol ameliorates liver metabolic damage caused by a high‐fat diet through Sirt1. Mol Metab. 2017;6(1):138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abdallah J, Assaf S, Das A, Hirani V. Effects of anti‐inflammatory dietary patterns on non‐alcoholic fatty liver disease: a systematic literature review. Eur J Nutr. 2023;62(4):1563–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wikan N, Tocharus J, Oka C, Sivasinprasasn S, Chaichompoo W, Suksamrarn A, et al. The capsaicinoid nonivamide suppresses the inflammatory response and attenuates the progression of steatosis in a NAFLD‐rat model. J Biochem Mol Toxicol. 2022;37:e23279. [DOI] [PubMed] [Google Scholar]
- 28. Zeng G, An H, Fang D, Wang W, Han Y, Lian C. Plantamajoside protects H9c2 cells against hypoxia/reoxygenation‐induced injury through regulating the akt/Nrf2/HO‐1 and NF‐κB signaling pathways. J Recept Signal Transduct Res. 2022;42(2):125–32. [DOI] [PubMed] [Google Scholar]
- 29. Xiao D, Yang R, Gong L, Zhang Y, Xie Y, Ni S. Plantamajoside inhibits high glucose‐induced oxidative stress, inflammation, and extracellular matrix accumulation in rat glomerular mesangial cells through the inactivation of Akt/NF‐κB pathway. J Recept Signal Transduct Res. 2021;41(1):45–52. [DOI] [PubMed] [Google Scholar]
- 30. Wu H, Zhao G, Jiang K, Chen X, Zhu Z, Qiu C, et al. Plantamajoside ameliorates lipopolysaccharide‐induced acute lung injury via suppressing NF‐κB and MAPK activation. Int Immunopharmacol. 2016;35:315–22. [DOI] [PubMed] [Google Scholar]
- 31. Son WR, Nam MH, Hong CO, Kim Y, Lee KW. Plantamajoside from Plantago asiatica modulates human umbilical vein endothelial cell dysfunction by glyceraldehyde‐induced AGEs via MAPK/NF‐κB. BMC Complement Altern Med. 2017;17(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin S, Lu J, Chen Q, Jiang H, Lou C, Lin C, et al. Plantamajoside suppresses the activation of NF‐κB and MAPK and ameliorates the development of osteoarthritis. Int Immunopharmacol. 2022;115:109582. [DOI] [PubMed] [Google Scholar]
- 33. Alannan M, Fayyad‐Kazan H, Trézéguet V, Merched A. Targeting lipid metabolism in liver cancer. Biochemistry. 2020;59(41):3951–64. [DOI] [PubMed] [Google Scholar]
- 34. Zhao S, Wang J, Song X, Zhang X, Ge C, Gao S. Impact of dietary protein on lipid metabolism‐related gene expression in porcine adipose tissue. Nutr Metab. 2010;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Deberardinis RJ, Lum JJ, Thompson CB. Phosphatidylinositol 3‐kinase‐dependent modulation of carnitine palmitoyltransferase 1A expression regulates lipid metabolism during hematopoietic cell growth. J Biol Chem. 2006;281(49):37372–80. [DOI] [PubMed] [Google Scholar]
- 36. Ye J, Zheng J, Tian X, Xu B, Yuan F, Wang B, et al. Fucoxanthin attenuates free fatty acid‐induced nonalcoholic fatty liver disease by regulating lipid metabolism/oxidative stress/inflammation via the AMPK/Nrf2/TLR4 signaling pathway. Mar Drugs. 2022;20(4):225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou J, Zheng Q, Chen Z. The Nrf2 pathway in liver diseases. Front Cell Dev Biol. 2022;10:826204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bukke VN, Moola A, Serviddio G, Vendemiale G, Bellanti F. Nuclear factor erythroid 2‐related factor 2‐mediated signaling and metabolic associated fatty liver disease. World J Gastroenterol. 2022;28(48):6909–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shen B, Wang Y, Cheng J, Peng Y, Zhang Q, Li Z, et al. Pterostilbene alleviated NAFLD via AMPK/mTOR signaling pathways and autophagy by promoting Nrf2. Phytomedicine. 2023;109:154561. [DOI] [PubMed] [Google Scholar]
- 40. Li Y, Liu J, Ye B, Cui Y, Geng R, Liu S, et al. Astaxanthin alleviates nonalcoholic fatty liver disease by regulating the intestinal flora and targeting the AMPK/Nrf2 signal axis. J Agric Food Chem. 2022;70(34):10620–34. [DOI] [PubMed] [Google Scholar]


