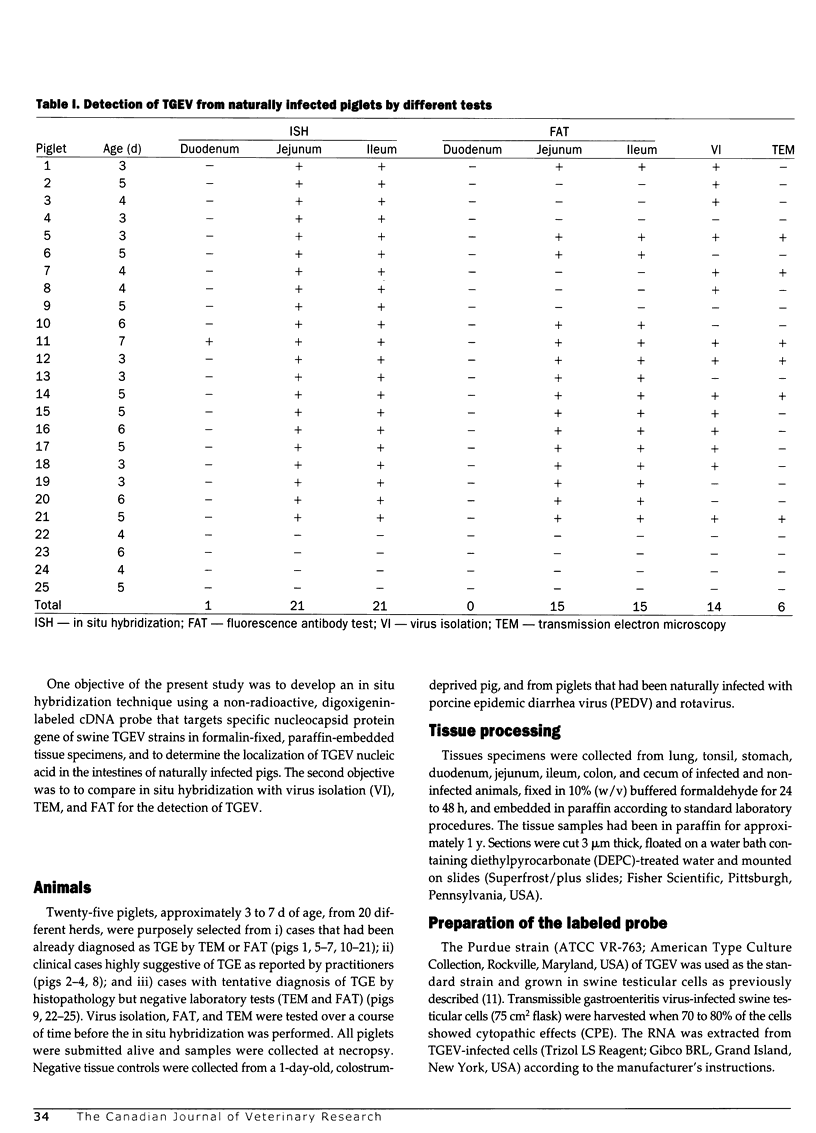
Abstract
Archived formalin-fixed, paraffin-embedded tissues from 25 pigs naturally infected with transmissible gastroenteritis virus (TGEV) were examined by in situ hybridization for TGEV nucleic acid using a nonradioactive digoxigenin-labeled cDNA probe that targeted the nucleocapsid sequence of TGEV strains. The results of in situ hybridization for the detection of TGEV were compared with virus isolation (VI), a fluorescent antibody test (FAT), and transmission electron microscopy (TEM). VI, FAT, and TEM were tested over a course of time before the in situ hybridization was performed. Positive hybridization signals were detected in duodenal, jejunal, and ileal enterocytes from 21 pigs. Hybridization signals were confined to the cytoplasm. Intestinal specimens from 25 piglets were evaluated by 4 tests. Twenty-one of 25 were positive by in situ hybridization. Of these 21 samples, 5 (24%) were positive for TGEV by all 4 tests, 15 (71%) were positive by FAT, 14 (67%) were positive by VI, and 6 (29%) were positive by TEM. In situ hybridization for the detection of TGEV in formalin-fixed, paraffin-embedded tissues provides a rapid means of confirmation of a histopathological diagnosis of TGEV without virus isolation, or when only formalin-fixed intestinal specimens were available.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chae C., Cheon D. S., Kwon D., Kim O., Kim B., Suh J., Rogers D. G., Everett K. D., Andersen A. A. In situ hybridization for the detection and localization of swine Chlamydia trachomatis. Vet Pathol. 1999 Mar;36(2):133–137. doi: 10.1354/vp.36-2-133. [DOI] [PubMed] [Google Scholar]
- Chasey D., Cartwright S. F. Virus-like particles associated with porcine epidemic diarrhoea. Res Vet Sci. 1978 Sep;25(2):255–256. doi: 10.1016/S0034-5288(18)32994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu R. M., Li N. J., Glock R. D., Ross R. F. Applications of peroxidase-antiperoxidase staining technique for detection of transmissible gastroenteritis virus in pigs. Am J Vet Res. 1982 Jan;43(1):77–81. [PubMed] [Google Scholar]
- Cubero M. J., Bernard S., Leon L., Berthon P., Contreras A. Pathogenicity and antigen detection of the Nouzilly strain of transmissible gastroenteritis coronavirus, in 1-week-old piglets. J Comp Pathol. 1992 Jan;106(1):61–72. doi: 10.1016/0021-9975(92)90068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dea S., Vaillancourt J., Elazhary Y., Martineau G. P. An outbreak of diarrhea in piglets caused by a coronavirus antigenically distinct from transmissible gastroenteritis virus. Can Vet J. 1985 Mar;26(3):108–111. [PMC free article] [PubMed] [Google Scholar]
- Frederick G. T., Bohl E. H., Cross R. F. Pathogenicity of an attenuated strain of transmissible gastroenteritis virus for newborn pigs. Am J Vet Res. 1976 Feb;37(2):165–169. [PubMed] [Google Scholar]
- Frederick G. T., Bohl E. H., Cross R. F. Pathogenicity of an attenuated strain of transmissible gastroenteritis virus for newborn pigs. Am J Vet Res. 1976 Feb;37(2):165–169. [PubMed] [Google Scholar]
- Haines D. M., Chelack B. J. Technical considerations for developing enzyme immunohistochemical staining procedures on formalin-fixed paraffin-embedded tissues for diagnostic pathology. J Vet Diagn Invest. 1991 Jan;3(1):101–112. doi: 10.1177/104063879100300128. [DOI] [PubMed] [Google Scholar]
- Kim O., Chae C. In situ hybridization for the detection and localization of porcine epidemic diarrhea virus in the intestinal tissues from naturally infected piglets. Vet Pathol. 2000 Jan;37(1):62–67. doi: 10.1354/vp.37-1-62. [DOI] [PubMed] [Google Scholar]
- Kim O., Choi C., Kim B., Chae C. Detection and differentiation of porcine epidemic diarrhoea virus and transmissible gastroenteritis virus in clinical samples by multiplex RT-PCR. Vet Rec. 2000 May 27;146(22):637–640. doi: 10.1136/vr.146.22.637. [DOI] [PubMed] [Google Scholar]
- Larochelle R., Magar R. The application of immunogold silver staining (IGSS) for the detection of transmissible gastroenteritis virus in fixed tissues. J Vet Diagn Invest. 1993 Jan;5(1):16–20. doi: 10.1177/104063879300500105. [DOI] [PubMed] [Google Scholar]
- Pospischil A., Hess R. G., Bachmann P. A. Light microscopy and ultrahistology of intestinal changes in pigs infected with epizootic diarrhoea virus (EVD): comparison with transmissible gastroenteritis (TGE) virus and porcine rotavirus infections. Zentralbl Veterinarmed B. 1981;28(7):564–577. doi: 10.1111/j.1439-0450.1981.tb01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickert R. R., Maliniak R. M. Intralaboratory quality assurance of immunohistochemical procedures. Recommended practices for daily application. Arch Pathol Lab Med. 1989 Jun;113(6):673–679. [PubMed] [Google Scholar]
- Saif L. J., Bohl E. H., Kohler E. M., Hughes J. H. Immune electron microscopy of transmissible gastroenteritis virus and rotavirus (reovirus-like agent) of swine. Am J Vet Res. 1977 Jan;38(1):13–20. [PubMed] [Google Scholar]
- Shoup D. I., Swayne D. E., Jackwood D. J., Saif L. J. Immunohistochemistry of transmissible gastroenteritis virus antigens in fixed paraffin-embedded tissues. J Vet Diagn Invest. 1996 Apr;8(2):161–167. doi: 10.1177/104063879600800204. [DOI] [PubMed] [Google Scholar]
- Siddell S. G., Anderson R., Cavanagh D., Fujiwara K., Klenk H. D., Macnaughton M. R., Pensaert M., Stohlman S. A., Sturman L., van der Zeijst B. A. Coronaviridae. Intervirology. 1983;20(4):181–189. doi: 10.1159/000149390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solorzano R. F., Morin M., Morehouse L. G. The use of immunofluorescence techniques for the laboratory diagnosis of transmissible gastroenteritis of swine. Can J Comp Med. 1978 Oct;42(4):385–391. [PMC free article] [PubMed] [Google Scholar]
- Witte K. H. Isolation of the virus of transmissible gastroenteritis (TGE) from naturally infected piglets in cell culture. Zentralbl Veterinarmed B. 1971 Dec;18(10):770–778. doi: 10.1111/j.1439-0450.1971.tb01654.x. [DOI] [PubMed] [Google Scholar]
- Zhu X. L., Paul P. S., Vaughn E., Morales A. Characterization and reactivity of monoclonal antibodies to the Miller strain of transmissible gastroenteritis virus of swine. Am J Vet Res. 1990 Feb;51(2):232–238. [PubMed] [Google Scholar]




