Abstract
Diabetic cardiomyopathy (DCM) is a serious cardiovascular complication of diabetes that severely affects the quality of life of diabetic patients. Long noncoding RNAs (lncRNAs) play important roles in the pathogenesis of DCM. However, the role of the lncRNA homeobox transcript antisense RNA (HOTAIR) in the progression of DCM remains unclear. The present study aimed to investigate the role of HOTAIR in high glucose (HG)‐induced pyroptosis in cardiomyocytes. The expression of the lncRNA HOTAIR, FUS, and SIRT3 in H9C2 cardiomyocytes was detected by RT–qPCR. Western blotting was used to evaluate the expression of FUS and SIRT3 as well as that of pyroptosis‐ and inflammation‐related proteins. RT–qPCR and ELISA were used to determine the expression and secretion of IL‐1β and IL‐18. RNA pulldown and RIP experiments were used to validate the binding relationship among HOTAIR, FUS, and SIRT3. Flow cytometry was performed to detect pyroptosis. HG induced pyroptosis and elevated the expression of proteins associated with pyroptosis and inflammation (NLRP3, GSDMD‐N, cleaved caspase‐1, IL‐1β, and IL‐18) in cardiomyocytes. HOTAIR and SIRT3 levels were decreased in HG‐exposed H9C2 cells. Additionally, overexpression of HOTAIR inhibited the HG‐induced pyroptosis and inflammatory response in cardiomyocytes. HOTAIR upregulated SIRT3 expression in H9C2 cells by targeting FUS. Moreover, SIRT3 upregulation suppressed HG‐mediated pyroptosis of cardiomyocytes. Notably, SIRT3 depletion reversed the inhibitory effect of HOTAIR on HG‐triggered pyroptosis in cardiomyocytes. Our research indicates that HOTAIR alleviates pyroptosis in diabetic cardiomyocytes through the FUS/SIRT3 axis, providing a potential marker for the diagnosis and treatment of DCM.
Keywords: diabetic cardiomyopathy, lncRNA HOTAIR, pyroptosis, SIRT3
Abbreviations
- DCM
diabetic cardiomyopathy
- ECL
enhanced chemiluminescence
- ELISA
enzyme‐linked immunosorbent assay
- HG
high glucose
- HOTAIR
homeobox transcript antisense RNA
- IL‐18
interleukin‐18
- IL‐1β
interleukin‐1β
- lncRNAs
long noncoding RNAs
- NAD+
nicotinamide adenine dinucleotide
- NLRP3
nucleotide oligomerization domain‐like receptor protein 3
- NLRs
NOD‐like receptors
- RBPs
RNA‐binding proteins
- RIP
RNA immunoprecipitation
- shRNAs
short hairpin RNAs
1. INTRODUCTION
Diabetic cardiomyopathy (DCM) is a unique heart disease caused by diabetes, one of the hallmarks of which is impaired myocardial glucose metabolism. 1 DCM is a major cause of increased morbidity and mortality in patients with diabetes and heart failure. 2 DCM can cause slight changes in myocardial structure in the early stage, gradually aggravate myocardial hypertrophy and fibrosis, lead to cardiac dysfunction, and eventually lead to the development of heart failure. 3 Therefore, finding effective targets for DCM is of great importance for the diagnosis and treatment of DCM. Pyroptosis is a form of cell death distinguished from necrosis and apoptosis. 4 The nucleotide oligomerization domain (NOD)‐like receptor protein 3 (NLRP3) inflammasome is a multiprotein heteromeric complex that can trigger the classical pathway of pyroptosis and the release of interleukin (IL)‐1β and IL‐18. 5 Studies have reported that DCM is associated with cardiomyocyte pyroptosis and the inflammatory response. 6 , 7 However, the exact mechanism in DCM remains unclear.
Long noncoding RNAs (lncRNAs) are a class of RNA transcripts longer than 200 nucleotides that participate in a variety of physiological and pathological processes. 8 Studies have confirmed that lncRNAs play important roles in various cardiovascular diseases, including hypertrophy, vascular disease and atherosclerosis. 9 Some lncRNAs participate in the pathophysiology of diabetes‐related complications, including cardiomyopathy, by regulating the inflammatory response, apoptosis and other processes. 10 The lncRNA homeobox transcript antisense RNA (HOTAIR) is transcribed from the antisense strand of the HOXC gene cluster and is overexpressed in a variety of malignancies. 11 One study found that the expression of HOTAIR was significantly reduced in the hearts of diabetic mice. 12 Nevertheless, whether HOTAIR regulates high glucose (HG)‐induced cardiomyocyte pyroptosis in DCM has not been reported.
The SIRT protein family, a group of nicotinamide adenine dinucleotide (NAD+)‐dependent deacetylases that deacetylate lysine residues to release acetylated substrates, is involved in DCM. 13 SIRT3 is a member of this family that is distributed mainly in mitochondria and plays important roles in mitochondrial oxidation, energy metabolism and apoptosis. 14 This protein is also a potential intervention target for various cardiovascular diseases, such as myocardial ischaemia–reperfusion injury, myocardial infarction and DCM. 15 Recent data indicate that melatonin effectively inhibits the development of DCM and promotes the expression of SIRT3 in DCM. 16 However, the role of SIRT3 in protecting against DCM has rarely been studied.
In the present study, we attempted to clarify the effect of HOTAIR on DCM and the underlying molecular mechanism. Bioinformatics analysis suggested that HOTAIR might regulate the expression of SIRT3 through FUS. Therefore, we speculate that HOTAIR can effectively alleviate the development of DCM through the FUS/SIRT3 signaling pathway.
2. MATERIALS AND METHODS
2.1. Cell culture
H9C2 cells were provided by the cell bank of the Chinese Academy of Sciences (Shanghai, China) and cultured in DMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 10% FBS (Invitrogen) and 1% penicillin–streptomycin (Sigma, St. Louis, MO, USA) at 37°C with 5% CO2. The cells were treated with normal glucose (NG, 5.5 mM) or HG (30 mM) (Sigma) for 24 h.
2.2. Cell transfection
Short hairpin RNAs (shRNAs) targeting HOTAIR (sh‐HOTAIR), FUS (sh‐FUS), or SIRT3 (sh‐SIRT3); a HOTAIR overexpression vector (OE‐HOTAIR); a SIRT3 overexpression vector (OE‐SIRT3); or their negative controls were obtained from GenePharma (Shanghai, China) and transfected into H9C2 cells using Lipofectamine 3000 (Invitrogen) following the manufacturer's instructions at approximately 80% cell confluence.
2.3. Real‐time quantitative PCR (RT–qPCR)
Total RNA was extracted using TRIzol reagent (Invitrogen). Reverse transcription of RNA was performed with a SuperScript II Reverse Transcriptase Kit (Invitrogen). The relative gene levels were determined with a SYBR Green PCR Kit (Takara, Tokyo, Japan) using a 7500 Thermocycler (Applied Biosystems, Foster City, CA, USA). The amplification primers were as follows: HOTAIR F: 5′‐TTGCCCCCAGCAAGAATCAT‐3′, R: 5′‐GTTCCGGAAATCAGGGCAGA‐3′; SIRT3 F: 5′‐AGCTGCCAGCAAGGTTCTTA‐3′, R: 5′‐CCTTTCCACACCCTGGACTA‐3′; FUS F: 5′‐GCTACTCCCAACAGAGCAGTCA‐3′, R: 5′‐GAGCTGACTGAGTTCCATAGCC‐3′; IL‐1β F: 5′‐CAGCAGCATCTCGACAAGAG‐3′, R: 5′‐CATCATCCCACGAGTCACAG‐3′; and IL‐6 F: 5′‐CCACCCACAACAGACCAGTA‐3′, R: 5′‐AACGGAACTCCAGAAGACCAG‐3′. GAPDH was used as an internal reference. The standard 2−∆∆Ct method was utilized to calculate the relative gene expression.
2.4. Western blot analysis
Cells were lysed using RIPA lysis buffer. The total protein was quantified by the BCA method. In addition, equivalent amounts of protein were run on 10% SDS–PAGE gels. The proteins were transferred to PVDF membranes (Millipore, Boston, MA, USA). The membranes were then blocked with 5% nonfat milk in TBST for 60 min and incubated with primary antibodies against the following proteins at 4°C overnight: FUS (#4885, 1:1000, Cell Signaling Technology, Danvers, MA, USA), SIRT3 (ab189860, 1:1000, Abcam, Cambridge, MA, USA), IL‐1β (sc‐52,012, 1:1000, Santa Cruz, DBA, Milan, Italy), cleaved IL‐1β (AF4006, 1:1000, Affinity Biosciences), IL‐18 (ab191860, 1:1000, Abcam), cleaved IL‐18 (AF119, 1:1000, MBL Life Science), NLRP3 (SC06‐23, 1:1000, Invitrogen), GSDMD‐N (ab215203, 1:1000, Abcam), caspase‐1 (#98033, 1:1000, Cell Signaling Technology), cleaved caspase‐1 (AF4005, 1:1000, Affinity Biosciences), and GAPDH (ab9484, 1:1000, Abcam). After washing, the membranes were incubated with HRP‐conjugated antibodies (#7074, #7076, 1:1000, Cell Signaling Technology) at room temperature for 1 h. The protein bands were visualized with an enhanced chemiluminescence (ECL) system (Beyotime, Shanghai, China). GAPDH was used as an internal loading control. The protein bands were analyzed using ImageJ software (National Institutes of Health).
2.5. Detection of pyroptosis by flow cytometry
Pyroptosis was evaluated with FAM‐FLICA Caspase‐1 Assay Kits (ImmunoChemistry, USA) in accordance with the manufacturer's protocol. 17 Samples were mixed with FLICA and propidium iodide (PI) and then incubated in the dark at 37°C for 1 h. Flow cytometry was then applied to analyze pyroptosis (BD Biosciences, San Jose, USA). Double‐positive cells were considered pyroptotic cells.
2.6. Enzyme‐linked immunosorbent assay (ELISA)
The secretion of IL‐1β and IL‐18 into the cell supernatant was detected using ELISA kits (Elabscience, China) in strict accordance with the manufacturer's instructions. The absorbance of the samples was measured using a microplate reader (Bio‐Rad Laboratories, Inc., Hercules, CA, USA) at a 450 nm wavelength.
2.7. RNA immunoprecipitation (RIP)
RIP was conducted using an EZ‐Magna RIP RNA‐Binding Protein Immunoprecipitation Kit (Millipore). After the cells were lysed in RIP lysis buffer, the cell lysates were incubated with magnetic beads conjugated with an anti‐FUS antibody (#4885, Cell Signaling Technology) or anti‐IgG (#2729, Cell Signaling Technology) as a control at 4°C overnight. The enrichment of HOTAIR and SIRT3 in the immunoprecipitated RNA was assessed with RT–qPCR.
2.8. RNA pulldown
Biotinylated RNA probes for HOTAIR and antisense transcripts were synthesized by RiboBio and transfected into cells for 48 h. Cells were harvested and lysed. Subsequently, the cell lysates were incubated with streptavidin magnetic beads (Invitrogen) at 4°C for 4 h and then washed with PBS. The bound proteins were detected by western blotting using an anti‐FUS antibody (#4885, 1:1000, Cell Signaling Technology).
2.9. Statistical analysis
Statistical analyses were conducted with GraphPad Prism 7 (GraphPad, La Jolla, CA, USA). The data are presented as the mean ± standard deviation (SD). Student's t test was employed to analyze the differences between two groups. Differences among multiple groups were evaluated with one‐way ANOVA. All the experiments were conducted three times. p < 0.05 was considered to indicate significance.
3. RESULTS
3.1. HG induced pyroptosis and the inflammatory response in cardiomyocytes
To investigate cardiomyocyte pyroptosis in vitro, we treated H9C2 rat cardiomyocytes with NG (5.5 mM) or HG (30 mM) for 24 h. RT–qPCR analysis indicated that compared to NG, HG reduced HOTAIR and SIRT3 expression but induced IL‐1β and IL‐18 expression in H9C2 cells (Figure 1A,B). Additionally, the secretion of IL‐1β and IL‐18 was markedly higher in HG‐treated H9C2 cells than in NG‐treated cells (Figure 1C). Flow cytometry showed that pyroptosis was promoted in the HG group compared with the NG group (Figure 1D). Furthermore, Western blot analysis demonstrated that HG treatment significantly elevated the levels of pyroptosis‐ and inflammation‐related proteins (NLRP3, GSDMD‐N, cleaved caspase‐1, IL‐1β, IL‐18) compared with those in the control group (Figure 1E). These results implied that HG promoted cardiomyocyte pyroptosis and inflammation.
FIGURE 1.

HG induced pyroptosis and the inflammatory response in cardiomyocytes. H9C2 cells were treated with NG (5.5 mM) or HG (30 mM) for 24 h. (A) RT–qPCR was used to evaluate HOTAIR and SIRT3 expression. (B) RT–qPCR was performed to analyze IL‐1β and IL‐18 levels. (C) ELISA was utilized to assess the secretion of IL‐1β and IL‐18. (D) Flow cytometry was conducted to detect pyroptosis. (E) Western blot analysis of pyroptosis‐ and inflammation‐related proteins. The results are expressed as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001.
3.2. Overexpression of HOTAIR inhibited the HG‐induced pyroptosis and inflammatory response in cardiomyocytes
To explore the function of HOTAIR in cardiomyocytes, a plasmid overexpressing HOTAIR was transfected into HG‐exposed H9C2 cells. The expression of HOTAIR was greater in the HOTAIR overexpression vector transfected cells than in the empty vector–transfected cells (Figure 2A). In addition, the expression and secretion of IL‐1β and IL‐18 in HG‐treated cells were markedly reduced by overexpression of HOTAIR (Figure 2B,C), and pyroptosis was suppressed in the HOTAIR upregulation group compared with the HG group (Figure 2D). Furthermore, compared to HG‐treated cells, cells overexpressing HOTAIR exhibited reduced levels of pyroptosis‐ and inflammation‐related proteins (Figure 2E). The above data suggested that pyroptosis and inflammation in HG‐treated cardiomyocytes were suppressed by HOTAIR overexpression.
FIGURE 2.

Overexpressed HOTAIR inhibited the HG‐induced pyroptosis and inflammatory response in cardiomyocytes. H9C2 cells were transfected with a HOTAIR overexpression plasmid and treated with HG. (A) The efficiency of HOTAIR overexpression was detected by RT–qPCR. (B) RT–qPCR was used to analyze IL‐1β and IL‐18 levels. (C) ELISA was performed to assess the secretion of IL‐1β and IL‐18. (D) Flow cytometry was utilized to detect pyroptosis. (E) Western blot analysis of pyroptosis‐ and inflammation‐related proteins. The results are expressed as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001.
3.3. HOTAIR upregulated the level of SIRT3 through FUS
Next, we used the online starBase database to predict the potential interaction of FUS and HOTAIR in H9C2 cells. RNA pulldown and RIP experiments confirmed the binding relationship between FUS and HOTAIR (Figure 3A,B). Furthermore, reduced HOTAIR expression was observed in cells transfected with sh‐HOTAIR (Figure 3C). Knockdown of HOTAIR inhibited the expression of FUS at the mRNA and protein levels (Figure 3D,E). FUS expression was confirmed to be increased after transfection of a FUS overexpression vector (Figure 3F). In addition, silencing HOTAIR reduced FUS expression, but FUS overexpression had the opposite effect (Figure 3G). Moreover, a RIP assay showed that there was an interaction between SIRT3 and FUS (Figure 3H). Overall, HOTAIR enhanced the stability of SIRT3 by recruiting FUS.
FIGURE 3.

HOTAIR upregulated the level of SIRT3 through FUS. (A, B) RNA pulldown and RIP experiments were conducted to verify the binding relationship between FUS and HOTAIR in H9C2 cells. (C) The efficiency of HOTAIR knockdown was detected by RT–qPCR. (D, E) RT–qPCR and Western blot analysis of FUS expression after knockdown of HOTAIR. (F) RT–qPCR detection of FUS overexpression efficiency. (G) The level of FUS was analyzed by Western blotting. (H) RIP analysis of the binding relationship between SIRT3 and FUS. The results are expressed as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001.
3.4. SIRT3 overexpression suppressed HG‐triggered cardiomyocyte pyroptosis
To further explore the role of SIRT3 in cardiomyocyte pyroptosis, the SIRT3 overexpression plasmid was transfected into HG‐exposed H9C2 cells. Compared to that in the control group, the expression of SIRT3 was induced in the SIRT3‐overexpressing group of H9C2 cells (Figure 4A,B). Moreover, the expression and secretion of IL‐1β and IL‐18 were suppressed by SIRT3 upregulation in HG‐treated cells (Figure 4C,D). Compared with the HG‐only group, the HG‐treated group overexpressing SIRT3 exhibited less pyroptosis (Figure 4E). In addition, the levels of proteins related to HG‐triggered pyroptosis‐ and inflammation were substantially abrogated by SIRT3 overexpression (Figure 4F). These results suggested that SIRT3 could affect HG‐mediated cardiomyocyte pyroptosis.
FIGURE 4.
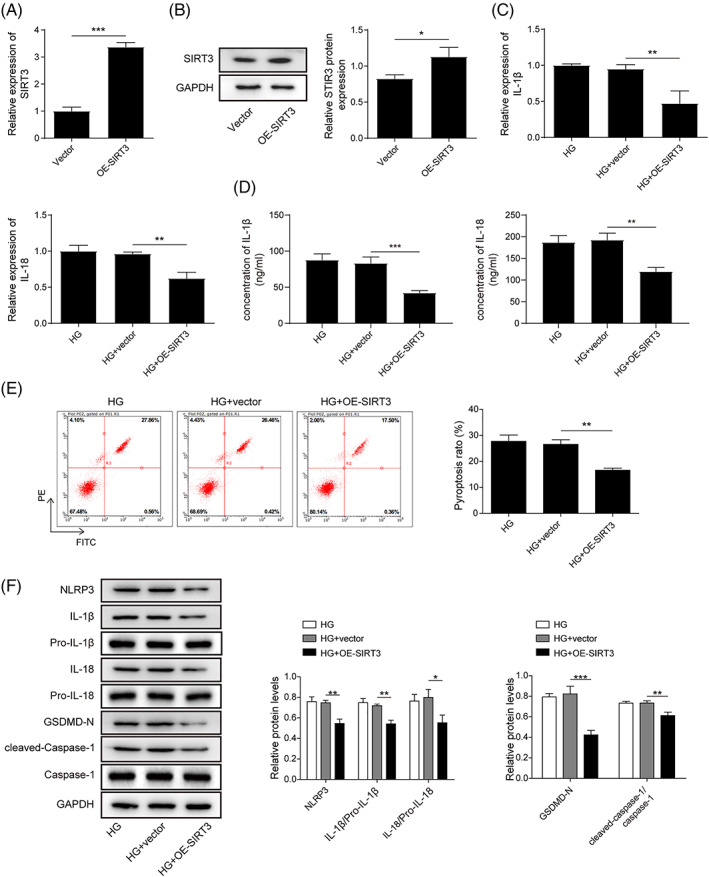
SIRT3 overexpression suppressed HG‐triggered cardiomyocyte pyroptosis. H9C2 cells were transfected with a SIRT3‐overexpressing plasmid and treated with HG. (A, B) The transfection efficiency of SIRT3 was detected by RT–qPCR and Western blotting. (C) RT–qPCR was performed to analyze IL‐1β and IL‐18 levels. (D) ELISA was used to assess the secretion of IL‐1β and IL‐18. (E) Flow cytometry detection of pyroptosis. (F) Western blot analysis of pyroptosis‐ and inflammation‐related proteins. The results are expressed as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001.
3.5. SIRT3 knockdown reversed the inhibitory effect of HOTAIR on HG‐induced cardiomyocyte pyroptosis
To further explore the potential mechanism of HOTAIR and SIRT3 in HG‐mediated cardiomyocyte pyroptosis, HOTAIR overexpression and SIRT3 knockdown plasmids were transfected into HG‐exposed H9C2 cells. The expression of SIRT3 was obviously lower in the sh‐SIRT3–transfected cells than in the empty vector–transfected cells (Figure 5A,B). Additionally, the expression and secretion of IL‐1β and IL‐18 in HG‐treated cells were downregulated by HOTAIR overexpression, an effect that was reversed by knockdown of SIRT3 (Figure 5C,D). On the other hand, overexpression of HOTAIR inhibited pyroptosis in HG‐treated cells, while SIRT3 silencing overturned this inhibitory effect (Figure 5E). In HG‐exposed cells, the HOTAIR upregulation‐mediated decrease in the expression of pyroptosis‐ and inflammation‐related proteins was abrogated by sh‐SIRT3 (Figure 5F). Altogether, the above data indicated that HOTAIR could regulate HG‐induced cardiomyocyte pyroptosis through SIRT3.
FIGURE 5.

SIRT3 knockdown reversed the inhibitory effect of HOTAIR on HG‐induced cardiomyocyte pyroptosis. HOTAIR overexpression and SIRT3 knockdown plasmids were transfected into HG‐exposed H9C2 cells. (A, B) The transfection efficiency of sh‐SIRT3 was detected by RT–qPCR and Western blotting. (C) RT–qPCR was utilized to analyze IL‐1β and IL‐18 levels. (D) ELISA was used to assess the secretion of IL‐1β and IL‐18. (E) Flow cytometry detection of pyroptosis. (F) Western blot analysis of pyroptosis‐ and inflammation‐related proteins. The results are expressed as the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001.
4. DISCUSSION
DCM is a specific type of heart disease caused by insulin resistance, compensatory hyperinsulinaemia, and hyperinsulinaemia in cardiometabolic tissues. 18 The disease is insidious with a high fatality rate, and there is a lack of specific therapeutic drugs in the clinic. 19 Intracellular NOD‐like receptors (NLRs) form inflammasomes that stimulate caspase‐1 and the release of various inflammatory substances, such as IL‐1 and IL‐18, leading to the occurrence of pyroptosis; the resulting pyroptosis is involved in DCM progression. 20 Recently, studies have shown that the occurrence and development of DCM can be alleviated by regulating the expression of related genes during pyroptosis. 21 In the current study, the lncRNA HOTAIR was upregulated in HG‐treated cardiomyocytes. Moreover, HOTAIR overexpression alleviated HG‐triggered cardiomyocyte pyroptosis and the inflammatory response by recruiting FUS to induce SIRT3 expression. Therefore, our findings reveal a vital role of HOTAIR in DCM.
The NLRP3 inflammasome is a key sensor mediating innate immune and inflammatory responses. 22 Increasing evidence is suggesting that the NLRP3 inflammasome participates in DCM. 23 Once activated, the NLRP3 inflammasome can promote the maturation and secretion of proinflammatory cytokines, such as Caspase 1, IL‐1β and IL‐18, and initiate pyroptosis. 24 In our study, we found that the expression levels of NLRP3, caspase‐1, IL‐1β and IL‐18 in cardiomyocytes were increased after HG stimulation, demonstrating that HG can directly trigger pyroptosis by activating the NLRP3 inflammasome in cardiomyocytes.
Silencing of lncRNA genes has been shown to significantly reduce the expression and release of related inflammatory factors in injured cardiomyocytes. 25 Multiple studies have shown that the lncRNA HOTAIR plays an important role in diabetes progression. 26 , 27 Qi et al. reported that HOTAIR improves DCM by inducing cardiomyocyte viability via activation of the PI3K/Akt signaling pathway. 28 In the current investigation, HG elevated the HOTAIR level in H9C2 cells. Additionally, HOTAIR overexpression downregulated the levels of inflammatory molecules and suppressed pyroptosis in HG‐treated cells, indicating that HOTAIR has a protective effect against HG‐triggered cardiomyocyte pyroptosis.
SIRT3 is a NAD+‐dependent deacetylase located in mitochondria that maintains diverse physiological functions, such as metabolism and ATP production. 29 SIRT3 deficiency has been implicated in the pathogeneses of metabolic disorders such as type 2 diabetes mellitus (T2DM). 30 For instance, polydatin alleviates DCM by activating SIRT3. 31 Salidroside protects cardiac function in DCM mice by activating SIRT3. 32 Most importantly, pinocembrin can reduce cardiomyocyte pyroptosis by activating the Nrf2/SIRT3 axis. 33 Our present study illustrates that SIRT3 is downregulated in HG‐stimulated H9C2 cells. We also showed that SIRT3 knockdown overturned the suppressive effect of HOTAIR on HG‐triggered cardiomyocyte pyroptosis, indicating that SIRT3 may serve as a downstream regulator of HOTAIR. Similarly, Zheng et al. have reported that SIRT3 decreases NLRP3 and IL‐1β expression in diabetic rats. 34 Zhao et al. have indicated that SIRT3 plays a protective role in sepsis‐induced acute kidney injury by inhibiting the NRLP3 inflammasome and downregulating IL‐1β and IL‐18. 35 Moreover, assembly of inflammasomes with caspase‐1 and NLRP3 has been detected in the brains of SIRT3‐deficient mice. 36 Emerging studies have shown that lncRNAs and RNA‐binding proteins (RBPs) can form complexes to regulate downstream gene expression. 37 We have further confirmed that HOTAIR promotes SIRT3 expression by targeting FUS, a multifunctional RNA binding protein. 38 These findings strongly suggested that the HOTAIR/FUS/SIRT3 signaling pathway mediates DCM progression.
In conclusion, we revealed that HOTAIR was specifically downregulated in HG‐exposed H9C2 cells. Moreover, HOTAIR alleviated HG‐induced pyroptosis in cardiomyocytes. Subsequent studies further found that in HG‐treated cardiomyocytes, HOTAIR activated SIRT3 expression by binding to FUS, thereby inhibiting pyroptosis. Therefore, the HOTAIR/FUS/SIRT3 signaling pathway participates in a new mechanism for DCM progression and may become a novel therapeutic target for the treatment of DCM.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
Xiong J, Zhou Q. The lncRNA HOTAIR attenuates pyroptosis of diabetic cardiomyocytes by recruiting FUS to regulate SIRT3 expression. Kaohsiung J Med Sci. 2023;39(5):458–467. 10.1002/kjm2.12676
REFERENCES
- 1. Dillmann WH. Diabetic cardiomyopathy. Circ Res. 2019;124(8):1160–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jia G, Whaley‐Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia‐ and insulin‐resistance‐induced heart disease. Diabetologia. 2018;61(1):21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tan Y, Zhang Z, Zheng C, Wintergerst KA, Keller BB, Cai L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence. Nat Rev Cardiol. 2020;17(9):585–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li X, Du N, Zhang Q, Li J, Chen X, Liu X, et al. MicroRNA‐30d regulates cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic cardiomyopathy. Cell Death Dis. 2014;5(10):e1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang F, Qin Y, Lv J, Wang Y, Che H, Chen X, et al. Silencing long non‐coding RNA Kcnq1ot1 alleviates pyroptosis and fibrosis in diabetic cardiomyopathy. Cell Death Dis. 2018;9(10):1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jarroux J, Morillon A, Pinskaya M. History, discovery, and classification of lncRNAs. Adv Exp Med Biol. 2017;1008:1–46. [DOI] [PubMed] [Google Scholar]
- 9. Uchida S, Dimmeler S. Long noncoding RNAs in cardiovascular diseases. Circ Res. 2015;116(4):737–50. [DOI] [PubMed] [Google Scholar]
- 10. Li M, Duan L, Li Y, Liu B. Long noncoding RNA/circular noncoding RNA‐miRNA‐mRNA axes in cardiovascular diseases. Life Sci. 2019;233:116440. [DOI] [PubMed] [Google Scholar]
- 11. Rajagopal T, Talluri S, Akshaya RL, Dunna NR. HOTAIR LncRNA: a novel oncogenic propellant in human cancer. Clin Chim Acta. 2020;503:1–18. [DOI] [PubMed] [Google Scholar]
- 12. Gao L, Wang X, Guo S, Xiao L, Liang C, Wang Z, et al. LncRNA HOTAIR functions as a competing endogenous RNA to upregulate SIRT1 by sponging miR‐34a in diabetic cardiomyopathy. J Cell Physiol. 2019;234(4):4944–58. [DOI] [PubMed] [Google Scholar]
- 13. Palomer X, Aguilar‐Recarte D, García R, Nistal JF, Vázquez‐Carrera M. Sirtuins: to be or not to be in diabetic cardiomyopathy. Trends Mol Med. 2021;27(6):554–71. [DOI] [PubMed] [Google Scholar]
- 14. Wu J, Zeng Z, Zhang W, Deng Z, Wan Y, Zhang Y, et al. Emerging role of SIRT3 in mitochondrial dysfunction and cardiovascular diseases. Free Radic Res. 2019;53(2):139–49. [DOI] [PubMed] [Google Scholar]
- 15. Murugasamy K, Munjal A, Sundaresan NR. Emerging roles of SIRT3 in cardiac metabolism. Front Cardiovasc Med. 2022;9:850340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang M, Lin J, Wang S, Cheng Z, Hu J, Wang T, et al. Melatonin protects against diabetic cardiomyopathy through Mst1/Sirt3 signaling. J Pineal Res. 2017;63(2). 10.1111/jpi.12418 [DOI] [PubMed] [Google Scholar]
- 17. Wang YW, Dong HZ, Tan YX, Bao X, Su YM, Li X, et al. HIF‐1α‐regulated lncRNA‐TUG1 promotes mitochondrial dysfunction and pyroptosis by directly binding to FUS in myocardial infarction. Cell Death Discov. 2022;8(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murtaza G, Virk HUH, Khalid M, Lavie CJ, Ventura H, Mukherjee D, et al. Diabetic cardiomyopathy – a comprehensive updated review. Prog Cardiovasc Dis. 2019;62(4):315–26. [DOI] [PubMed] [Google Scholar]
- 19. Paolillo S, Marsico F, Prastaro M, Renga F, Esposito L, De Martino F, et al. Diabetic cardiomyopathy: definition, diagnosis, and therapeutic implications. Heart Fail Clin. 2019;15(3):341–7. [DOI] [PubMed] [Google Scholar]
- 20. Xue Y, Enosi Tuipulotu D, Tan WH, Kay C, Man SM. Emerging activators and regulators of inflammasomes and pyroptosis. Trends Immunol. 2019;40(11):1035–52. [DOI] [PubMed] [Google Scholar]
- 21. Wu A, Sun W, Mou F. lncRNA‐MALAT1 promotes high glucose‐induced H9C2 cardiomyocyte pyroptosis by downregulating miR‐141‐3p expression. Mol Med Rep. 2021;23(4):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seoane PI, Lee B, Hoyle C, Yu S, Lopez‐Castejon G, Lowe M, et al. The NLRP3‐inflammasome as a sensor of organelle dysfunction. J Cell Biol. 2020;219(12):e202006194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun Y, Ding S. NLRP3 inflammasome in diabetic cardiomyopathy and exercise intervention. Int J Mol Sci. 2021;22(24):13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol. 2021;18(9):2114–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang W, Xu W, Feng Y, Zhou X. Non‐coding RNA involvement in the pathogenesis of diabetic cardiomyopathy. J Cell Mol Med. 2019;23(9):5859–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chu PM, Yu CC, Tsai KL, Hsieh PL. Regulation of oxidative stress by long non‐coding RNAs in vascular complications of diabetes. Life (Basel). 2022;12(2):274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sargazi S, Ravanbakhsh M, Nia MH, Mirinejad S, Sheervalilou R, Majidpour M, et al. Association of polymorphisms within HOX transcript antisense RNA (HOTAIR) with type 2 diabetes mellitus and laboratory characteristics: a preliminary case‐control study. Dis Markers. 2022;2022:4327342 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qi K, Zhong J. LncRNA HOTAIR improves diabetic cardiomyopathy by increasing viability of cardiomyocytes through activation of the PI3K/Akt pathway. Exp Ther Med. 2018;16(6):4817–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang S, Zhang J, Deng X, Zhao Y, Xu K. Advances in characterization of SIRT3 deacetylation targets in mitochondrial function. Biochimie. 2020;179:1–13. [DOI] [PubMed] [Google Scholar]
- 30. Kitada M, Ogura Y, Monno I, Koya D. Sirtuins and type 2 diabetes: role in inflammation, oxidative stress, and mitochondrial function. Front Endocrinol (Lausanne). 2019;10:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang M, Wang S, Cheng Z, Xiong Z, Lv J, Yang Z, et al. Polydatin ameliorates diabetic cardiomyopathy via Sirt3 activation. Biochem Biophys Res Commun. 2017;493(3):1280–7. [DOI] [PubMed] [Google Scholar]
- 32. Li Y, Wei X, Liu SL, Zhao Y, Jin S, Yang XY. Salidroside protects cardiac function in mice with diabetic cardiomyopathy via activation of mitochondrial biogenesis and SIRT3. Phytother Res. 2021;35(8):4579–91. [DOI] [PubMed] [Google Scholar]
- 33. Gu J, Huang H, Liu C, Jiang B, Li M, Liu L, et al. Pinocembrin inhibited cardiomyocyte pyroptosis against doxorubicin‐induced cardiac dysfunction via regulating Nrf2/Sirt3 signaling pathway. Int Immunopharmacol. 2021;95:107533. [DOI] [PubMed] [Google Scholar]
- 34. Zheng J, Shi L, Liang F, Xu W, Li T, Gao L, et al. Sirt3 ameliorates oxidative stress and mitochondrial dysfunction after intracerebral hemorrhage in diabetic rats. Front Neurosci. 2018;12:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao WY, Zhang L, Sui MX, Zhu YH, Zeng L. Protective effects of sirtuin 3 in a murine model of sepsis‐induced acute kidney injury. Sci Rep. 2016;6:33201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tyagi A, Nguyen CU, Chong T, Michel CR, Fritz KS, Reisdorph N, et al. SIRT3 deficiency‐induced mitochondrial dysfunction and inflammasome formation in the brain. Sci Rep. 2018;8(1):17547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ferrè F, Colantoni A, Helmer‐Citterich M. Revealing protein‐lncRNA interaction. Brief Bioinform. 2016;17(1):106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ghanbarpanah E, Kohanpour MA, Hosseini‐Beheshti F, Yari L, Keshvari M. Structure and function of FUS gene in prostate cancer. Bratisl Lek Listy. 2018;119(10):660–3. [DOI] [PubMed] [Google Scholar]


