Abstract
Papillary thyroid cancer (PTC) has attracted much attention due to its high morbidity and severe metastasis. Long noncoding RNA ENST00000504230 (LncRNA ENST00000504230, known as LINC00958) was overexpressed in many cancers and associated with cancer development. However, its underlying mechanism in PTC remains unclear. PTC tissues and corresponding adjacent tissues were collected for measuring the expression of LINC00958 and miR‐627. MiR‐627 and TRIM44 expressions were measured in in vitro cultured PTC cell lines (B‐cpap and IHH4 cells) transfected with sh‐LINC00958 or miR‐627 mimic using RT‐qPCR and western blot. Cell proliferation, migration, and invasion were measured by 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl‐tetrazolium bromide (MTT) and Transwell assays, respectively. Dual‐luciferase reporter assay was performed to evaluate the target association between miR‐627 and TRIM44. LINC00958 was up‐regulated in PTC tissues and cells, while the expression of miR‐627 was lowly expressed. Knockdown of LINC00958 inhibited the proliferation, migration, and invasion by elevating miR‐627 expression in PTC cells. TRIM44 was confirmed as a target of miR‐627. Overexpression of miR‐627 in PTC inhibited the proliferation, migration, and invasion by down‐regulating the expression of TRIM44. LINC00958 promoted proliferation, migration, and invasion in PTC by down‐regulating miR‐627 and activating TRIM44, indicating the potential therapeutic effect of LINC00958 on PTC.
Keywords: LINC00958, microRNA‐627, papillary thyroid cancer, proliferation and migration, tripartite motif 44
1. INTRODUCTION
Thyroid carcinoma, a heterogeneous disease characterized by different histopathological and biological properties across subtypes, is the most common tumor in the endocrine system. 1 , 2 Thyroid carcinoma happens most commonly in women, and makes up for about 1% of all malignant diseases. Over the past decades, the incidence of thyroid carcinoma has increased dramatically worldwide. 3 Most thyroid cancers can be divided into papillary thyroid cancer (PTC) and follicular thyroid cancer, of which PTC accounts for 80%–90%. 4 PTC is a high proportion of thyroid cancer, so PTC has become a research hotspot. Ten percent of PTC patients die of the disease due to relapse and distant metastases. 5 , 6 A strikingly high frequency of lymph node metastasis in PTC is the leading cause of these clinical risk factors. 7 , 8 Here, we focused on the mechanism of proliferation and migration, which are beneficial for targeted molecular therapy and early diagnosis.
Long noncoding RNAs (lncRNAs) in tumors has been studied more and more. lncRNAs are molecules more than 200 nucleotides long and not involved in coding proteins. 9 The dysregulation of lncRNAs are related to the occurrence of many cancers such as gastric cancer, breast cancer, lung cancer, and so on, and can regulate a series of biological processes of tumor cells, including DNA damage repair, cell cycle, apoptosis, autophagy, and interference with signal transduction pathways. 10 , 11 There are few studies on the lncRNA ENST000004230 (LINC00958). LINC00958 was originally identified as a potential oncogene in bladder cancer. 12 Studies also showed LINC00958 was upregulated in pancreatic cancer and glioma. 13 , 14 Nevertheless, little is known about whether LINC00958 is dysregulated in PTC and how to regulate tumor development.
MicroRNAs (miRNAs) are small, noncoding single‐chained RNAs, which comprise a series of regulators by targeting mRNAs, and prevent mRNA translation or cause mRNA degradation. 15 miRNAs play an important role in cancer. miR‐627 is a kind of tumor suppressor, which has been shown to inhibit the progression of cancer. 16 , 17 , 18 In the previous study, miR‐627 can inhibited proliferation, migration, and invasion in glioma, which presents low‐level expression in a variety of cancers. 19 , 20 LINC00958 has been shown to promote tumor progression through miR‐627 in previous studies of oral squamous cell carcinoma (OSCC), 9 but it never has been investigated in PTC.
Tripartite motif 44 (TRIM44) functions as an important regulator in several types of malignant tumors (such as nonsmall cell lung cancer [NSCLC], prostate cancer), which is a member of the TRIM family. 21 , 22 , 23 It is previously studied that the expression of TRIM44 was markedly upregulated in human NSCLC tissues, and ectopic expression of TRIM44 significantly promoted the metastasis and the epithelial‐mesenchymal transition (EMT) process in lung cancer cells. 24 Overexpression of TRIM44 led to malignant lesions of gastric cancer. 25 What's more, in the study of PTC, it was found that knockdown of TRIM44 inhibited the proliferation and invasion of tumor cells. 21 However, the mechanism by which TRIM44 specifically regulates tumor proliferation and migration has not been studied in PTC.
In this study, we firstly detected the expression of LINC00958 and miR‐627 in 10 clinical PTC tissues. Furthermore, we analyzed whether the mechanism of LINC00958 regulating cell proliferation, migration, and invasion in thyroid papillary cells was related to miR‐627/TRIM44. This would indicate a novel potential target for treatment of PTC.
2. MATERIALS AND METHODS
2.1. Clinical samples
Ten pairs of frozen thyroid cancer tissues and matched paracancerous tissues were obtained from the Renmin Hospital of Hubei University of Medicine. These patients did not receive chemotherapy or radiotherapy before the operation. Patients with infectious diseases, autoimmune diseases or multiple primary cancers were excluded from the study.
2.2. Cell culture
The human PTC cell lines, K1, TPC‐1, and B‐cpap cells were suspended in RPMI 1640 complete medium containing 10% FBS and IHH‐4 cells were maintained in a 1:1 mixture of RPMI 1640 and DMEM, containing 10% FBS. The normal human thyroid cell line, Htori‐3 were cultured in DMEM supplemented with 10% FBS. All cells were cultured at 37°C, in a humidified atmosphere with 5% CO2.
2.3. Cell viability assay
Cell viability was determined based on the ability that living cells can convert 3‐(4,5‐dimethyl‐2‐thiazolyl)‐2,5‐diphenyl‐2‐H‐tetrazolium bromide (MTT) to formazan catalyzed by succinate dehydrogenase. Cells were suspended in complete medium containing 5 mg/ml MTT, then incubated for 4 h. After incubation, the medium was discarded and DMSO was used to dissolve formed formazan within cells. The absorbance at 490 nm was measured using a microplate reader (Bioteke, Winooski). Relative cell viability was determined as absorbance value relative to that of the control.
2.4. Cell invasion and migration assay
Cell invasion and migration were conducted with 24‐well transwell plates (8 μm pore size, Corning Co. Ltd., USA). One hundred micro liter of serum‐free medium containing 3 × 104 cells from each group were added to the upper chamber with or without matrigel, and 500 μl of medium containing 10% FBS were added into each lower chamber. After incubation at 37°C for 12 h, the cells remaining on the upper membranes were removed with a cotton swab. The cells that had migrated through the filters were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. Cells were observed and photographed under a microscope.
2.5. Quantitative real‐time polymerase chain reaction
Total RNA was extracted by using TRIzol reagent, and cDNA was synthesized using reverse transcriptase kits according to manufacturer's instruction (Toyobo, Japan). The cDNA was used for quantitative real‐time polymerase chain reaction (qRT‐PCR) with specific primers for different genes. Reactions were carried out on an Eppendorf MasterCycler RealPlex4 (Wesseling‐Berzdorf, Germany) with a SYBR ExScript PCR kit (Toyobo, Japan). Relative expression levels were calculated by the 2−ΔΔCT method (4), and normalized to the expression of the glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) gene and U6. The primers were listed as: LINC00958: Forward, 5′‐TCCCTGCACTTCACAGTGTC‐3′; Reverse, 5′‐CAGAGCTTGAGCAGTGATCG‐3′; miR‐627: Forward, 5′‐GCGGCGGAGGAGAAAAGAATCTC‐3′; Reverse, 5′‐ATCCAGTGCAGGGTCCGAGG‐3′; TRIM44: Forward, 5′‐GTGGACATCCAAGAGGCAATG‐3′; Reverse, 5′‐GCCGTTACATACACGCTGTC‐3′; GAPDH: Forward, 5′‐CTGACTTCAACAGCGACACC‐3′; Reverse, 5′‐GTGGTCCAGGGGTCTTACTC‐3′; U6: Forward, 5′‐CTCGCTTCGGCAGCACA‐3′; Reverse, 5′‐AACGCTTCACGAATTTGCGT‐3′.
2.6. Plasmid constructs and transfection
The short hairpin RNA against LINC00958 (sh‐LINC00958), the short hairpin RNA against TRIM44 (sh‐TRIM44) and overexpression plasmid of LINC00958 (LINC00958) as well as their negative controls (sh‐NC and vector) were synthesized by Sangon (Shanghai, China). For in vitro transfection, cells at a density of 1 × 105 cells/well were transfected with sh‐LINC00958, sh‐TRIM44, or LINC00958 and their negative controls using Lipofectamine™ 3000 (Invitrogen) according to the manufacturer's instructions. Plasmid DNA‐encoding TRIM44 was constructed by inserting the cDNA clone of TRIM44 into the pcDNA3.1 vector. MiR‐627 mimic and mimic negative control (mimic NC), miR‐627 inhibitor and inhibitor negative control (inhibitor NC) were generated by Genomeditech (Shanghai, China). Cell transfection was conducted in cells by lipofecter liposomal transfection reagent (Beyotime) for 24 h.
2.7. Dual‐luciferase reporter assay
The online target prediction website TargetScan was used to predict the base sequence of the binding sites of miR‐627 and TRIM44. The wildtype (wt)‐ or mutant (mut)‐ 3′‐untranslated region (3′UTR) sequence of TRIM44 mRNA was amplified by polymerase chain reaction (PCR) and was cloned into both the firefly luciferase reporter plasmid pGL3‐Basic (Promega) following XhoI and HindIII restriction enzyme digestion. After site‐directed mutagensis of the potential complementary binding site, the pGL3 vector was double‐enzyme digested by XhoI and HindIII. The mutant fragment was inserted into the vector to construct mutant recombinant dual luciferase reporter vector. For dual‐luciferase reporter assay, cells were co‐transfected with wt‐TRIM44 or mut‐TRIM44 and inhibitor NC, miR‐627 inhibitor, mimic NC or miR‐627 mimic. After 24 h, the transfected cells were collected and luciferase activity was detected with a dual‐luciferase assay kit (Promega).
2.8. Protein extraction and western blotting
The proteins were isolated from cells by using RIPA lysis buffer mixed with 1% protease inhibitor and phosphorylase inhibitor, and the concentrations were measured using BCA Protein Assay Kit (Beyotime Institute of Biotechnology, China). The lysate was mixed with 5× SDS sample buffer and boiled for 10 min. Lysate samples were separated on 6% and 12% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE), and transferred to a PVDF membrane. The blots were blocked with 5% milk blocking solution for 1 h at room temperature and then incubated overnight with antibodies against TRIM44 (Abcam, USA). Anti‐GADPH antibody was purchased from Sigma‐Aldrich, and served as a loading control. After extensive washing with PBS‐T, membranes were further incubated with an HRP‐labeled secondary antibody (dilution ratio: 1:10,000) for 1 h at room temperature. The blots were visualized by ECL Western Blotting Detection Reagents (Beyotime Institute of Biotechnology) and the images were performed by GEL imaging system (Bio‐Rad, USA). The quantification of proteins was analyzed by the Image‐Pro Plus v.6.0 software (Media Cybernetics, USA).
2.9. Data analysis
Results in figures are expressed as mean ± standard deviation (SD). Statistical analysis was performed using GraphPad 5.0 Prism. Differences between normally distributed data were analyzed by one way of analysis of variance (ANOVA) and Student t‐tests. The p values less than 0.05 were considered significant.
3. RESULTS
3.1. LINC00958 is highly expressed in PTC, while miR‐627 is lowly expressed
We firstly tested the expression of LINC00958 in PTC tissues and 10 corresponding adjacent tissues. The results displayed that LINC00958 expression in PTC was higher than that in adjacent tumors. The expression of miR‐627, low in PTC and high in adjacent tumors, showing the opposite trend (P <0.05; Figure 1A,B). Then, the expressions of LINC00958 and miR‐627 in normal thyroid cells and four thyroid papillary cancer cell lines were detected. Compared with normal thyroid cells, the expression of LINC00958 was up‐regulated, while miR‐627 was down‐regulated in PTC cell lines (P <0.05; Figure 1C,D). This effect is more significant in B‐cpap and IHH4 cells, so our next research is carried out in these two cell lines. (Figure 1C,D).
FIGURE 1.

LINC00958 is highly expression in PTC, while miR‐627 is lowly expressed. (A,B) The expressions of LINC00958 and miR‐627 in 10 PTC tissues and corresponding adjacent tissues were detected using qRT‐PCR assay. (C,D) The expressions of LINC00958 and miR‐627 in normal thyroid cells and four PTC cell lines were determined using qRT‐PCR assay. *P <0.05, **P <0.01
3.2. LINC00958 promotes the proliferation, migration, and invasion of B‐cpap and IHH4
To determine the role of LINC00958 in regulating tumor cell proliferation, migration, and invasion, we used shRNA to knock down LINC00958 in B‐cpap and IHH4. As shown in Figure 2A, the expression of LINC00958 was obviously decreased by sh‐LINC00958 in both B‐cpap and IHH4, suggesting that the transfection was successful. MiR‐627 expression was found to be markedly up‐regulated in B‐cpap and IHH4 cells following LINC00958 knockdown (Figure 2B). MTT was used to evaluate the effect of knockdown of LINC00958 on cell proliferation, and results showed that knockdown of LINC00958 resulted in significantly reduced cell proliferation of B‐cpap and IHH4 compared with shNC group (Figure 2C). The results of cell migration and invasion detected by Transwell analysis showed that the silencing of LINC00958 significantly suppressed the migration and invasion of B‐cpap and IHH4 cells compared with the control group (Figure 2D,E).
FIGURE 2.
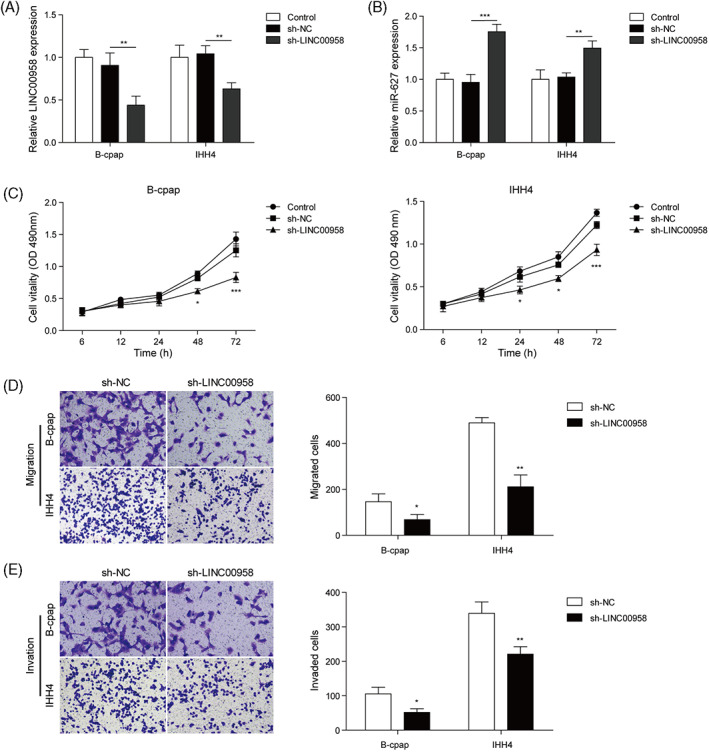
LINC00958 promotes the proliferation, migration, and invasion of B‐cpap and IHH4. B‐cpap, and HH4 cells were transfected with sh‐LINC00958 for 48 h, respectively. (A,B) LINC00958 and miR‐627 expressions in B‐cpap and IHH4 cells were detected using qRT‐PCR. (C) Cell proliferation of B‐cpap and IHH4 cells was assessed using MTT assay. (D,E) Cell migration and invasion of B‐cpap and IHH4 cells were evaluated using Transwell assay. *P <0.05, **P <0.01
3.3. Overexpression of miR‐627 inhibits proliferation, migration, and invasion of B‐cpap and IHH4
In order to determine the role of miR‐627 in regulating tumor cell proliferation, migration, and invasion, miR‐627 was overexpressed in B‐cpap and IHH4 transfected with miR‐627 mimic (Figure 3A). Results showed that overexpression of miR‐627 greatly down‐regulated cell proliferation of B‐cpap and IHH4 (Figure 3B). What's more, up regulation of miR‐627 significantly reduced cell migration and invasion of B‐cpap and IHH4 (Figure 3C,D).
FIGURE 3.

Overexpression of miR‐627 inhibits proliferation, migration, and invasion of B‐cpap and IHH4. B‐cpap and HH4 cells were transfected with miR‐627 overexpression plasmid for 48 h, respectively. (A) miR‐627 expression in B‐cpap and IHH4 cells was determined using qRT‐PCR. (B) Cell proliferation of B‐cpap and IHH4 cells was assessed using MTT assay. (C,D) Transwell assay was employed to evaluate cell migration and invasion of B‐cpap and IHH4 cells. *P <0.05, **P <0.01
3.4. LINC00958 promotes TRIM44 expression by regulating miR‐627
We first detected the expression of TRIM44 in both mRNA and protein levels in normal thyroid cells and B‐cpap, IHH4. Results showed that the expression was increased both in levels of mRNA and protein in B‐cpap and IHH4 compared with normal thyroid cells (Figure 4A,B). Then we investigated the regulatory effects of LINC00958 on TRIM44. Knockdown of LINC00958 reduced TRIM44 expression in levels of mRNA and protein compared with shNC group in B‐cpap and IHH4 (Figure 4C,D). To explore how to interact between miR‐627 and TRIM44, we used TargetScan to predict online the potential complementary sequence between miR‐627 and TRIM44 (Figure 4E). We used luciferase luminescence to detect whether miR‐627 bound directly to TRIM44. The wt and mut‐TRIM44 3′UTR were generated and co‐transfected with miR‐627 mimic and inhibitor and NC into cells. The results of dual‐luciferase reporter assay revealed that miR‐627 mimic bound to wt‐TRIM44 3′UTR and inhibited the activity of luciferase (Figure 4F). The results of dual‐luciferase reporter assay revealed that miR‐627 mimic bound to wt‐TRIM44 3′UTR and inhibited the activity of luciferase, while it was significantly increased luciferase activity by miR‐627 inhibition. The luciferase activity was not changed in Mut‐TRIM44 group (Figure 4F). These data suggested that TRIM44 acted as a target of miR‐627 in PTC cells.
FIGURE 4.

LINC00958 promotes TRIM44 expression by regulating miR‐627. (A) TRIM44 expression in normal thyroid cells, B‐cpap, and IHH4 cells was evaluated using qRT‐PCR. (B) The protein level of TRIM44 in normal thyroid cells, B‐cpap and IHH4 was assessed using western blot. B‐cpap and IHH4 cells were transfected with sh‐LINC00958 for 48 h, respectively. (C) TRIM44 expression was determined using qRT‐PCR assay. (D) Western blot was performed to evaluate the protein level of TRIM44 in B‐cpap and IHH4 cells. (E) The binding site of TRIM44 and miR‐627 were predicted by TargetScan. (F) Dual luciferase reporter gene assay was performed to verify the binding relationship between TRIM44 and miR‐627. *P <0.05, **P <0.01
3.5. LINC00958 promotes cell proliferation and migration through miR‐627/TRIM44
To investigate the effect of LINC00958/miR‐627/TRIM44 signal axis on the tumor proliferation and migration, we simultaneously knocked down LINC00958 and overexpressed TRIM44. We used shRNA to knock down LINC00958 and the vector to overexpress TRIM44 in PTC cells (Figure 5A). In addition, MTT assay revealed that the suppressive effect of knockdown of LINC00958 on proliferation was reversed by overexpression of TRIM44 (Figure 5B). Transwell analysis showed that overexpression of TRIM44 in B‐cpap and IHH4 reversed the knockdown effect of LINC00958 on cell invasion and migration (Figure 5C,D). To further verify the role of LINC00958/ TRIM44 axis in PTC, we overexpressed LINC00958 in B‐cpap and IHH4 cells, and set up a functional rescue experiment: LINC00958 and sh‐TRIM44 group. We found that TRIM44 expression was significantly elevated by LINC00958 overexpression, whereas TRIM44 knockdown reversed it (Figure 5E). Additionally, as shown in Figure 5F–H, TRIM44 knockdown abolished the effects of LINC00958 overexpression on cell proliferation, migration, and invasion. In summary, our results illustrated the key roles of LINC00958/miR‐627/TRIM44 axis in PTC.
FIGURE 5.

LINC00958 promotes cell proliferation and migration through miR‐627/TRIM44 axis. B‐cpap and IHH4 cells were transfected with sh‐LINC00958 and TRIM44 overexpression plasmid for 48 h. (A) TRIM44 expression in B‐cpap and IHH4 cells was assessed using qRT‐PCR assay. (B) Cell proliferation B‐cpap and IHH4 cells was determined using MTT assay. C and D, Transwell assay was employed to evaluate cell migration and invasion of B‐cpap and IHH4 cells. PTC cells were divided into six groups: control, vector, LINC00958, shNC group, sh‐TRIM44, and LINC00958 + sh‐TRIM44 group. (E) TRIM44 expression in B‐cpap and IHH4 cells was evaluated using qRT‐PCR assay. (F,G) Transwell assay was performed to evaluate cell migration and invasion of B‐cpap and IHH4 cells. *P <0.05, **P <0.01
4. DISSCUSION
PTC is a well‐differentiated thyroid carcinoma that originates from the thyroid follicular cells and accounts for 80%–90% of all thyroid cancer. 26 , 27 PTC spreads through lymphatics easily, that leads to tumor recurrence, metastases, and even death. 28 , 29 Therefore, it is necessary to study the mechanism of regulating the metastasis of PTC. The purpose of this study is to investigate the molecular mechanism of LINC00958 on cell proliferation, migration, and invasion in PTC. What's more, our study first found the effect of LINC00958 on proliferation, migration, and invasion in thyroid papillary carcinoma cells and the molecular mechanism of its specific regulation.
Today, more and more lncRNAs are found to be involved in tumorigenesis and tumor development. 30 LINC00958 is a new type of lncRNA that has entered people's field of vision, which has been reported as oncogene in the human cancer. 31 LINC00958 was up‐regulated in a variety of tumors, 13 , 32 which was consistent with our results that LINC00958 was up‐regulated in PTC. What's more, LINC00958 promoted the proliferation, invasion and reduced the apoptosis of OSCC cells in vitro. 32 LINC00958 was also identified candidate oncogenes in bladder cancer, which can promote the proliferation of bladder cancer. 31 In additional, miR‐627 in particular has been reported to act as a tumor suppressor by means of its role in the progression of OSCC. 9 Here, in vitro experiments of PTC have confirmed that LINC00958 regulates the regulatory mechanism of miR‐627 on cell proliferation, migration, and invasion, providing a new target for the prevention and treatment of PTC.
TRIM44 functions as a critical regulator in several types of malignancy. 21 , 33 , 34 In the study of human esophageal cancer, TRIM44 was found to affect the EMT of cells through Akt/mTOR pathway. 33 It has also been reported in PTC that the knockdown of TRIM44 inhibits the proliferation and invasion of cancer cells through Wnt/β‐Catenin pathway. 21 However, there was no report on the possible regulatory mechanism of TRIM44 upstream in the study of PTC. Our study confirmed for the first time that TRIM44 was regulated by upstream lncRNA and miRNA in PTC. On basis of literature research, our research aimed at the high expression of LINC00958 in cells and found its downstream regulated miRNA. Then the downstream target protein TRIM44 was studied, and the regulatory signal axis of LINC00958/miR‐627/TRIM44 was found. Our research perfected the regulatory network that regulates PTC migration and proliferation, highlighted the novelty of the article, and has important implications for the discovery of new markers for PTC. However, we found that there was only a partial reversal of both TRIM44 expression level and migration and invasion in cells receiving sh‐LINC000958 and TRIM44. We speculated that TRIM44 was only one of the key targets of LINC00958/miR‐627 axis in regulating PTC progression that was to say LINC00958/miR‐627 axis might have multiple targets that affected the development of PTC. For example, He et al. displayed that miR‐627 could inhibit osteosarcoma cell proliferation and metastasis by targeting PTN. 35 Wang et al. also revealed that miR‐627 suppressed the proliferation of hepatocellular carcinoma cells by targeting BCL3 transcription coactivator. 36 Our study only confirmed that TRIM44 was only one of the key targets of LINC00958/miR‐627 axis in regulating PTC progression.
In conclusion, this study reported here provided evidence that LINC00958 functions as an oncogene in PTC. Knockdown of LINC00958 inhibited tumor proliferation, migration, and invasion. Luciferase experiments verify that miR‐627 binds directly to TRIM44. At the same time, the knockdown of LINC00958 will cause the decreased expression of TRIM44 in PTC. In total, we showed LncRNA LINC00958/miR‐627 signal axis regulated the proliferation and migration of PTC by TRIM44. Thus, LINC00958 may be a potential therapeutic target for the treatment of PTC.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Li Y‐Q, Wang L‐C, Li A‐X, Huang W, Song Y, Wang W. LINC00958/miR‐627 signal axis regulates the proliferation, migration, and invasion of thyroid papillary carcinoma cells by TRIM44 . Kaohsiung J Med Sci. 2022;38:415–424. 10.1002/kjm2.12502
Funding information Education Department of Hubei Province, Grant/Award Number: B2015479
REFERENCES
- 1. Huang C, Yang X, Han L, Fan Z, Liu B, Zhang C, et al. The prognostic potential of alpha‐1 type I collagen expression in papillary thyroid cancer. Biochem Biophys Res Commun. 2019;515(1):125–32. [DOI] [PubMed] [Google Scholar]
- 2. Liu H, Deng H, Zhao Y, Li C, Liang Y. LncRNA XIST/miR‐34a axis modulates the cell proliferation and tumor growth of thyroid cancer through MET‐PI3K‐AKT signaling. J Exp Clin Cancer Res. 2018;37(1):279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013;2013:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schmittgen TD, Livak KJ. Analyzing real‐time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–8. [DOI] [PubMed] [Google Scholar]
- 5. Kim SJ, Lee KE, Myong JP, Park JH, Jeon YK, Min HS, et al. BRAF V600E mutation is associated with tumor aggressiveness in papillary thyroid cancer. World J Surg. 2012;36(2):310–7. [DOI] [PubMed] [Google Scholar]
- 6. Hay ID. Papillary thyroid carcinoma. Endocrinol Metab Clin North Am. 1990;19(3):545–76. [PubMed] [Google Scholar]
- 7. Jing H, Su X, Gao B, Shuai Y, Chen J, Deng Z, et al. Epigenetic inhibition of Wnt pathway suppresses osteogenic differentiation of BMSCs during osteoporosis. Cell Death Dis. 2018;9(2):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schlumberger MJ. Papillary and follicular thyroid carcinoma. N Engl J Med. 1998;338(5):297–306. [DOI] [PubMed] [Google Scholar]
- 9. Chen F, Liu M, Yu Y, Sun Y, Li J, Hu W, et al. LINC00958 regulated miR‐627‐5p/YBX2 axis to facilitate cell proliferation and migration in oral squamous cell carcinoma. Cancer Biol Ther. 2019;20(9):1270–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abu N, Hon KW, Jeyaraman S, Jamal R. Long noncoding RNAs as biotargets in cisplatin‐based drug resistance. Future Oncol. 2018;14(29):3085–95. [DOI] [PubMed] [Google Scholar]
- 11. Mao C, Wang X, Liu Y, Wang M, Yan B, Jiang Y, et al. A G3BP1‐interacting lncRNA promotes ferroptosis and apoptosis in cancer via nuclear sequestration of p53. Cancer Res. 2018;78(13):3484–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kier MG, Hansen MK, Lauritsen J, Mortensen MS, Bandak M, Agerbaek M, et al. Second malignant neoplasms and cause of death in patients with germ cell cancer: a Danish Nationwide cohort study. JAMA Oncol. 2016;2(12):1624–7. [DOI] [PubMed] [Google Scholar]
- 13. Guo E, Liang C, He X, Song G, Liu H, Lv Z, et al. Long noncoding RNA LINC00958 accelerates gliomagenesis through regulating miR‐203/CDK2. DNA Cell Biol. 2018;37(5):465–72. [DOI] [PubMed] [Google Scholar]
- 14. Chen S, Chen JZ, Zhang JQ, Chen HX, Qiu FN, Yan ML, et al. Silencing of long noncoding RNA LINC00958 prevents tumor initiation of pancreatic cancer by acting as a sponge of microRNA‐330‐5p to down‐regulate PAX8. Cancer Lett. 2019;446:49–61. [DOI] [PubMed] [Google Scholar]
- 15. Jazdzewski K, Liyanarachchi S, Swierniak M, Pachucki J, Ringel MD, Jarzab B, et al. Polymorphic mature microRNAs from passenger strand of pre‐miR‐146a contribute to thyroid cancer. Proc Natl Acad Sci U S A. 2009;106(5):1502–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhong L, Sun S, Shi J, Cao F, Han X, Chen Z. MicroRNA‐125a‐5p plays a role as a tumor suppressor in lung carcinoma cells by directly targeting STAT3. Tumour Biol. 2017;39(6):1010428317697579. [DOI] [PubMed] [Google Scholar]
- 17. Dong W, Li B, Wang J, Song Y, Zhang Z, Fu C. MicroRNA‐337 inhibits cell proliferation and invasion of cervical cancer through directly targeting specificity protein 1. Tumour Biol. 2017;39(6):1010428317711323. [DOI] [PubMed] [Google Scholar]
- 18. He H, Jazdzewski K, Li W, Liyanarachchi S, Nagy R, Volinia S, et al. The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci U S A. 2005;102(52):19075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fan Z, Zheng J, Xue Y, Liu X, Wang D, Yang C, et al. NR2C2‐uORF targeting UCA1‐miR‐627‐5p‐NR2C2 feedback loop to regulate the malignant behaviors of glioma cells. Cell Death Dis. 2018;9(12):1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Padi SK, Zhang Q, Rustum YM, Morrison C, Guo B. MicroRNA‐627 mediates the epigenetic mechanisms of vitamin D to suppress proliferation of human colorectal cancer cells and growth of xenograft tumors in mice. Gastroenterology. 2013;145(2):437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou Z, Liu Y, Ma M, Chang L. Knockdown of TRIM44 inhibits the proliferation and invasion in papillary thyroid cancer cells through suppressing the Wnt/β‐catenin signaling pathway. Biomed Pharmacother. 2017;96:98–103. [DOI] [PubMed] [Google Scholar]
- 22. Yamada Y, Takayama KI, Fujimura T, Ashikari D, Obinata D, Takahashi S, et al. A novel prognostic factor TRIM44 promotes cell proliferation and migration, and inhibits apoptosis in testicular germ cell tumor. Cancer Sci. 2017;108(1):32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li L, Shao MY, Zou SC, Xiao ZF, Chen ZC. MiR‐101‐3p inhibits EMT to attenuate proliferation and metastasis in glioblastoma by targeting TRIM44. J Neurooncol. 2019;141(1):19–30. [DOI] [PubMed] [Google Scholar]
- 24. Xing Y, Meng Q, Chen X, Zhao Y, Liu W, Hu J, et al. TRIM44 promotes proliferation and metastasis in non‐small cell lung cancer via mTOR signaling pathway. Oncotarget. 2016;7(21):30479–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kashimoto K, Komatsu S, Ichikawa D, Arita T, Konishi H, Nagata H, et al. Overexpression of TRIM44 contributes to malignant outcome in gastric carcinoma. Cancer Sci. 2012;103(11):2021–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DeLellis RA, Lloyd RV, Heitz PU. Pathology and genetics of tumours of endocrine organs. Vol 8. World Health Organization: IARC; 2004. [Google Scholar]
- 27. Zhang K, Li C, Liu J, Li Z, Ma C. Down‐regulation of APTR and it's diagnostic value in papillary and anaplastic thyroid cancer. Pathol Oncol Res. 2020;26(1):559–65. [DOI] [PubMed] [Google Scholar]
- 28. Giordano D, Frasoldati A, Kasperbauer JL, Gabrielli E, Pernice C, Zini M, et al. Lateral neck recurrence from papillary thyroid carcinoma: predictive factors and prognostic significance. Laryngoscope. 2015;125(9):2226–31. [DOI] [PubMed] [Google Scholar]
- 29. Scheumann GF, Gimm O, Wegener G, Hundeshagen H, Dralle H. Prognostic significance and surgical management of locoregional lymph node metastases in papillary thyroid cancer. World J Surg. 1994;18(4):559–68. [DOI] [PubMed] [Google Scholar]
- 30. Peng L, Yuan X, Jiang B, Tang Z, Li GC. LncRNAs: key players and novel insights into cervical cancer. Tumour Biol. 2016;37(3):2779–88. [DOI] [PubMed] [Google Scholar]
- 31. Seitz AK, Christensen LL, Christensen E, Faarkrog K, Ostenfeld MS, Hedegaard J, et al. Profiling of long non‐coding RNAs identifies LINC00958 and LINC01296 as candidate oncogenes in bladder cancer. Sci Rep. 2017;7(1):395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang Z, Zhu X, Dong P, Cai J. Long noncoding RNA LINC00958 promotes the oral squamous cell carcinoma by sponging miR‐185‐5p/YWHAZ. Life Sci. 2020;242:116782. [DOI] [PubMed] [Google Scholar]
- 33. Xiong D, Jin C, Ye X, Qiu B, Jianjun X, Zhu S, et al. TRIM44 promotes human esophageal cancer progression via the AKT/mTOR pathway. Cancer Sci. 2018;109(10):3080–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou X, Yang Y, Ma P, Wang N, Yang D, Tu Q, et al. TRIM44 is indispensable for glioma cell proliferation and cell cycle progression through AKT/p21/p27 signaling pathway. J Neurooncol. 2019;145(2):211–22. [DOI] [PubMed] [Google Scholar]
- 35. He M, Shen P, Qiu C, Wang J. miR‐627‐3p inhibits osteosarcoma cell proliferation and metastasis by targeting PTN. Aging (Albany, NY). 2019;11(15):5744–56. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36. Wang J, Chen T, Wang L, Yao B, Sun L, Chen S, et al. MicroRNA‐627‐5p inhibits the proliferation of hepatocellular carcinoma cells by targeting BCL3 transcription coactivator. Clin Exp Pharmacol Physiol. 2020;47(3):485–94. [DOI] [PubMed] [Google Scholar]


