Abstract
Recently, the underlying mechanism of vascular calcification (VC) has been partially elucidated. However, it is still high incidence, and no effective treatment has been found. This study aims at figuring out the underlying mechanisms of microRNA‐708‐5p (miR‐708‐5p)/sodium‐phosphate transporter 1 (Pit‐1) axis in high phosphate (HP)‐induced VC of T/G HA‐VSMCs. Alizarin Red S staining was used to evaluate calcium salt deposition, and the activity of alkaline phosphatase (ALP) was determined by measuring the absorbance at 405 nm. RT‐qPCR and Western blot were performed to assess the levels of miR‐708‐5p and Pit‐1, the levels of ALP, Pit‐1, β‐catenin, glycogen synthesis kinase 3 β (GSK3β), and p‐GSK3β proteins, respectively. The interaction between miR‐708‐5p and Pit‐1 was validated by luciferase reporter assay. Our findings illustrated that miR‐708‐5p was downregulated and Pit‐1was upregulated in HP‐induced VC. MiR‐708‐5p mimics inhibited HP‐induced VC. Further experiments demonstrated that miR‐708‐5p targets Pit‐1. In addition, miR‐708‐5p inactivates the Wnt8b/β‐catenin pathway via targeting Pit‐1 to reduce HP‐induced VC. MiR‐708‐5p has a crucial effect on VC via targeting Pit‐1 and inhibiting Wnt8b/β‐catenin pathway, it may serve as a new target for VC treatment.
Keywords: high phosphate, MiR‐708‐5p, pit‐1, vascular calcification
Abbreviations
- ALP
alkaline phosphatase
- ARS
alizarin red S
- β‐GP
β‐glycerophosphate
- CKD
chronic kidney disease
- DMEM
Dulbecco's modified Eagle medium
- FBS
fetal bovine serum
- GSK3β
glycogen synthesis kinase 3 β
- HP
high phosphate
- miR‐708‐5p
microRNA‐708‐5p
- miRNAs
microRNAs
- Pit‐1
phosphate transporter
- PVDF
polyvinylidene fluoride
- VC
vascular calcification
- VSMCs
vascular smooth muscle cells
1. INTRODUCTION
In patients with chronic kidney disease (CKD), renal failure is one of the main causes of cardiovascular disease and patient death. According to reports, vascular calcification (VC) is common in CKD patients, which may lead to a continuous increase in the overall mortality of cardiovascular disease patients. 1 VC is characterized by the accumulation of calcium and phosphate in the cardiovascular system. Therefore, high phosphate is considered as a model of induced calcification. 2 Meanwhile, studies have shown that VC in vascular smooth muscle cells (VSMC) may be one of the main causes of CKD, and VC of VSMCs in turn increases the absorption of phosphate, in which some important signal pathways may be activated, such as Wnt8b/β‐catenin pathways. 3 , 4 Therefore, the identification of possible molecular targets of VC in CKD may have potential clinical significance for finding new therapies for this disease.
MicroRNA (miRNA) is defined as a set of noncoding RNA sequences approximately 18–21 bp in length. 5 According to reports, miRNAs play a crucial role in gene expression by regulating the post‐transcriptional translation of target genes. Therefore, miRNA acts as a key regulator, affecting many physiological and pathological processes. 6 Particularly, studies have shown that miRNA is involved in the regulation of VC progress. For example, miR‐204 and miR‐211 are closely related to VC and aging regulated by melatonin. 7 MiR‐34a promotes VC of VSMCs by downregulating the SIRT1/Axl axis. 8 MiR‐708‐5p has been reported to participate in various pathological processes, including pancreatic ductal adenocarcinoma, breast carcinoma, and osteoporosis. 9 , 10 , 11 Furthermore, the mechanism of miR‐708‐5p regulating disease progression through Wnt8b3a/β‐catenin pathway has been elucidated preliminarily. 12 , 13 However, the specific molecular mechanism of miR‐708‐5p in phosphate‐induced VC has not been elucidated yet.
The sodium phosphate transporter (Pit‐1), which involves in the Pit‐Oct‐Unc (POU) transcription factor family, was first discovered in the pituitary. 14 The human Pit‐1 gene is located on chromosome 2q13 and contains 11 exons and 10 introns, with a length of 18 kb. Its translation initiation site was identified in exon II, and the translation termination codon was identified in exon XI. 15 Pit‐1 is widely distributed in tissues, including bone, aorta, and aortic smooth muscle cells. According to its amino acid sequence, the molecular weight of Pit‐1 is approximately 74 kDa. 16 Research has shown that Pit‐1 is involved in the VC of VSMCs induced by high phosphate (HP), and phosphate uptake by Pit‐1 is essential for VC and phenotypic regulation in response to elevated phosphate in VSMCs. 15 In this article, we will further explore the specific mechanism of Pit‐1 promoting VC of VSMCs.
Wnt8b/β‐catenin pathway family, involved in various physiological and pathological processes, is a group of proteins involved in many cellular functions, including organogenesis and cell survival. 17 , 18 It is reported that Mg2+ reduces the VC of VSMC by inactivating Wnt8b /β‐catenin pathway. 19 Pioglitazone alleviates β‐glycerophosphate (β‐GP)‐induced VC in rat VSMCs. 20 In this article, we will explore the specific mechanism of Wnt8b/β‐catenin pathway in VC of VSMCs.
In summary, both miR‐708‐5p and Pit‐1 are involved in the regulation of VC in VSMCs, and the specific mechanism may be mediating the Wnt8b/β‐catenin pathway. In this article, we aimed to investigate whether miR‐708‐5p regulates the Wnt8b/β‐catenin pathway via targeted regulation of Pit‐1, thereby mediating the VC of VSMCs induced by HP.
2. MATERIALS AND METHODS
2.1. Cell culture
Human T/G HA‐VSMCs were purchased from Fudan University (Shanghai, China). T/G HA‐VSMCs were cultured in Dulbecco modified Eagle media (DMEM, Gibco, Thermo Fisher Scientific, Inc.), which containing 10% fetal bovine serum (FBS, Solarbio, Beijing, China) and 1% penicillin‐streptomycin solution. The T/G HA‐VSMCs were cultured at 37°C in a damp culture tank containing 5% CO2.
2.2. Cell transfection
Sangon Biotech (Shanghai, China) synthesize the random sequences of miR‐708‐5p inhibitor (5′‐CCCAGCUAGAUUGUAAGCUCCUU‐3′)/ mimics (sense 5′‐AAGGAGCUUACAAUCUAGCUGGG‐3′; antisense 5′‐CAGCUAGAUUGUAAGCUCCUUUU‐3′) and pc‐Pit‐1 plasmids, mimics NC (sense 5′‐UUCUCCGAACGUGUCACGUCU‐3′; antisense 5′‐ACGUGACACGUUCGGAGAAAU‐3′) served as the negative control for miR‐708‐5p mimics, inhibitor NC (5′‐CAGUACUUUUGUGUAGUACAA‐3′) served as the negative control for miR‐708‐5p inhibitor, pcDNA3.1 plasmids served as the negative control for pc‐Pit‐1 plasmids. Lipofectamine™ 3000 Transfection Reagent (Takara, Kusatsu, Japan) was performed to transfect the plasmids. Following 48 h transfection, RT‐qPCR was used to determine transfection efficiency, and T/G HA‐VSMCs were applied to subsequent experiments.
2.3. Cell treatment
When T/G HA‐VSMCs covered the medium, the cells were washed with PBS, separated with 0.125% trypsin and 0.001% EDTA, and preheated at 37°C for subculture. T/G HA‐VSMCs were randomly divided into six groups, control group (cells cultured in normal medium); HP group, cells were treated 10 mmol/L β‐GP (Sigma Aldrich, St. Louis, MO); HP + mimics NC group; HP + miR‐708‐5p mimics group; HP + miR‐708‐5p mimics + pcDNA3.1 group; HP + miR‐708‐5p mimics + pc‐Pit‐1 group. After 7 days of culture, T/G HA‐VSMCs were used for functional research.
2.4. Alizarin red S (ARS) staining
The treated T/G HA‐VSMCs were randomly added to a 6‐well cell culture plate. When the cells reached 80% of the bottom of the flask, the culture medium was discarded and washed with 1 × PBS for three times. At room temperature, the cells were fixed with 4% formaldehyde for 10 min, then stained with 2% ARS (Sigma Aldrich; pH = 4.2) for 5 min. During observation, if orange–red nodules appear, the staining is considered positive. Finally, wash the cells with distilled water and take pictures under a microscope.
2.5. Alkaline phosphatase (ALP) activity detection
According to the instructions, the ALP activity determination kit (Sigma‐Aldrich, St. Louis, MO) was used to determine the ALP activity of T/G HA‐VSMCs. In detail, 100 μl substrate buffer and 20 μl sample were added to the 96‐well plate, and the 96‐well plate was placed on the shaker and shaken for 1 min, and the cells were incubated at 37°C for 15 min. At the end of the min, 80 μl termination solution was added to each pore to terminate the reaction, and the absorbance at 405 nm was measured by microplate reader.
2.6. Extracellular calcium levels detection
The treated T/G HA‐VSMC was randomly added to the 6‐well cell culture plate. When the cell reaches 80% of the bottom of the flask, discard the culture medium and wash it with 1 × PBS for three times. Subsequently, the cells were separated and collected in a hydrochloric acid solution with a volume fraction of 6%, and were treated with ultrasound at intervals of 3 s each time for three times in a row. Then, centrifuge at 12,000 rpm/min for 15 min, and collect the supernatant. Finally, the o‐cresol phthalein complex method and the Coomassie brilliant blue dye‐binding method were performed to detect the calcium concentration and the protein concentration, respectively, to standardize to obtain the value of intracellular calcium level.
2.7. RT‐qPCR
Total RNA was extracted with Trizol reagent (Invitrogen, Carlsbad, California, USA). cDNA was synthesized with M‐MLV reverse transcriptase (RNase H) kit. RT‐qPCR was performed by using SYBR Green PCR Master Mix (Takara, Kusatsu, Japan). β‐actin served as negative control for mRNA detection and U6 as a negative control for miRNA detection. The relative expression was calculated by 2−ΔΔCT. The synthesized primers have the sequence shown in Table 1.
TABLE 1.
Primers used in this paper
| Primer name | Primer sequences |
|---|---|
| F‐Pit‐1 | 5′‐TACCATCCTCATCTCGGTGG‐3′ |
| R‐Pit‐1 | 5′‐TGACGGCTTGACTGAACTGG‐3′ |
| F‐miR‐708‐5p | 5′‐CCGCACGAAGGAGCTTAC‐3′ |
| R‐miR‐708‐5p | 5′‐GTGCAGGGTCCGAGGTAT‐3′ |
| F‐U6 | 5′‐GCTCGCTTCGGCAGCACA‐3′ |
| R‐U6 | 5′‐GAGGTATTCGCACCAGAGGA‐3′ |
| F‐β‐Actin | 5′‐GAGTCAACGGATTTGGTCGT‐3′ |
| R‐β‐Actin | 5′‐TTGATTTTGGAGGGATCTCG‐3’ |
2.8. Western blot
Total proteins were lysed in RIPA buffer and quantified by BCA analysis (Beyotime, Shanghai, China). Proteins were analyzed with 10% SDS‐PAGE and then transferred to 5% skimmed milk TBST sealed polyvinylidene fluoride (PVDF) membrane for 1 h. PVDF membrane was incubated with primary antibodies (anti‐ALP, anti‐Pit‐1, anti‐β‐catenin, anti‐p‐GSK3β/GSK3β, and anti‐β‐actin) at 4°C overnight. β‐actin served as internal control. All antibodies were purchased from (1:1000, Santa Cruz, San Francisco, USA). After incubation with HRP‐conjugated secondary antibodies (Santa Cruz) at room temperature for 1 h. After washing the membrane with TBST, it was developed in the dark with enhanced chemiluminescence (ECL) reagent. A Fuji LAS1000 plus chemiluminescence imaging system (Fuji Film, Tokyo, Japan) was used to detect the ECL signal on the western blot. The optical density of protein bands was measured with Image J software (NIH, Bethesda, MD).
2.9. Bioinformatics and dual‐luciferase reporter gene assay
First, Starbase bioinformatics software was used to predict the potential binding sites of miR‐708‐5p downstream. The wild‐type (WT) or mutant‐type (MUT) sequence of Pit‐1 3′‐UTR was cloned into the pGL3‐M vector (Promega, WI, USA), Pit‐1‐3′‐UTR‐WT or Pit‐1‐3′‐UTR‐MUT were generated. These vectors and miR‐708‐5p mimics or NC mimics were co‐transfected into T/G HA‐VSMCs by using Lipofectamine™ 3000 Transfection Reagent (Invitrogen, California, USA). After 48 h, luciferase activity was tested by dual‐luciferase reporter gene system.
2.10. Statistical analysis
Mean ± SD represents data from three independent experiments. GraphPad Prism 7.0 software (GraphPad Software, Inc.), was used for the statistical analysis of all data. The comparison between the two groups was performed by t‐test or one‐way ANOVA, and the comparison between multiple groups was performed by Tukey's posterior. p < 0.05, the difference is statistically significant.
3. RESULTS
3.1. MiR‐708‐5p was downregulated and Pit‐1 was upregulated in HP‐induced VC
Studies have shown that miRNAs are involved in a variety of physiological and pathological processes, including VC. 7 To investigate the role of miR‐708‐5p in HP‐induced VC, 10 mmol/L β‐GP was used to induce VC in T/G HA‐VSMCs. Findings indicated that in control group, there was no staining of the extracellular matrix and no calcification was observed, whereas in the HP group showed red staining, and the nodules were obviously calcified, indicating that positive extracellular matrix calcium deposition (Figure 1A, B). Subsequently, the changes of extracellular calcium level and ALP activity were detected. ALP is a marker molecule for osteoblast differentiation, which can catalyze the combination of matrix protein and calcium phosphate to promote VC. Results indicated that calcium salt content levels and ALP activity were both significantly increased in HP‐induced T/G HA‐VSMCs, and HP increased ALP activity in a dose‐dependent manner (Figures 1C, D and S1A). These findings suggest that β‐GP treatment increased ALP activity, calcium salt content, and calcification degree. Namely, β‐GP successfully induced calcification in T/G HA‐VSMCs. Furthermore, Western blot analysis showed that the levels of ALP and Pit‐1 proteins were upregulated in HP group (Figure 1E, F). RT‐qPCR analysis indicated that the level of miR‐708‐5p in HP group was lower than that in controls (Figure 1G), and Pit‐1 was significantly up‐regulated following miR‐708‐5p decrease (Figure S1B, C). These findings showed that miR‐708‐5p was inhibited and Pit‐1 was increased in HP‐induced VC, indicating Pit‐1 may play a positive role in HP‐induced VC, while miR‐708‐5p had the opposite effect.
FIGURE 1.

The levels of miR‐708‐5p and Pit‐1 in HP‐induced VC. (A, B) ARS staining was performed to detect calcium salt deposition (×100); (C) the levels of extracellular matrix calcium. (D) ALP activity was measured by optical density values at 405 nm. E, F, Western blot was performed to measure the levels of ALP and Pit‐1 proteins. G, RT‐qPCR was performed to assess miR‐708‐5p levels. N = 3, *p < 0.05, **p < 0.01, ***p < 0.001
3.2. MiR‐708‐5p overexpression inhibits HP‐induced VC
To further investigate the roles of miR‐708‐5p in HP‐induced VC, miR‐708‐5p mimics were transfected into T/G HA‐VSMCs for miR‐708‐5p overexpression. RT‐qPCR analysis showed that after miR‐708‐5p was overexpressed, the level of miR‐708‐5p was significantly up‐regulated (Figure 2A). Subsequently, following using 10 mmol/L β‐GP to induce calcification and overexpression of miR‐708‐5p in T/G HA‐VSMCs, ARS staining analysis showed that the extracellular matrix of the HP group was stained red, and the nodules were obviously calcified, indicating that the extracellular matrix calcium deposits were positive, while miR‐708‐5p overexpression alleviated this trend (Figure 2B, C). Then, the changes of ALP activity and extracellular calcium level were detected after miR‐708‐5p overexpression. Findings showed that ALP activity and calcium salt content were both increased in HP group, whereas overexpression of miR‐708‐5p reversed this outcome (Figure 2D, E). Consistently, Western blot analysis indicated that ALP and Pit‐1 levels were upregulated by HP induction were antagonized by miR‐708‐5p overexpression (Figure 2F, G). Besides, as shown in Figure S2A, miR‐708‐5p mimics alleviated HP‐induced inhibitory effect, and further treatment of LiCl had no effect on miR‐708‐5p expression. Inversely, the levels of Pit‐1, Wnt8b, and β‐catenin proteins were significantly increased HP‐induced T/G HA‐VSMCs, while the upward trend was reversed miR‐708‐5p mimics, and the inhibitory effect of miR‐708‐5p overexpression on the expression of Pit‐1, Wnt8b and β‐catenin proteins was alleviated by LiCl treatment (Figure S2B). These findings suggest that miR‐708‐5p overexpression may reduce VC degree in HP‐induced T/G HA‐VSMCs.
FIGURE 2.

MiR‐708‐5p overexpression inhibits HP‐induced VC. (A) RT‐qPCR was performed to assess the transfection efficiency of miR‐708‐5p mimics. (B, C) ARS staining was performed to detect calcium salt deposition (×100). (D) The levels of extracellular matrix calcium. (E) The optical density values at 405 nm were measured to determine ALP activity. F‐G, Western blot was performed to measure the levels of ALP and Pit‐1 proteins. N = 3, *p < 0.05, **p < 0.01, ***p < 0.001
3.3. MiR‐708‐5p targets Pit‐1
In order to further study the molecular mechanism of miR‐708‐5p in HP‐induced VC, Starbase V2.0 (http://starbase.sysu.edu.cn/) was used to predict the effective target downstream of miR‐708‐5p. The binding site between miR‐708‐5P and Pit‐1 was shown in Figure 3A. Subsequently, the dual‐luciferase reporter experiment showed that co‐transfection of miR‐708‐5p mimics inhibited the luciferase activity of the Pit‐1‐WT reporter gene, but co‐transfection with miR‐708‐5p mimics did not cause the luciferase activity of the Pit‐1‐MUT reporter gene changed (Figure 3B). What's more, RT‐qPCR analysis showed that miR‐708‐5p was significantly inhibited by miR‐708‐5p inhibitor, and miR‐708‐5p inhibitor promoted the level of Pit‐1 in T/G HA‐VSMCs, whereas miR‐708‐5p mimics had the opposite effect on Pit‐1 mRNA level (Figure S3 and 3C). Meanwhile, western blot further confirmed the inhibitory effect of miR‐708‐5p on Pit‐1 (Figure 3D). As shown Figure S4A, B, Pit‐1 knockdown had no effect on miR‐708‐5p expression in T/G HA‐VSMCs, but the decrease of miR‐708‐5p in HP‐induced T/G HA‐VSMCs was up‐regulated by Pit‐1 knockdown. These findings revealed that miR‐708‐5p targets Pit‐1 in HP‐induced T/G HA‐VSMCs.
FIGURE 3.

MiR‐708‐5p targets Pit‐1. (A) StarBase was applied to predict the potential targets of miR‐708‐5p downstream. (B) The dual‐luciferase reporter assay was performed to confirm the direct binding relationship between miR‐708‐5p and Pit‐1. (C) RT‐qPCR was performed to assess the level of Pit‐1 mRNA. (D) Western blot was performed to measure the levels of Pit‐1 protein. N = 3, *p < 0.05, **p < 0.01, ***p < 0.001
3.4. MiR‐708‐5p inhibits the Wnt8b/β‐catenin pathway via targeting Pit‐1 to reduce HP‐induced VC
Researches show that the Wnt8b/β‐catenin pathway involves the regulation of VC process. 21 Initially, to verify whether miR‐708‐5p regulates the VC process via targeting Pit‐1. pc‐Pit‐1 was transfected into T/G HA‐VSMCs for Pit‐1 overexpression (Figure S5A). Subsequently, HP‐induced T/G HA‐VSMCs in which miR‐708‐5p overexpressed were transfected with pc‐Pit‐1 for the overexpression of Pit protein. ARS staining analysis showed that HP + miR‐708‐5p mimics group weakly stained in red with calcified nodules, compared with HP group. However, overexpression of Pit‐1 reversed the inhibition of calcified nodules by miR‐708‐5p (Figure 4A, B). Next, the changes of ALP activity and extracellular calcium levels were detected. Results showed that ALP activity and calcium salt content in HP group were increased significantly, compared with the control. The levels of ALP activity and calcium salt content were inhibited by miR‐708‐5p overexpression. Whereas the effect was further reversed by overexpression of Pit‐1 (Figure 4C, D). What's more, Western blot analysis showed that ALP and Pit‐1 proteins levels in HP group were increased significantly, compared with the control. Whereas miR‐708‐5p mimics inhibited the levels of ALP and Pit‐1 induced by HP, and further overexpression of Pit‐1 reversed this effect (Figure 4E, F). As shown in Figure S5B, the decrease of α‐SMA in HP‐induced T/G HA‐VSMCs was up‐regulated by miR‐708‐5p overexpression, but the effect of miR‐708‐5p was reversed by Pit‐1 overexpression. Whereas the levels of Runx2 and BMP2 showed the opposite trend. Based on the results, we suggested miR‐708‐5p mimics reduced VC degree in HP‐induced T/G HA‐VSMCs, whereas the inhibitory effect was alleviated by Pit‐1 overexpression. Furthermore, western blot analysis showed that β‐catenin and p‐GSK3β proteins levels were upregulated in HP‐induced T/G HA‐VSMCs, whereas the promoting effect was inhibited by miR‐708‐5p overexpression, and the effect of miR‐708‐5p overexpression was alleviated by Pit‐1 overexpression (Figure 4G, H). Similarly, the up‐regulated levels of Wnt8b and β‐catenin in HP‐induced T/G HA‐VSMCs were inhibited by miR‐708‐5p mimics, while Pit‐1 overexpression reversed the effect of miR‐708‐5p mimics (Figure S6). These findings indicated that miR‐708‐5p might inhibit Wnt8b/β‐catenin pathway via targeting Pit‐1 to reduce HP‐induced VC.
FIGURE 4.
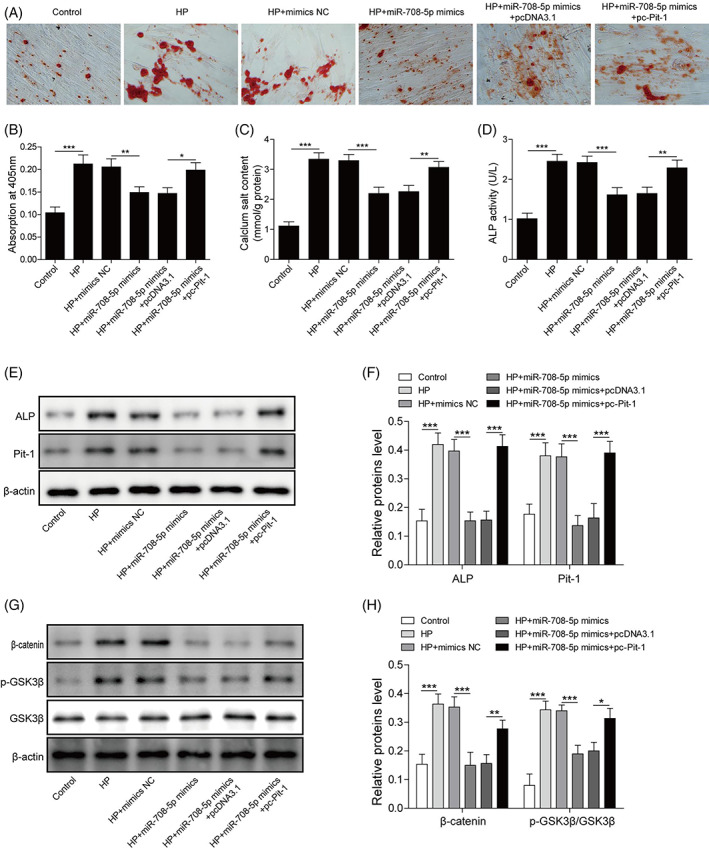
MiR‐708‐5p inhibits the Wnt8b/β‐catenin pathway via targeting Pit‐1 to reduce HP‐induced vascular calcification. (A, B) ARS staining was performed to detect calcium salt deposition (×100). (C) The levels of extracellular matrix calcium. (D) The optical density values at 405 nm were measured to determine ALP activity. (E, F) Western blot was performed to measure the levels of ALP and Pit‐1 proteins. (G, H) Western blot was performed to measure the levels of Wnt8b/β‐catenin pathway associated proteins, including β‐catenin, p‐GSK3β, and GSK3β. N = 3, *p < 0.05, **p < 0.01, ***p < 0.001
4. DISCUSSION
VC is a process in which the phenotype of VSMCs is transformed into osteoblasts, and it is one of the risk factors for accelerating the process of arteriosclerosis in CKD, and phosphate is an important regulator of VC. 22 A previous study confirmed that the miRNA/Pit axis in human VSMCs is related to the Wnt8b/β‐catenin pathway, which may be achieved by regulating‐related proteins, including β‐catenin and p‐GSK3β. 23 Therefore, this study aimed to explore the mechanism of miR‐708‐5p/Pit‐1 axis in HP‐induced VC of T/G HA‐VSMC.
MiRNAs are short noncoding RNAs that bind to their 3′/5′‐untranslated regions at the post‐transcriptional level to regulate gene expression. 24 Studies have shown that miRNAs play an important role in gene expression by regulating post‐transcriptional translation, which may be involved in the regulation of a variety of disease progression. 6 Without exception, studies have reported that miR‐708‐5p plays an important role in a variety of physiological and pathological processes. 10 Sun et al. researches show that miR‐708‐5p, as a sponge of lncRNA LOXL1‐AS1, is involved in the regulation of gastric cancer progression. 25 In colorectal cancer, miR‐708‐5p promotes the spread, migration, and invasion of tumor cells. 26 In this paper, our findings showed that miR‐708‐5p level was inhibited in HP‐induced VC of T/G HA‐VSMCs, and miR‐708‐5p overexpression alleviated the degree of VC in HP‐induced T/G HA‐VSMCs, suggesting that miR‐708‐5p plays a negative role in VC progression in HP‐induced T/G HA‐VSMCs. According to reports, miR‐708‐5p plays a crucial role in gene expression through transcriptional regulation. 25 Bioinformatics analysis indicated that there was a potential target between miR‐708‐5p and Pit‐1, which was confirmed by double luciferase report assay. Pit‐1 has been identified as a key transporter of phosphate‐induced VSMC calcification. 27 It has been reported that Pit‐1 has a vital effect on VC of VSMCs in multiple diseases, including CKD and osteoporosis. 28 , 29 In this article, our findings illustrated that Pit‐1 was upregulated in HP‐induced VC, indicating Pit‐1 plays a positive role in the process of VC in HP‐induced T/G HA‐VSMCs. What's more, miR‐708‐5p reduced HP‐induced VC in T/G HA‐VSMCs may via targeted regulation of Pit‐1. Wnt8b/β‐catenin pathway is a family of proteins involved in a variety of important cellular functions, such as stem cell regeneration and organisms. 30 Studies have shown that Wnt8b/β‐catenin pathway induces calcification by mediating HP in VSMCs. 4 The evidence provided in this study showed that in HP‐induced T/G HA‐VSMCs, the expression of Wnt8b/β‐catenin pathway‐related proteins β‐catenin and p‐GSK3β were upregulated, while the overexpression of miR‐708‐5p could be antagonized the upregulation effect, and the overexpression of miR‐708‐5p reduced the impact of Pit‐1 overexpression, suggesting that miR‐708‐5p may inhibit the Wnt8b/β‐catenin pathway by targeting Pit‐1.
In conclusion, our findings illuminated that miR‐708‐5p was inhibited and Pit‐1 was upregulated in HP‐induced VC. MiR‐708‐5p overexpression inhibited HP‐induced VC. Further experiments demonstrated that miR‐708‐5p targets Pit‐1. In addition, miR‐708‐5p inhibits Wnt8b/β‐catenin pathway via targeting Pit‐1 to reduce HP‐induced VC. It is a pity that at present, no animal research has been done in this study, and these data from in vitro experiments give us confidence in animal experiments in vivo. In the follow‐up study, we will conduct further research in animal experiments.
CONFLICT OF INTEREST
All authors declare no conflict of interest.
Supporting information
Figure S1 A, ALP activity in T/G HA‐VSMCs was detected following treated with LP (1 mmol/L), MP (5 mmol/L) and HP (10 mmol/L), respectively. B and C, miR‐708‐5p level and Pit‐1 protein in T/G HA‐VSMCs was detected by RT‐qPCR and western blot following treated with HP (10vmmol/L) at 0, 1, 2, 3, 4, 5, 6, 7 days. N = 3, *p < 0.05, **p < 0.01, ***P < 0.001.
Figure S2 HP‐induced T/G HA‐VSMCs were transfected with miR‐708‐5p mimics and furtherly treated with LiCl. A, miR‐708‐5p level was detected by RT‐qPCR. B, the levels of Pit‐1, Wnt8b and β‐catenin proteins were measured by western blot. N = 3, *p < 0.05, **p < 0.01, ***p < 0.001.
Figure S3 miR‐708‐5p level in T/G HA‐VSMCs was detected by RT‐qPCR following transfected with miR‐708‐5p inhibitor. N = 3, **p < 0.01.
Figure S4 A, miR‐708‐5p level in T/G HA‐VSMCs was detected by RT‐qPCR following transfected with si‐Pit‐1. B, miR‐708‐5p level in HP‐induced T/G HA‐VSMCs was detected by RT‐qPCR following transfected with si‐Pit‐1. N = 3, *p < 0.05, **p < 0.01.
Figure S5 A, A, pc‐Pit‐1 was transfected into T/G HA‐VSMCs for Pit‐1 overexpression, Pit‐1 level was detected by Western blot. B, the levels α‐SMA, Runx2 and BMP2 proteins were measured by Western blot. N = 3, **p < 0.01, ***p < 0.001.
Figure S6 the levels of Wnt8b and β‐catenin proteins were detected by Western blot. N = 3, **p < 0.01, ***p < 0.001.
Wu N, Liu G‐B, Zhang Y‐M, Wang Y, Zeng H‐T, Xiang H. MiR‐708‐5p/Pit‐1 axis mediates high phosphate‐induced calcification in vascular smooth muscle cells via Wnt8b/β‐catenin pathway. Kaohsiung J Med Sci. 2022;38(7):653–661. 10.1002/kjm2.12542
Funding information This work was supported by Natural Science Foundation of Hunan Province‐Science and Health Joint Project, Grant/Award Number: 2019JJ80112
REFERENCES
- 1. Sonou T, Ohya M, Yashiro M, Masumoto A, Nakashima Y, Ito T, et al. Magnesium prevents phosphate‐induced vascular calcification via TRPM7 and Pit‐1 in an aortic tissue culture model. Hypertens Res. 2017;40(6):562–7. [DOI] [PubMed] [Google Scholar]
- 2. Fakhry M, Skafi N, Fayyad‐Kazan M, Kobeissy F, Hamade E, Mebarek S, et al. Characterization and assessment of potential microRNAs involved in phosphate‐induced aortic calcification. J Cell Physiol. 2018;233(5):4056–67. [DOI] [PubMed] [Google Scholar]
- 3. Tian BY, Yao L, Sheng ZT, Wan PZ, Qiu XB, Wang J, et al. Specific knockdown of WNT8b expression protects against phosphate‐induced calcification in vascular smooth muscle cells by inhibiting the WNT‐β‐catenin signaling pathway. J Cell Physiol. 2019;234(4):3469–77. [DOI] [PubMed] [Google Scholar]
- 4. He F, Wang H, Ren WY, Ma Y, Liao YP, Zhu JH, et al. BMP9/COX‐2 axial mediates high phosphate‐induced calcification in vascular smooth muscle cells via Wnt/β‐catenin pathway. J Cell Biochem. 2018;119(3):2851–63. [DOI] [PubMed] [Google Scholar]
- 5. Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11(9):597–610. [DOI] [PubMed] [Google Scholar]
- 6. Kabekkodu SP, Shukla V, Varghese VK, D'Souza J, Chakrabarty S, Satyamoorthy K. Clustered miRNAs and their role in biological functions and diseases. Biol Rev Camb Philos Soc. 2018;93(4):1955–86. [DOI] [PubMed] [Google Scholar]
- 7. Xu F, Zhong JY, Lin X, Shan SK, Guo B, Zheng MH, et al. Melatonin alleviates vascular calcification and ageing through exosomal miR‐204/miR‐211 cluster in a paracrine manner. J Pineal Res. 2020;68(3):e12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Badi I, Mancinelli L, Polizzotto A, Ferri D, Zeni F, Burba I, et al. miR‐34a promotes vascular smooth muscle cell calcification by downregulating SIRT1 (Sirtuin 1) and AXL (AXL receptor tyrosine kinase). Arterioscler Thromb Vasc Biol. 2018;38(9):2079–90. [DOI] [PubMed] [Google Scholar]
- 9. Huang S, Guo H, Cao Y, Xiong J. MiR‐708‐5p inhibits the progression of pancreatic ductal adenocarcinoma by targeting Sirt3. Pathol Res Pract. 2019;215(4):794–800. [DOI] [PubMed] [Google Scholar]
- 10. Dong HT, Liu Q, Zhao T, Yao F, Xu Y, Chen B, et al. Long non‐coding RNA LOXL1‐AS1 drives breast cancer invasion and metastasis by antagonizing miR‐708‐5p expression and activity. Mol Ther Nucleic Acids. 2020;19:696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De‐La‐Cruz‐Montoya AH, Ramírez‐Salazar EG, Martínez‐Aguilar MM, González‐de‐la‐Rosa PM, Quiterio M, Abreu‐Goodger C, et al. Identification of miR‐708‐5p in peripheral blood monocytes: potential marker for postmenopausal osteoporosis in Mexican‐mestizo population. Exp Biol Med (Maywood). 2018;243(13):1027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu J, Fan W, Ma L, Geng X. miR‐708‐5p promotes fibroblast‐like synoviocytes' cell apoptosis and ameliorates rheumatoid arthritis by the inhibition of Wnt3a/β‐catenin pathway. Drug Des Devel Ther. 2018;12:3439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yu Y, Chang Z, Han C, Zhuang L, Zhou C, Qi X, et al. Long non‐coding RNA MINCR aggravates colon cancer via regulating miR‐708‐5p‐mediated Wnt/β‐catenin pathway. Biomed Pharmacother. 2020;129:110292. [DOI] [PubMed] [Google Scholar]
- 14. Sendon‐Lago J, Seoane S, Eiro N, Bermudez MA, Macia M, Garcia‐Caballero T, et al. Cancer progression by breast tumors with Pit‐1‐overexpression is blocked by inhibition of metalloproteinase (MMP)‐13. Breast Cancer Res. 2014;16(6):505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gonzalez M, Martínez R, Amador C, Michea L. Regulation of the sodium‐phosphate cotransporter Pit‐1 and its role in vascular calcification. Curr Vasc Pharmacol. 2009;7(4):506–12. [DOI] [PubMed] [Google Scholar]
- 16. Crouthamel MH, Lau WL, Leaf EM, Chavkin NW, Wallingford MC, Peterson DF, et al. Sodium‐dependent phosphate cotransporters and phosphate‐induced calcification of vascular smooth muscle cells: redundant roles for PiT‐1 and PiT‐2. Arterioscler Thromb Vasc Biol. 2013;33(11):2625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta‐catenin pathway in cancer: update on effectors and inhibitors. Cancer Treat Rev. 2018;62:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Song L, Li ZY, Liu WP, Zhao MR. Crosstalk between Wnt/β‐catenin and hedgehog/Gli signaling pathways in colon cancer and implications for therapy. Cancer Biol Ther. 2015;16(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Montes de Oca A, Guerrero F, Martinez‐Moreno JM, Madueño JA, Herencia C, Peralta A, et al. Magnesium inhibits Wnt/β‐catenin activity and reverses the osteogenic transformation of vascular smooth muscle cells. PLoS One 2014;9(2):e89525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gao M, Chen T, Wu L, Zhao X, Mao H, Xing C. Effect of pioglitazone on the calcification of rat vascular smooth muscle cells through the downregulation of the Wnt/β‐catenin signaling pathway. Mol Med Rep. 2017;16(5):6208–13. [DOI] [PubMed] [Google Scholar]
- 21. Zhou P, Zhang X, Guo M, Guo R, Wang L, Zhang Z, et al. Ginsenoside Rb1 ameliorates CKD‐associated vascular calcification by inhibiting the Wnt/β‐catenin pathway. J Cell Mol Med. 2019;23(10):7088–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rong S, Zhao X, Jin X, Zhang Z, Chen L, Zhu Y, et al. Vascular calcification in chronic kidney disease is induced by bone morphogenetic protein‐2 via a mechanism involving the Wnt/β‐catenin pathway. Cell Physiol Biochem. 2014;34(6):2049–60. [DOI] [PubMed] [Google Scholar]
- 23. Yan JY, Zhou Q, Yu HM, Hou ML, Lu LH. High glucose promotes vascular smooth muscle cell calcification by activating WNT signaling pathway. Nan Fang Yi Ke Da Xue Xue Bao. 2015;35(1):29–33. [PubMed] [Google Scholar]
- 24. Wang B, Wang X, Tong X, Zhang Y. Schisandrin B inhibits cell viability and migration, and induces cell apoptosis by circ_0009112/miR‐708‐5p Axis through PI3K/AKT pathway in osteosarcoma. Front Genet. 2020;11:588670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sun Q, Li J, Li F, Li H, Bei S, Zhang X, et al. LncRNA LOXL1‐AS1 facilitates the tumorigenesis and stemness of gastric carcinoma via regulation of miR‐708‐5p/USF1 pathway. Cell Prolif. 2019;52(6):e12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu X, Cui F, Chen Y, Zhu Y, Liu F. Long non‐coding RNA LOXL1‐AS1 enhances colorectal cancer proliferation, migration and invasion through miR‐708‐5p/CD44‐EGFR Axis. Onco Targets Ther. 2020;13:7615–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lau WL, Festing MH, Giachelli CM. Phosphate and vascular calcification: emerging role of the sodium‐dependent phosphate co‐transporter PiT‐1. Thromb Haemost. 2010;104(3):464–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Belmokhtar K, Ortillon J, Jaisson S, Massy ZA, Boulagnon Rombi C, Doué M, et al. Receptor for advanced glycation end products: a key molecule in the genesis of chronic kidney disease vascular calcification and a potential modulator of sodium phosphate co‐transporter PIT‐1 expression. Nephrol Dial Transplant. 2019;34(12):2018–30. [DOI] [PubMed] [Google Scholar]
- 29. Platko K, Lebeau PF, Gyulay G, Lhoták Š, MacDonald ME, Pacher G, et al. TDAG51 (T‐cell death‐associated gene 51) is a key modulator of vascular calcification and osteogenic Transdifferentiation of arterial smooth muscle cells. Arterioscler Thromb Vasc Biol. 2020;40(7):1664–79. [DOI] [PubMed] [Google Scholar]
- 30. Deng F, Peng L, Li Z, Tan G, Liang E, Chen S, et al. YAP triggers the Wnt/β‐catenin signalling pathway and promotes enterocyte self‐renewal, regeneration and tumorigenesis after DSS‐induced injury. Cell Death Dis. 2018;9(2):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 A, ALP activity in T/G HA‐VSMCs was detected following treated with LP (1 mmol/L), MP (5 mmol/L) and HP (10 mmol/L), respectively. B and C, miR‐708‐5p level and Pit‐1 protein in T/G HA‐VSMCs was detected by RT‐qPCR and western blot following treated with HP (10vmmol/L) at 0, 1, 2, 3, 4, 5, 6, 7 days. N = 3, *p < 0.05, **p < 0.01, ***P < 0.001.
Figure S2 HP‐induced T/G HA‐VSMCs were transfected with miR‐708‐5p mimics and furtherly treated with LiCl. A, miR‐708‐5p level was detected by RT‐qPCR. B, the levels of Pit‐1, Wnt8b and β‐catenin proteins were measured by western blot. N = 3, *p < 0.05, **p < 0.01, ***p < 0.001.
Figure S3 miR‐708‐5p level in T/G HA‐VSMCs was detected by RT‐qPCR following transfected with miR‐708‐5p inhibitor. N = 3, **p < 0.01.
Figure S4 A, miR‐708‐5p level in T/G HA‐VSMCs was detected by RT‐qPCR following transfected with si‐Pit‐1. B, miR‐708‐5p level in HP‐induced T/G HA‐VSMCs was detected by RT‐qPCR following transfected with si‐Pit‐1. N = 3, *p < 0.05, **p < 0.01.
Figure S5 A, A, pc‐Pit‐1 was transfected into T/G HA‐VSMCs for Pit‐1 overexpression, Pit‐1 level was detected by Western blot. B, the levels α‐SMA, Runx2 and BMP2 proteins were measured by Western blot. N = 3, **p < 0.01, ***p < 0.001.
Figure S6 the levels of Wnt8b and β‐catenin proteins were detected by Western blot. N = 3, **p < 0.01, ***p < 0.001.


