Abstract
Allergic rhinitis (AR) is a chronic inflammatory disease of the nasal mucosa. M2 macrophage polarization can reduce inflammation and repair tissue injury during AR development. Studies have substantiated the involvement of miRNAs in AR pathogenesis. Herein, the molecular mechanism of miR‐214‐3p in AR development was explored. To mimic the AR environment, ovalbumin (OVA) was used to treat macrophages. MiR‐214‐3p and glycogen synthase kinase 3 beta (GSK3B) expression in nasal mucus tissues and macrophages was assessed by RT‐qPCR. The M2 phenotypic signature of CD206 in macrophages was assessed by flow cytometry. The protein expression of GSK3B and M2 macrophage markers (ARG‐1 and IL‐10) was evaluated by western blotting. The correlation between miR‐214‐3p and GSK3B was validated by a luciferase reporter assay. We found that miR‐214‐3p was overexpressed in macrophages and nasal mucus tissues from AR patients. MiR‐214‐3p facilitated M2 polarization of macrophages upon OVA stimulation. Mechanistically, miR‐214‐3p targeted the GSK3B 3′ untranslated region in macrophages. In addition, GSK3B was downregulated in macrophages and nasal mucus tissues from AR patients. In rescue assays, GSK3B downregulation reversed the inhibitory effects of miR‐214‐3p silencing on M2 polarization of macrophages treated with OVA. Overall, miR‐214‐3p facilitates M2 macrophage polarization by targeting GSK3B.
Keywords: AR, GSK3B, M2 macrophage polarization, MiR‐214‐3p
1. INTRODUCTION
Allergic rhinitis (AR) is one of the most common allergic diseases characterized by nasal inflammation after the nasal mucosa comes in contact with immunoglobulin E (IgE)‐mediated allergens. 1 In recent years, AR incidence has exhibited a global upward trend due to air pollution, an accelerating life rhythm, increasing pressure in life and work, and lack of physical exercise. 2 Although AR is not fatal, nasal symptoms and systemic discomfort caused by the disease are very obvious, seriously affecting the physical health and quality of life of AR patients. 3 At present, corticosteroids, antihistamines, mast cell stabilizers, anticholinergics, decongestants, and immunotherapy (desensitization therapy) are mainly used for the treatment of AR in clinical practice. 4 However, these AR therapies are not efficient enough, and long‐term medication may contribute to drug dependence and side effects. 5 Therefore, exploring a therapy that can improve efficacy, shorten the course of treatment and lower the recurrence rate for the treatment of AR is an important task.
As phagocytic and antigen‐presenting cells in the body, macrophages play an important role in processing exogenous pathogenic microorganisms and sending out danger signals. 6 According to differences in phenotypes and secreted cytokines, macrophages can be divided into two polarization types, namely, alternatively activated M2 macrophages and classically activated M1 macrophages. M1 macrophages mainly exert proinflammatory functions by secreting proinflammatory cytokines. 7 Conversely, M2 macrophages can reduce inflammation and repair tissue injury. The common surface markers of M2‐type macrophages include CD209, CD206, and CD301. 8 After stimulation with IL‐4, IL‐3, IL‐10, TGF, and ARG‐1, macrophages polarize to the M2 type and secrete TGF‐3, VEGF, ECF, and other factors to inhibit inflammation and promote wound repair and fibrosis degeneration, especially in the late stage of the inflammatory response. 9 Some studies have demonstrated that M2 macrophages can influence the IgE class switch process and the release of IL‐4 in AR mice sensitized by cedar pollen, 10 suggesting that M2 macrophages participate in AR initiation and development.
MicroRNAs (miRNAs) are noncoding RNAs 22–24 nucleotides in length that exist widely in multicellular organisms. 11 MiRNAs modulate gene expression to affect the stability and translation of target mRNAs at the posttranscriptional level. 12 Accumulating studies have revealed the involvement of various miRNAs in AR development. 13 MiR‐31 is downregulated in AR nasal mucosa, and miR‐31 upregulation attenuates nasal rubbing, sneezing and IL‐13‐induced nasal epithelial inflammation during AR pathogenesis. 14 Local delivery of miRNA‐146a through chitosan hydrogel doped with PEG–PLA nanoparticles improves drug delivery ability in nasal mucosa and pharmacodynamic effects for AR treatment induced by ovalbumin. 15 MiR‐214‐3p has been reported to be involved in the development of diverse diseases, such as atherosclerosis, 16 osteoarthritis, 17 and recurrent pregnancy loss. 18 Interestingly, miR‐214‐3p was demonstrated to be overexpressed in nasal mucus tissues from AR patients, 19 which indicates that miR‐214‐3p may participate in AR development. Nevertheless, the role and specific mechanism of miR‐214‐3p in AR progression are still uncharacterized.
In this study, the functions and molecular mechanism of miR‐214‐3p in macrophages and nasal mucus tissues isolated from AR patients were explored, which may provide promising insight into AR treatment.
2. MATERIALS AND METHODS
2.1. Bioinformatic analysis
Potential target genes (LSM12, RC3H1, NAA15, AMMECR1L, ZFAND3, NEO1, DOLPP1, GSK3B, and MED19) of miR‐214‐3p were identified using miRDB (http://www.mirdb.org/) (screen condition: target rank ≤ 9). Binding sites between miR‐214‐3p and the GSK3B 3′UTR were predicted by TargetScan (http://www.targetscan.org/).
2.2. Participants
According to the 2016 revision of Allergic Rhinitis and its Impact on Asthma (ARIA) Guidelines, participants were diagnosed with AR based on symptoms, skin prick test, and serum IgE measurement. A total of 12 healthy controls (male 85.0%, mean age 31.3 years, range 18–54 years) and 28 AR patients (male 75.0%, mean age 33.5 years, range 19–55 years) were recruited at Hubei Provincial Hospital of Traditional Chinese Medicine (Hubei, China). Informed consent from the participants was obtained. Participants with infectious rhinitis, chronic rhinosinusitis and nasal polyps and an age over 60 and below 4 years were excluded.
2.3. Tissue sampling and macrophage isolation
Nasal lavage was conducted to collect nasal mucus. In brief, subjects bent their heads low and flushed their nostrils with 5 ml preheated saline. Flush solution was sucked into the suction collector by a nasal endoscope and then centrifuged (4000g, 5 min) to obtain the supernatant and cell pellet. CD14 microbeads (Miltenyi Biotec, Cologne) were used for positive selection of human macrophages from the cell mixtures.
2.4. Flow cytometry
Monoclonal anti‐human antibodies against proteins such as CD206 (15‐2, Bio‐Rad), CD14 (HCD14, BioLegend), CD3 (HIT3a, BD Pharmingen), and CD45 (HI30, BioLegend) for the identification of macrophages were incubated with the cells on ice for 30 min. Nonspecific binding was blocked with TruStain FcX (eBioscience). A FACS Canto II (BD Biosciences) was used to perform the fluorescence‐activated cell sorting (FACS) to sort the macrophages of the nasal mucus of healthy controls and AR patients. CD3−CD14+ macrophages were gated on CD45+ leukocyte cells, and a marker for M2 macrophages (CD206) was further sorted and analyzed with FlowJo v.10 (Treestar, Inc.).
2.5. Cell culture and treatment
Sorted macrophages were cultured in DMEM (Gibco) supplemented with 10% FBS (Gibco) and maintained in 5% CO2 at 37°C. OVA can be used to induce the AR model 20 and was used to treat sorted macrophages from healthy controls for 0, 6, 12, 24, or 48 h at a concentration of 5 mg/ml. 21 In some cases, macrophage M2 polarization was induced by IL‐4 at a concentration of 20 ng/ml. 22
2.6. Cell transfection
Short hairpin RNA (shRNA) targeting GSK3B (sh‐GSK3B) (GenePharma) was used to knock down GSK3B with sh‐NC (GenePharma) as a negative control. MiR‐214‐3p inhibitor was applied to downregulate miR‐214‐3p with NC inhibitor as a negative control. MiR‐214‐3p mimic was used to enhance endogenous miR‐214‐3p expression with NC mimic as a negative control. MiR‐214‐3p inhibitor, NC inhibitor, miR‐214‐3p mimic, NC mimic, sh‐GSK3B, and sh‐NC were transfected into macrophages using Lipofectamine 2000 (Invitrogen). The transfection efficiency was examined using RT‐qPCR after 48 h.
2.7. RT‐qPCR
Total RNA isolated from nasal mucus tissues and macrophages by TRIzol reagent (Invitrogen) was reverse transcribed to complementary DNA using a reverse transcription cDNA synthesis kit (Vazyme), and then the PrimeScript RT reagent Kit (Invitrogen) was used for RT‐qPCR analysis through an IQ5 real‐time PCR system (Bio‐Rad). The levels of miR‐214‐3p and GSK3B were normalized to U6 and GAPDH, respectively, and analyzed using the 2−ΔΔCt method. The primers used are presented in Table 1.
TABLE 1.
The primer sequences
| Target | Sequences |
|---|---|
| miR‐214‐3p | Forward, 5′‐AGCAGGCACAGACAGGCA‐3′ |
| Reverse, 5′‐GCAGGGTCCGAGGTATTC‐3′; | |
| GSK3B | Forward, 5′‐AGCTATACAGACACTAAAGT‐3′ |
| Reverse, 5′‐GATGAGGTCTATCTTAATC‐3′ | |
| GAPDH | Forward, 5′‐TCAAGATCATCAGCAATGCC‐3′ |
| Reverse, 5′‐CGATACCAAAGTTGTCATGGA‐3′ | |
| U6 | Forward, 5′‐ATACAGAGAAAGTTAGCACGG‐3′ |
| Reverse, 5′‐GGAATGCTTCAAAGAGTTGTG‐3′ |
2.8. Western blotting
After total proteins were isolated from macrophages by RIPA lysis buffer (Beyotime), a PierceTM BCA Assay Kit (Thermo Fisher Scientific) was used to assess the concentrations. After 12% SDS‐PAGE and transfer onto PVDF membranes, the proteins were coincubated with primary antibodies at 4°C overnight and then with a horseradish peroxidase‐labeled IgG secondary antibody (ab6721; 1:1000) at room temperature for 2 h. Primary antibodies included anti‐ARG‐1 (ab269541; 1:500), anti‐IL‐10 (ab133575; 1:500), anti‐IL‐4 (ab62351; 1:1000), anti‐IL‐5 (ab134935; 1:1000), anti‐IL‐13 (ab133353; 1:2000), anti‐GSK3B (ab277904; 1:500), and anti‐GAPDH (ab8245; 1:1000). All antibodies were purchased from Abcam. The protein bands were imaged by an ECL chemiluminescence kit (Pierce) and analyzed by ImageJ software.
2.9. Luciferase reporter assay
The wild‐type or mutant type GSK3B 3′UTR containing the binding site of miR‐214‐3p was subcloned into the pmirGLO vector (Promega) to generate the GSK3B 3′UTR‐Wt/Mut reporter. Next, the pmirGLO vector carrying the GSK3B 3′UTR and miR‐214‐3p inhibitor/mimic were cotransfected into macrophages via Lipofectamine 2000 (Invitrogen) for 48 h. A luciferase reporter assay system (Promega) was used to test luciferase activity levels.
2.10. Statistical analysis
Data are presented as the mean ± standard and were statistically analyzed by Student's t test (comparison between two groups) and by ANOVA (comparison among multiple groups) using GraphPad Prism 5.0 software. Pearson correlation analysis was used to analyze the correlation between miR‐214‐3p expression and GSK3B expression in nasal mucus tissues from AR patients. The results were considered statistically significant when the p value was <0.05.
3. RESULTS
3.1. MiR‐214‐3p is overexpressed in nasal mucus tissues and macrophages from AR patients
The participants were divided into healthy controls (n = 12) and AR patients (n = 28). Next, miR‐214‐3p expression in nasal mucus tissues from healthy controls and AR patients was assessed via RT‐qPCR, which demonstrated that the miR‐214‐3p level in AR nasal mucus tissues was significantly higher than that in normal nasal mucus tissues (Figure 1A). After FACS was used to sort macrophages derived from nasal mucus of healthy controls and AR patients (Figure 1B), miR‐214‐3p was observed to be upregulated in the macrophages of AR patients (Figure 1C). Next, OVA (5 mg/ml) was used to stimulate the sorted macrophages from healthy controls for 0, 6, 12, 24, or 48 h, and the results indicated that miR‐214‐3p levels were elevated with increasing OVA treatment time (Figure 1D). Overall, miR‐214‐3p is overexpressed in the macrophages and nasal mucus tissues of AR patients.
FIGURE 1.
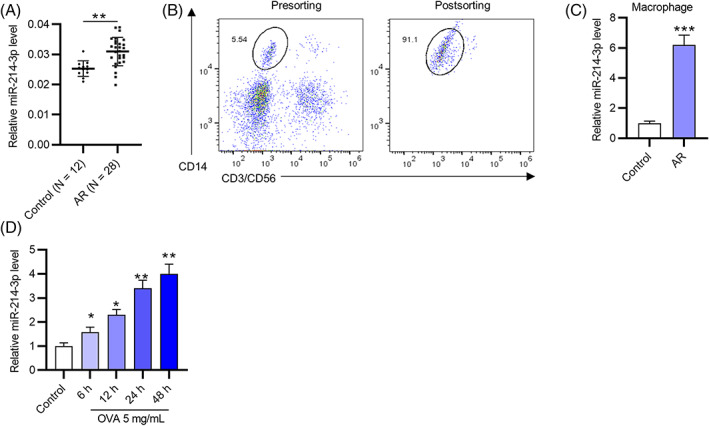
MiR‐214‐3p is overexpressed in nasal mucus tissues and macrophages from AR patients. (A) MiR‐214‐3p levels in nasal mucus tissues from healthy controls and AR patients were assessed via RT‐qPCR. (B) FACS was used to sort macrophages derived from the nasal mucus of healthy controls or AR patients. (C) MiR‐214‐3p expression in macrophages of healthy controls and AR patients was assessed by RT‐qPCR. (D) OVA (5 mg/ml) was used to stimulate the sorted macrophages from healthy controls for 6, 12, 24, or 48 h. *p < 0.05, **p < 0.01, ***p < 0.001
3.2. MiR‐214‐3p facilitates M2 macrophages polarization
To investigate whether dysregulated miR‐214‐3p is related to M2 polarization during AR development, the following experiments were conducted. The transfection efficiency of the miR‐214‐3p inhibitor was assessed by RT‐qPCR, which showed that miR‐214‐3p was downregulated in macrophages from the nasal mucus of healthy controls after silencing miR‐214‐3p (Figure 2A). Next, to confirm M2 polarization of macrophages, the intensity of the M2 phenotypic signature marker CD206 was evaluated by flow cytometry. After OVA was used to treat macrophages from the nasal mucus of healthy controls to simulate the AR environment, the proportion of CD206 on macrophages was elevated (Figure 2B,C), suggesting that OVA stimulation induced M2 macrophage polarization. However, OVA‐induced CD206 upregulation on macrophages was attenuated by miR‐214‐3p inhibition (Figure 2B,C), which indicated that downregulated miR‐214‐3p inhibited the M2‐prone phenotype of macrophages upon OVA stimulation. Furthermore, the protein levels of M2 macrophage markers (IL‐4, IL‐5, IL‐13, ARG‐1, and IL‐10) in macrophages from the nasal mucus of healthy controls were increased by OVA treatment, which was reversed by miR‐214‐3p repression (Figure 2D–F). IL‐4 stimulation also upregulated miR‐214‐3p repression in macrophages (Figure S1A). The miR‐214‐3p inhibitor rescued the IL‐4‐induced M2 polarization of macrophages (Figure S1B–F). In summary, miR‐214‐3p facilitates M2 polarization of macrophages.
FIGURE 2.
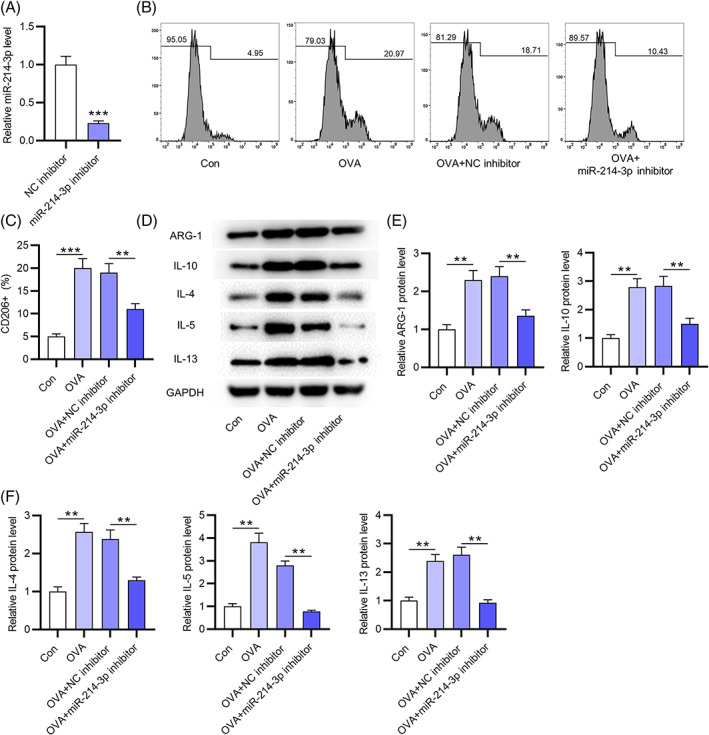
MiR‐214‐3p facilitates OVA‐induced M2 macrophage polarization. (A) Transfection efficiency of miR‐214‐3p inhibitor into macrophages from nasal mucus of healthy controls was assessed by RT‐qPCR. (B,C) proportion of CD206+ macrophages from nasal mucus of healthy controls in four groups (con, OVA, OVA+NC inhibitor, and OVA+miR‐214‐3p inhibitor) by flow cytometry. (D–F) Western blotting was conducted to evaluate M2 macrophage markers (IL‐4, IL‐5, IL‐13, IL‐10, and ARG‐1) protein expression in macrophages from nasal mucus of healthy controls in four groups (con, OVA, OVA+NC inhibitor, and OVA+miR‐214‐3p inhibitor). **p < 0.01, ***p < 0.001
3.3. MiR‐214‐3p targets GSK3B
To explore the mechanism of miR‐214‐3p in AR pathogenesis, its potential downstream mRNAs (LSM12, RC3H1, NAA15, AMMECR1L, ZFAND3, NEO1, DOLPP1, GSK3B, and MED19) were predicted by miRDB (Figure 3A). After downregulating miR‐214‐3p, only GSK3B levels were markedly upregulated in macrophages among these candidates (Figure 3B). A binding site between miR‐214‐3p and GSK3B was predicted using TargetScan (Figure 3C). A luciferase reporter assay showed that miR‐143‐3p silencing increased the luciferase activity of GSK3B 3′UTR‐Wt but not that of GSK3B 3′UTR‐Mut, further confirming the binding relationship between miR‐214‐3p and the GSK3B 3’UTR (Figure 3D). Additionally, GSK3B protein expression in macrophages was elevated by miR‐214‐3p knockdown (Figure 3E). Moreover, we successfully overexpressed miR‐214‐3p by transfection of miR‐214‐3p mimics into macrophages (Figure 3F). MiR‐214‐3p overexpression reduced GSK3B mRNA and protein levels (Figure 3G,H). The luciferase activity of plasmids containing the GSK3B 3’UTR was suppressed by miR‐214‐3p mimics, while that of plasmids containing the mutated GSK3B 3′UTR was not significantly influenced under the same conditions (Figure 3I). Taken together, miR‐214‐3p targets GSK3B in macrophages of nasal mucus.
FIGURE 3.
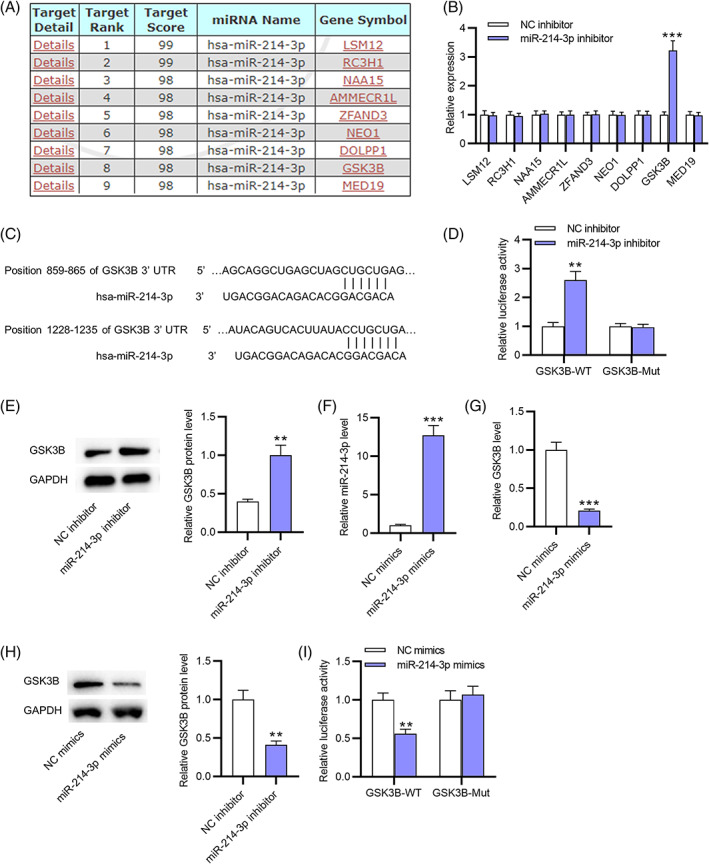
MiR‐214‐3p targets GSK3B. (A) Potential target genes (LSM12, RC3H1, NAA15, AMMECR1L, ZFAND3, NEO1, DOLPP1, GSK3B, and MED19) of miR‐214‐3p were predicted by miRDB. (B) The expression of potential downstream mRNAs of miR‐214‐3p in macrophages of nasal mucus after downregulating miR‐214‐3p was assessed by RT‐qPCR. (C) A binding site between miR‐214‐3p and GSK3B was predicted using TargetScan. (D) The interaction between miR‐214‐3p and the GSK3B 3’UTR was validated by a luciferase reporter assay. (E) Western blotting was performed to assess GSK3B protein expression in macrophages from nasal mucus after miR‐214‐3p knockdown. (F) The miR‐214‐3p mimic‐mediated overexpression efficiency of miR‐214‐3p was assessed by RT‐qPCR. (G,H) GSK3B expression in miR‐214‐3p mimic‐transfected macrophages was assessed by RT‐qPCR and western blotting. (I) A luciferase reporter assay was performed to reveal the luciferase activity of GSK3B 3′UTR‐Wt/Mut reporter vectors under the condition of miR‐214‐3p overexpression. **p < 0.01, ***p < 0.001
3.4. GSK3B is downregulated in nasal mucus tissues and macrophages from AR patients
As Figure 4A indicates, GSK3B expression in nasal mucus tissues from AR patients (n = 28) was lower than that in healthy controls (n = 28). In addition, the mRNA and protein levels of GSK3B were markedly downregulated in macrophages derived from the nasal mucus of AR patients compared with macrophages of healthy controls (Figure 4B,C). After OVA (5 mg/ml) was utilized to stimulate macrophages from the nasal mucus of healthy controls for 0, 6, 12, 24, or 48 h, the expression of GSK3B mRNA and protein decreased with increasing OVA treatment time (Figure 4D–F). According to the results from Pearson correlation analysis, miR‐214‐3p expression was negatively related to GSK3B expression in nasal mucus tissues from AR patients (n = 28) (Figure 4G).
FIGURE 4.
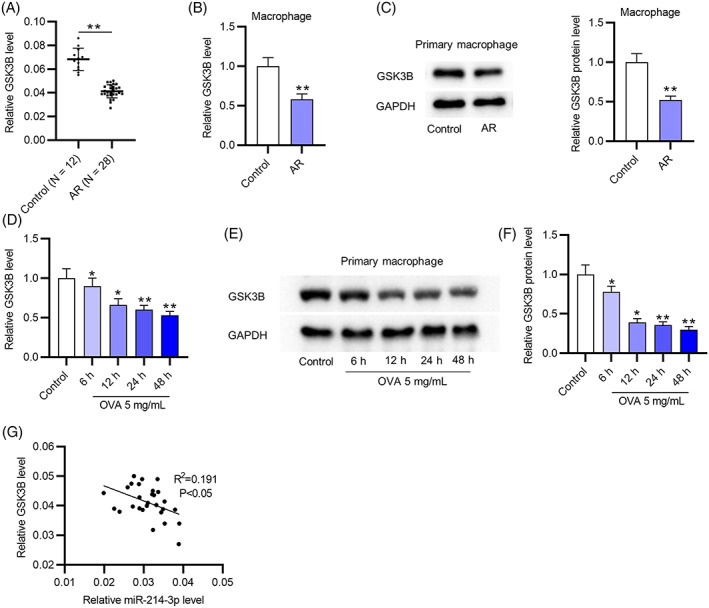
GSK3B is downregulated in nasal mucus tissues and macrophages from AR patients. (A) GSK3B expression in nasal mucus tissues from healthy controls and AR patients was assessed by RT‐qPCR. (B,C) The mRNA and protein levels of GSK3B in macrophages derived from the nasal mucus of AR patients or healthy controls were assessed by RT‐qPCR and western blotting. (D–F) After OVA (5 mg/ml) treatment for 6, 12, 24, or 48 h, the mRNA and protein levels of GSK3B in macrophages from the nasal mucus of healthy controls were examined by RT‐qPCR and western blotting. (G) The correlation between miR‐214‐3p and GSK3B in nasal mucus tissues from AR patients (n = 28) was analyzed by Pearson correlation analysis. *p < 0.05, **p < 0.01
3.5. GSK3B downregulation reverses the effects of miR‐214‐3p silencing on M2 macrophage polarization
To determine the role of the miR‐214‐3p/GSK3B axis in M2 macrophage polarization, rescue assays were conducted. Western blotting was applied to assess the transfection efficiency of sh‐GSK3B, and the results showed that GSK3B protein expression was downregulated in OVA‐treated macrophages after silencing GSK3B (Figure 5A). Flow cytometry demonstrated that the decrease in the M2 phenotypic signature marker CD206 caused by miR‐214‐3p depletion was counteracted by GSK3B inhibition in macrophages upon OVA stimulation (Figure 5B,C). Simultaneously, miR‐214‐3p silencing reduced the protein expression of M2 macrophage markers (IL‐4, IL‐5, IL‐13, ARG‐1, and IL‐10) in OVA‐stimulated macrophages, which was offset by GSK3B knockdown (Figure 5D,E). Collectively, GSK3B downregulation reverses the inhibitory effects of miR‐214‐3p silencing on M2 polarization of macrophages treated with OVA.
FIGURE 5.
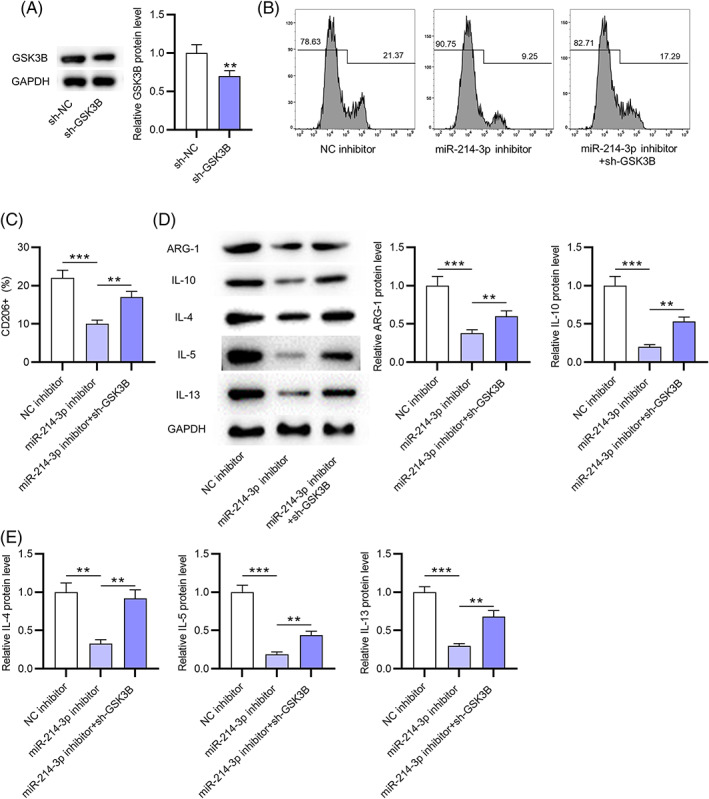
GSK3B downregulation reverses the effects of miR‐214‐3p knockdown on M2 macrophage polarization. (A) GSK3B protein expression in OVA‐treated macrophages after silencing GSK3B was assessed by western blotting. (B,C) M2 phenotypic signature marker CD206 in macrophages upon OVA stimulation after silencing miR‐214‐3p or GSK3B was assessed by flow cytometry. (D,E) the protein expression of M2 macrophage markers (IL‐4, IL‐5, IL‐13, IL‐10, and ARG‐1) in OVA‐stimulated macrophages after inhibiting GSK3B or miR‐214‐3p was examined by western blotting. **p < 0.01, ***p < 0.001
4. DISCUSSION
AR is a chronic allergic disease inflammation characterized by an inflammatory response of the nasal mucosa. 23 Some reports have demonstrated that miRNAs bind to the 3′UTR of their target genes to exert functional roles in AR pathogenesis. For example, miR‐338‐3p overexpression suppresses PM2.5‐induced autophagy of human nasal epithelium cells and alleviates nasal symptoms of AR rats by targeting UBE2Q1. 24 MiR‐345‐5p inhibits apoptosis and fibrosis of nasal epithelial cells and ameliorates the inflammatory response in AR mice by negatively regulating TLR4 expression. 25 In AR pathogenesis, miR‐202‐5p targets MATN2 to facilitate M2 phenotype polarization. 21 Consistent with a previous study, 19 miR‐214‐3p was found to be overexpressed in nasal mucus tissues and macrophages from AR patients in this study, suggesting that miR‐214‐3p may be associated with AR development. MiR‐214, miR‐145, miR‐544, miR‐138, and miR‐371 can modulate the balance between Type 1 helper T/Type 2 helper T cells by regulating Runx3 in a combinatorial manner in asthma, a disease that can be triggered by AR. 26 Caspase‐1 signaling, a pathway regulated by miR‐214‐3p, 16 has been validated to exert critical functions in AR models. 27 , 28 Herein, macrophages of nasal mucus were treated with OVA to mimic AR conditions. MiR‐214‐3p expression in macrophages of nasal mucus was elevated with increasing OVA treatment time. Functionally, miR‐214‐3p downregulation attenuated the OVA‐induced increase in the M2 phenotypic signature marker CD206 and the protein expression of M2 macrophage markers (IL‐10 and ARG‐1) in macrophages, indicating that miR‐214‐3p facilitates M2 macrophage polarization.
Next, glycogen synthase kinase 3 beta (GSK3B) was confirmed as the target gene of miR‐214‐3p via bioinformatics analysis and a luciferase reporter assay. As one of the serine/threonine protein kinases, glycogen synthase kinase 3 (GSK3) is divided into two subgroups, GSK3A and GSK3B. 29 GSK3 is a negative regulator of glucose homeostasis and is involved in mitochondrial dysfunction, ER stress, inflammation, energy metabolism, and apoptotic pathways. 30 GSK3B has been implicated in Alzheimer's disease, 31 schizophrenia, 32 obesity, 33 and various cancers. 34 , 35 Previously, GSK3B was revealed to be involved in M2 polarization. The IL‐10/GSK3B/PTEN axis is regulated by regulatory T lymphocytes to modulate macrophage polarization toward the M2 phenotype, thus ameliorating ICH‐induced inflammatory injury. 36 HDAC depletion prevents white matter injury by regulating microglia/macrophage polarization via the GSK3B/PTEN/Akt axis. 37 Herein, GSK3B was downregulated in macrophages and nasal mucus tissues from AR patients, which suggested that GSK3B may be associated with AR development. Furthermore, the mRNA and protein expression of GSK3B was reduced with increasing OVA treatment time. MiR‐214‐3p expression was negatively related to GSK3B expression in nasal mucus tissues from AR patients. In rescue assays, GSK3B downregulation reversed the inhibitory effects of miR‐214‐3p silencing on M2 polarization of macrophages treated with OVA, indicating that GSK3B suppresses M2 macrophage polarization and is involved in miR‐214‐3p‐mediated M2 macrophage polarization.
In conclusion, miR‐214‐3p facilitates M2 macrophage polarization by targeting GSK3B, which may provide a better understanding of novel therapeutic agents of AR. However, there are some limitations in this study. First, the samples from AR patients and healthy controls were too small. Second, some signaling pathways, such as IL‐33/ST2 signaling, 38 the NF‐κB pathway, 20 and the p38/ERK1/2 MAPK pathway, 39 have been reported to participate in AR development. Thus, whether the miR‐214‐3p/GSK3B axis can regulate these signaling pathways in AR should be investigated in future studies.
CONFLICT OF INTERESTS
The authors declare no conflict of interests.
Supporting information
Figure S1 MiR‐214‐3p facilitates IL‐4‐induced M2 macrophage polarization. (A) IL‐4 (20 ng/ml) was used to stimulate the sorted macrophages from healthy controls for 6, 12, 24, or 48 h. (B) Transfection efficiency of miR‐214‐3p inhibitor into IL‐4‐stimulated macrophages was assessed by RT‐qPCR. (C,D) Proportion of CD206+ macrophages in the four groups (Con, IL‐4, IL‐4 + NC inhibitor, and IL‐4 + miR‐214‐3p inhibitor) by flow cytometry. (E,F) Western blotting was conducted to evaluate M2 macrophage marker (IL‐4, IL‐5, IL‐13, IL‐10, and ARG‐1) protein expression in macrophages in the four groups (Con, IL‐4, IL‐4 + NC inhibitor, and IL‐4 + miR‐214‐3p inhibitor). *p < 0.05, **p < 0.01, ***p < 0.001
Peng L‐Y, Li B‐B, Deng K‐B, Wang W‐G. MicroRNA‐214‐3p facilitates M2 macrophage polarization by targeting GSK3B . Kaohsiung J Med Sci. 2022;38:347–356. 10.1002/kjm2.12487
Ling‐Yan Peng and Bi‐Bao Li contributed equally to this study.
REFERENCES
- 1. Lee BW, Lee CS, Lim ER, Tham AC. Intranasal steroid use and satisfaction in allergic rhinitis: a cross‐sectional study from an Asian perspective. ORL J Otorhinolaryngol Relat Spec. 2021:1–7. [DOI] [PubMed] [Google Scholar]
- 2. Chen YS, Mirzakhani H, Lu M, Zeiger RS, O'Connor GT, Sandel MT, et al. The association of prenatal vitamin d sufficiency with aeroallergen sensitization and allergic rhinitis in early childhood. J Allergy Clin Immunol Pract. 2021;9(10):3788–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vasileiadou S, Ekerljung L, Bjerg A, Goksör E. Asthma increased in young adults from 2008‐2016 despite stable allergic rhinitis and reduced smoking. PLoS One. 2021;16(6):e0253322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bilstein A, Werkhäuser N, Rybachuk A, Mösges R. The effectiveness of the bacteria derived extremolyte ectoine for the treatment of allergic rhinitis. Biomed Res Int. 2021;2021:5562623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhardwaj B, Singh J. Efficacy of vitamin D supplementation in allergic rhinitis. Indian J Otolaryngol Head Neck Surg. 2021;73(2):152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang J, Ma CR, Hua YQ, Li L, Ni JY, Huang YT, et al. Contradictory regulation of macrophages on atherosclerosis based on polarization, death and autophagy. Life Sci. 2021;276:118957. [DOI] [PubMed] [Google Scholar]
- 7. Mathiesen CBK, Rudjord‐Levann AM, Gad M, Larsen J, Sellebjerg F, Pedersen AE. Cladribine inhibits secretion of pro‐inflammatory cytokines and phagocytosis in human monocyte‐derived M1 macrophages in‐vitro. Int Immunopharmacol. 2021;91:107270. [DOI] [PubMed] [Google Scholar]
- 8. Motz K, Lina I, Murphy MK, Drake V, Davis R, Tsai HW, et al. M2 macrophages promote collagen expression and synthesis in Laryngotracheal stenosis fibroblasts. Laryngoscope. 2021;131(2):E346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han GH, Kim SJ, Ko WK, Lee D, Han IB, Sheen SH, et al. Transplantation of tauroursodeoxycholic acid‐inducing M2‐phenotype macrophages promotes an anti‐neuroinflammatory effect and functional recovery after spinal cord injury in rats. Cell Prolif. 2021;54(6):e13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tyurin YA, Lissovskaya SA, Fassahov RS, Mustafin IG, Shamsutdinov AF, Shilova MA, et al. Cytokine profile of patients with allergic rhinitis caused by pollen, mite, and microbial allergen sensitization. J Immunol Res. 2017;2017:3054217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang G, Yu X, Xia J, Sun J, Huang H, Liu Y. MicroRNA‐9 restrains the sharp increase and boost apoptosis of human acute myeloid leukemia cells by adjusting the hippo/YAP signaling pathway. Bioengineered. 2021;12(1):2906–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fu M, Zhang K. MAPK interacting serine/threonine kinase 1 (MKNK1), one target gene of miR‐223‐3p, correlates with neutrophils in sepsis based on bioinformatic analysis. Bioengineered. 2021;12(1):2550–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang R, Xue S, Liu Y, Peng M, Guo B. The correlation of long non‐coding RNA NEAT1 and its targets microRNA (miR)‐21, miR‐124, and miR‐125a with disease risk, severity, and inflammation of allergic rhinitis. Medicine. 2021;100(4):e22946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou F, Liu P, Lv H, Gao Z, Chang W, Xu Y. miR‐31 attenuates murine allergic rhinitis by suppressing interleukin‐13‐induced nasal epithelial inflammatory responses. Mol Med Rep. 2021;23(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Su Y, Sun B, Gao X, Liu S, Hao R, Han B. Chitosan hydrogel doped with PEG‐PLA nanoparticles for the local delivery of miRNA‐146a to treat allergic rhinitis. Pharmaceutics. 2020;12(10):907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li T, Tu P, Bi J, Sun Y, Yu D, Wang J, et al. LncRNA Miat knockdown alleviates endothelial cell injury through regulation of miR‐214‐3p/Caspase‐1 signalling during atherogenesis. Clin Exp Pharmacol Physiol. 2021;48(9):1231–8. [DOI] [PubMed] [Google Scholar]
- 17. Xiao P, Zhu X, Sun J, Zhang Y, Qiu W, Li J, et al. Cartilage tissue miR‐214‐3p regulates the TrkB/ShcB pathway paracrine VEGF to promote endothelial cell migration and angiogenesis. Bone. 2021;151:116034. [DOI] [PubMed] [Google Scholar]
- 18. Al‐Rubaye S, Ghaderian SMH, Salehpour S, Salmani T, Vojdani S, Yaseen R, et al. Aberrant expression of BAX, MEG3, and miR‐214‐3P genes in recurrent pregnancy loss. Gynecol Endocrinol. 2021;37(7):660–4. [DOI] [PubMed] [Google Scholar]
- 19. Huang Y, Guo ZQ, Zhang RX, Zhao RW, Dong WY, Wang H, et al. Effect of PM2.5 on MicroRNA expression and function in nasal mucosa of rats with allergic rhinitis. Am J Rhinol Allergy. 2020;34(4):543–53. [DOI] [PubMed] [Google Scholar]
- 20. Piao CH, Song CH, Lee EJ, Chai OH. Saikosaponin a ameliorates nasal inflammation by suppressing IL‐6/ROR‐γt/STAT3/IL‐17/NF‐κB pathway in OVA‐induced allergic rhinitis. Chem Biol Interact. 2020;315:108874. [DOI] [PubMed] [Google Scholar]
- 21. Wang L, Liu X, Song X, Dong L, Liu D. MiR‐202‐5p promotes M2 polarization in allergic rhinitis by targeting MATN2. Int Arch Allergy Immunol. 2019;178(2):119–27. [DOI] [PubMed] [Google Scholar]
- 22. Liang CL, Jiang H, Feng W, Liu H, Han L, Chen Y, et al. Total glucosides of paeony ameliorate pristane‐induced lupus nephritis by inducing PD‐1 ligands+ macrophages via activating IL‐4/STAT6/PD‐L2 signaling. Front Immunol. 2021;12:683249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Song J. Effects of Yu‐ping‐feng granules combined with loratadine tablets on treatment efficacy and immune factor levels in allergic rhinitis patients. Am J Transl Res. 2021;13(5):5192–9. [PMC free article] [PubMed] [Google Scholar]
- 24. Wang JC, Huang Y, Zhang RX, Han ZJ, Zhou LL, Sun N, et al. miR‐338‐3p inhibits autophagy in a rat model of allergic rhinitis after PM2.5 exposure through AKT/mTOR signaling by targeting UBE2Q1. Biochem Biophys Res Commun. 2021;554:1–6. [DOI] [PubMed] [Google Scholar]
- 25. Liu J, Jiang Y, Han M, Jiang L, Liang D, Li S, et al. MicroRNA‐345‐5p acts as an anti‐inflammatory regulator in experimental allergic rhinitis via the TLR4/NF‐κB pathway. Int Immunopharmacol. 2020;86:106522. [DOI] [PubMed] [Google Scholar]
- 26. Qiu YY, Zhang YW, Qian XF, Bian T. miR‐371, miR‐138, miR‐544, miR‐145, and miR‐214 could modulate Th1/Th2 balance in asthma through the combinatorial regulation of Runx3. Am J Transl Res. 2017;9(7):3184–99. [PMC free article] [PubMed] [Google Scholar]
- 27. Moon PD, Han NR, Lee JS, Kim HY, Hong S, Kim HJ, et al. β‐Eudesmol inhibits thymic stromal lymphopoietin through blockade of caspase‐1/NF‐κB signal cascade in allergic rhinitis murine model. Chem Biol Interact. 2018;294:101–6. [DOI] [PubMed] [Google Scholar]
- 28. Kim HY, Jeong HJ, Kim HM. Anti‐allergic and anti‐inflammatory effects of the Bcl‐2 inhibitor ABT‐737 on experimental allergic rhinitis models. Eur J Pharmacol. 2018;833:34–43. [DOI] [PubMed] [Google Scholar]
- 29. Noori MS, Courreges MC, Bergmeier SC, McCall KD, Goetz DJ. Modulation of LPS‐induced inflammatory cytokine production by a novel glycogen synthase kinase‐3 inhibitor. Eur J Pharmacol. 2020;883:173340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wadhwa P, Jain P, Jadhav HR. Glycogen synthase kinase 3 (GSK3): its role and inhibitors. Curr Top Med Chem. 2020;20(17):1522–34. [DOI] [PubMed] [Google Scholar]
- 31. D'Mello SR. When good kinases go rogue: GSK3, p38 MAPK and CDKs as therapeutic targets for Alzheimer's and Huntington's disease. Int J Mol Sci. 2021;22(11):5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen Y, Hua S, Wang W, Fan W, Tang W, Zhang Y, et al. A comprehensive analysis of GSK3B variation for schizophrenia in Han Chinese individuals. Asian J Psychiatr. 2020;47:101832. [DOI] [PubMed] [Google Scholar]
- 33. Ge D, Dauchy RT, Liu S, Zhang Q, Mao L, Dauchy EM, et al. Insulin and IGF1 enhance IL‐17‐induced chemokine expression through a GSK3B‐dependent mechanism: a new target for melatonin's anti‐inflammatory action. J Pineal Res. 2013;55(4):377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu C, Du Z, Ren S, Pian Y. Downregulation of GSK3B by miR‐132‐3p enhances etoposide‐induced breast cancer cell apoptosis. Ann Clin Lab Sci. 2021;51(3):285–94. [PubMed] [Google Scholar]
- 35. Taylan E, Zayou F, Murali R, Karlan BY, Pandol SJ, Edderkaoui M, et al. Dual targeting of GSK3B and HDACs reduces tumor growth and improves survival in an ovarian cancer mouse model. Gynecol Oncol. 2020;159(1):277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhou K, Zhong Q, Wang YC, Xiong XY, Meng ZY, Zhao T, et al. Regulatory T cells ameliorate intracerebral hemorrhage‐induced inflammatory injury by modulating microglia/macrophage polarization through the IL‐10/GSK3β/PTEN axis. J Cereb Blood Flow Metab. 2017;37(3):967–79. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37. Wang G, Shi Y, Jiang X, Leak RK, Hu X, Wu Y, et al. HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3β/PTEN/Akt axis. Proc Natl Acad Sci U S A. 2015;112(9):2853–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu G, Liu F. Advances of IL‐33/ST2 signaling pathway in allergic rhinitis. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020;34(6):565–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Paiva Ferreira LKD, Paiva Ferreira LAM, Bezerra Barros GC, Mozzini Monteiro T, de Araújo Silva LA, Pereira RA, et al. MHTP, a synthetic alkaloid, attenuates combined allergic rhinitis and asthma syndrome through downregulation of the p38/ERK1/2 MAPK signaling pathway in mice. Int Immunopharmacol. 2021;96:107590. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 MiR‐214‐3p facilitates IL‐4‐induced M2 macrophage polarization. (A) IL‐4 (20 ng/ml) was used to stimulate the sorted macrophages from healthy controls for 6, 12, 24, or 48 h. (B) Transfection efficiency of miR‐214‐3p inhibitor into IL‐4‐stimulated macrophages was assessed by RT‐qPCR. (C,D) Proportion of CD206+ macrophages in the four groups (Con, IL‐4, IL‐4 + NC inhibitor, and IL‐4 + miR‐214‐3p inhibitor) by flow cytometry. (E,F) Western blotting was conducted to evaluate M2 macrophage marker (IL‐4, IL‐5, IL‐13, IL‐10, and ARG‐1) protein expression in macrophages in the four groups (Con, IL‐4, IL‐4 + NC inhibitor, and IL‐4 + miR‐214‐3p inhibitor). *p < 0.05, **p < 0.01, ***p < 0.001


