Abstract
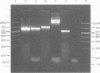
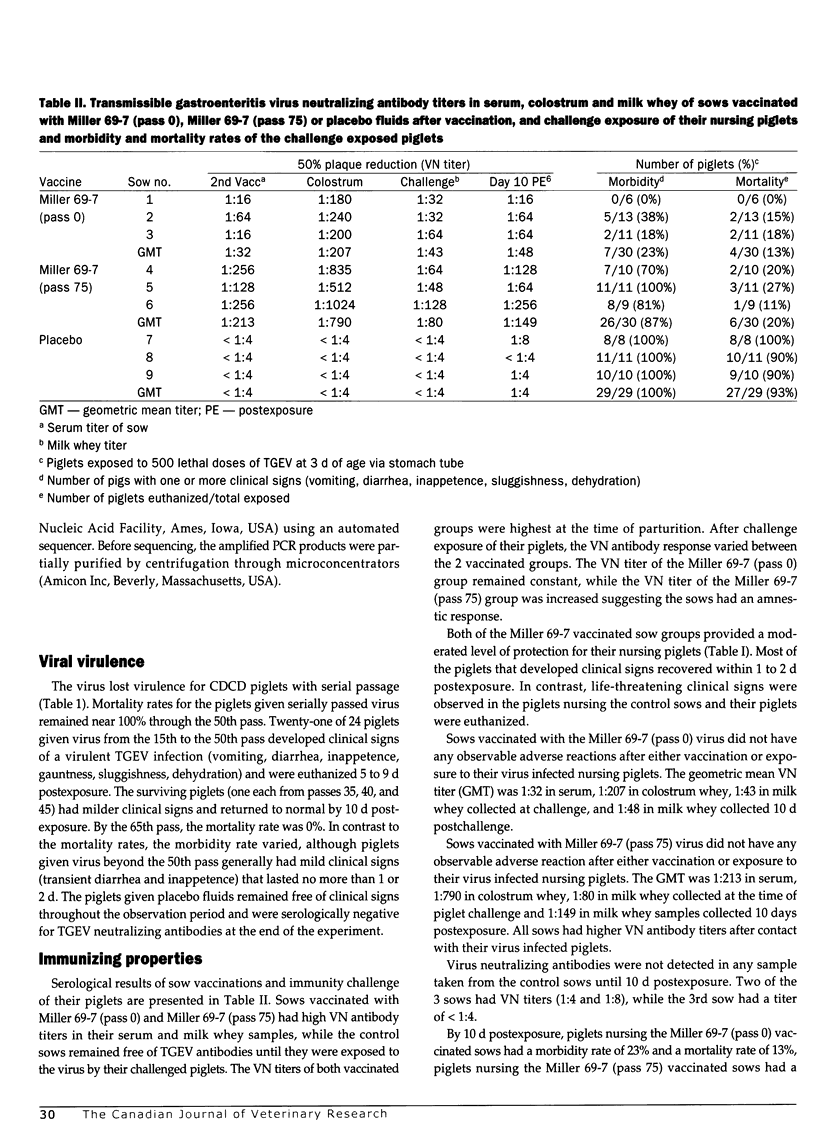
Serial passage of virulent transmissible gastroenteritis virus through cell culture reduced its virulence in 3-day-old piglets. Intramuscular inoculation of pregnant gilts with 2 doses of this modified-live virus elicited a level of lactogenic immunity that protected their nursing piglets against a lethal dose of challenge virus. Sequence analysis of a 637-bp fragment of the spike gene containing most of the aminopeptidase receptor and the 4 major antigenic sites from the original and the serially passed viruses were nearly identical. Gel analysis revealed that the fragment from the ORF-3 gene of virulent virus was smaller than the corresponding fragment from the serially passed virus. Sequence analysis of the fragment from the passed virus revealed that the sequence between nt 5310 and nt 5434 was replaced by a 636-bp fragment from the polymerase 1A gene. This replacement resulted in the loss of the CTAAACTT leader RNA-binding site and ATG start codon for the ORF-3A gene but it did not affect the ORF-3B gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAY W. W., DOYLE L. P., HUTCHINGS L. M. Some properties of the causative agent of transmissible gastroenteritis in swine. Am J Vet Res. 1952 Jul;13(48):318–321. [PubMed] [Google Scholar]
- Bohl E. H., Gupta R. K., Olquin M. V., Saif L. J. Antibody responses in serum, colostrum, and milk of swine after infection or vaccination with transmissible gastroenteritis virus. Infect Immun. 1972 Sep;6(3):289–301. doi: 10.1128/iai.6.3.289-301.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eleouet J. F., Rasschaert D., Lambert P., Levy L., Vende P., Laude H. Complete sequence (20 kilobases) of the polyprotein-encoding gene 1 of transmissible gastroenteritis virus. Virology. 1995 Feb 1;206(2):817–822. doi: 10.1006/viro.1995.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick G. T., Bohl E. H. Local and systemic cell-mediated immunity against transmissible gastroenteritis, an intestinal viral infection of swine. J Immunol. 1976 Apr;116(4):1000–1004. [PubMed] [Google Scholar]
- Kwon H. M., Saif L. J., Jackwood D. J. Field isolates of transmissible gastroenteritis virus differ at the molecular level from the Miller and Purdue virulent and attenuated strains and from porcine respiratory coronaviruses. J Vet Med Sci. 1998 May;60(5):589–597. doi: 10.1292/jvms.60.589. [DOI] [PubMed] [Google Scholar]
- McGoldrick A., Lowings J. P., Paton D. J. Characterisation of a recent virulent transmissible gastroenteritis virus from Britain with a deleted ORF 3a. Arch Virol. 1999;144(4):763–770. doi: 10.1007/s007050050541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. W., Kemeny L. J., Lambert G., Stark S. L., Booth G. D. Age-dependent resistance to transmissible gastroenteritis of swine. III. Effects of epithelial cell kinetics on coronavirus production and on atrophy of intestinal villi. Vet Pathol. 1975;12(5-6):434–445. doi: 10.1177/0300985875012005-00610. [DOI] [PubMed] [Google Scholar]
- Pedersen N. C., Ward J., Mengeling W. L. Antigenic relationship of the feline infectious peritonitis virus to coronaviruses of other species. Arch Virol. 1978;58(1):45–53. doi: 10.1007/BF01315534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif L. J., van Cott J. L., Brim T. A. Immunity to transmissible gastroenteritis virus and porcine respiratory coronavirus infections in swine. Vet Immunol Immunopathol. 1994 Oct;43(1-3):89–97. doi: 10.1016/0165-2427(94)90124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddell S. G., Anderson R., Cavanagh D., Fujiwara K., Klenk H. D., Macnaughton M. R., Pensaert M., Stohlman S. A., Sturman L., van der Zeijst B. A. Coronaviridae. Intervirology. 1983;20(4):181–189. doi: 10.1159/000149390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamoglia T. W. Present status of products available for use against transmissible gastroenteritis. J Am Vet Med Assoc. 1972 Feb 15;160(4):554–558. [PubMed] [Google Scholar]
- Vaughn E. M., Halbur P. G., Paul P. S. Sequence comparison of porcine respiratory coronavirus isolates reveals heterogeneity in the S, 3, and 3-1 genes. J Virol. 1995 May;69(5):3176–3184. doi: 10.1128/jvi.69.5.3176-3184.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley R. D., Woods R. D., Correa I., Enjuanes L. Lack of protection in vivo with neutralizing monoclonal antibodies to transmissible gastroenteritis virus. Vet Microbiol. 1988 Dec;18(3-4):197–208. doi: 10.1016/0378-1135(88)90087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods R. D. Development of PCR-based techniques to identify porcine transmissible gastroenteritis coronavirus isolates. Can J Vet Res. 1997 Jul;61(3):167–172. [PMC free article] [PubMed] [Google Scholar]