Abstract
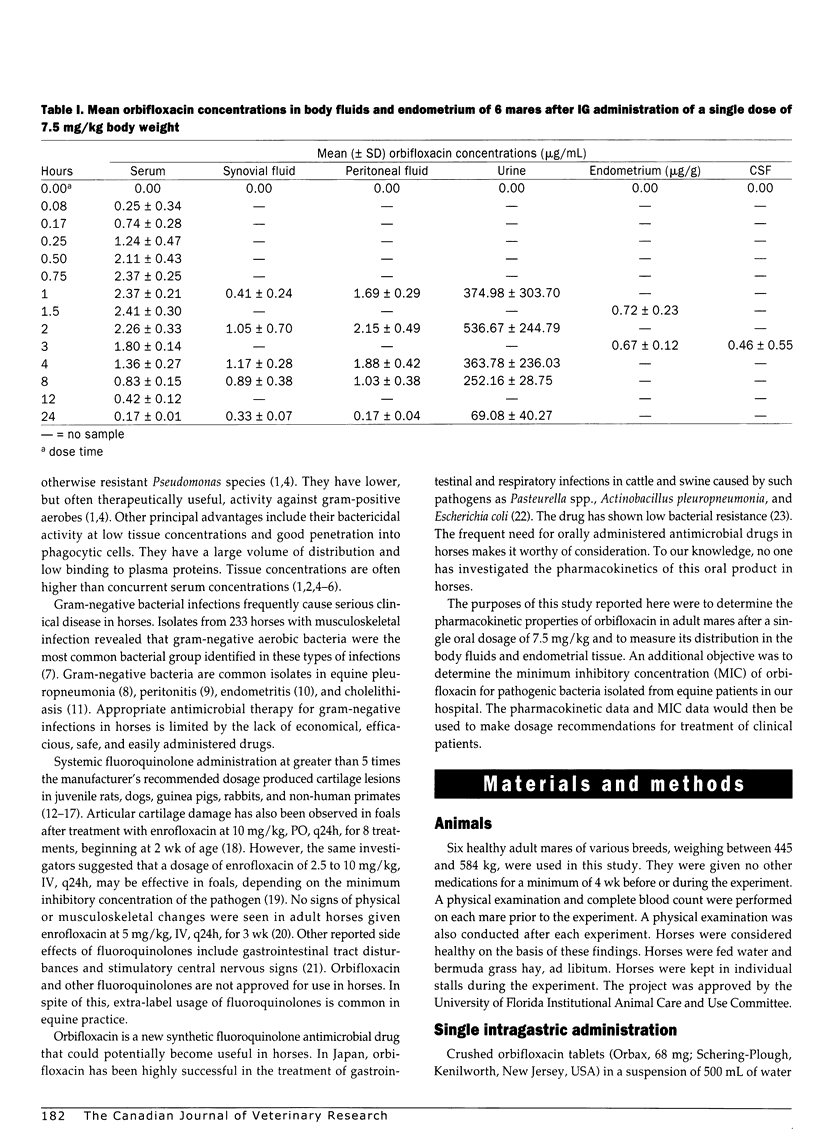
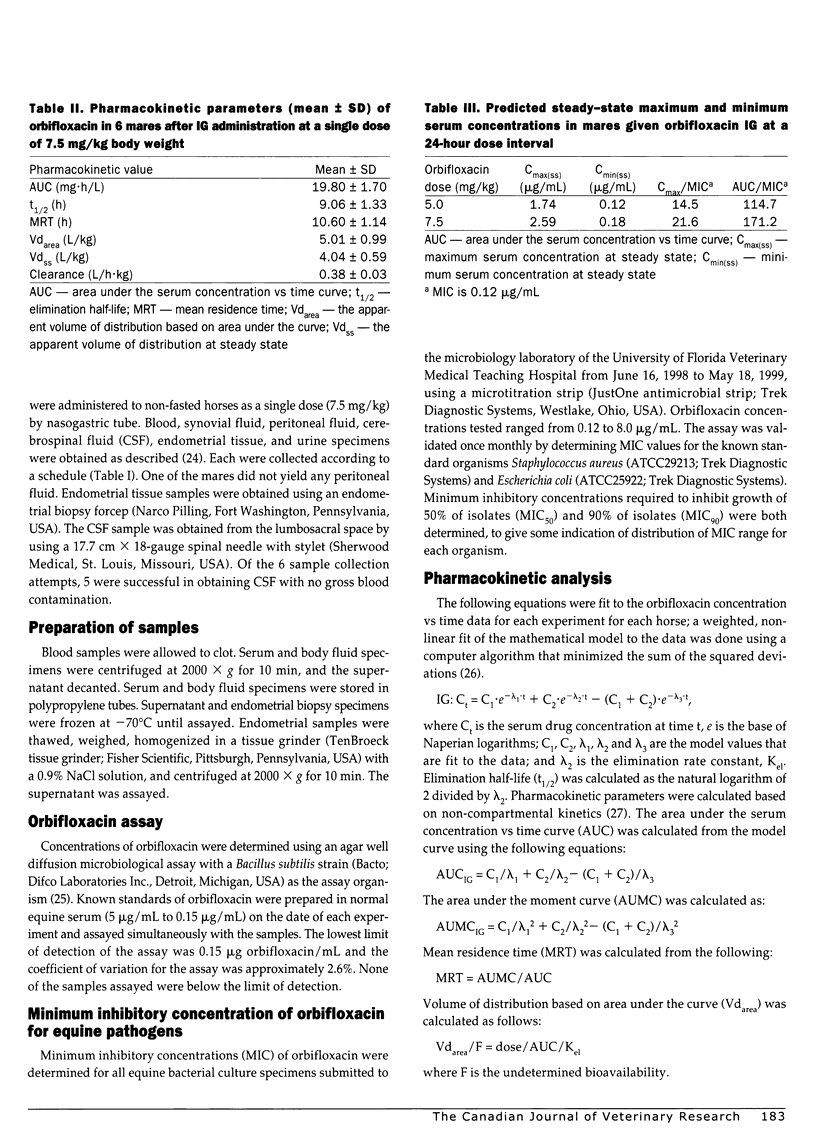
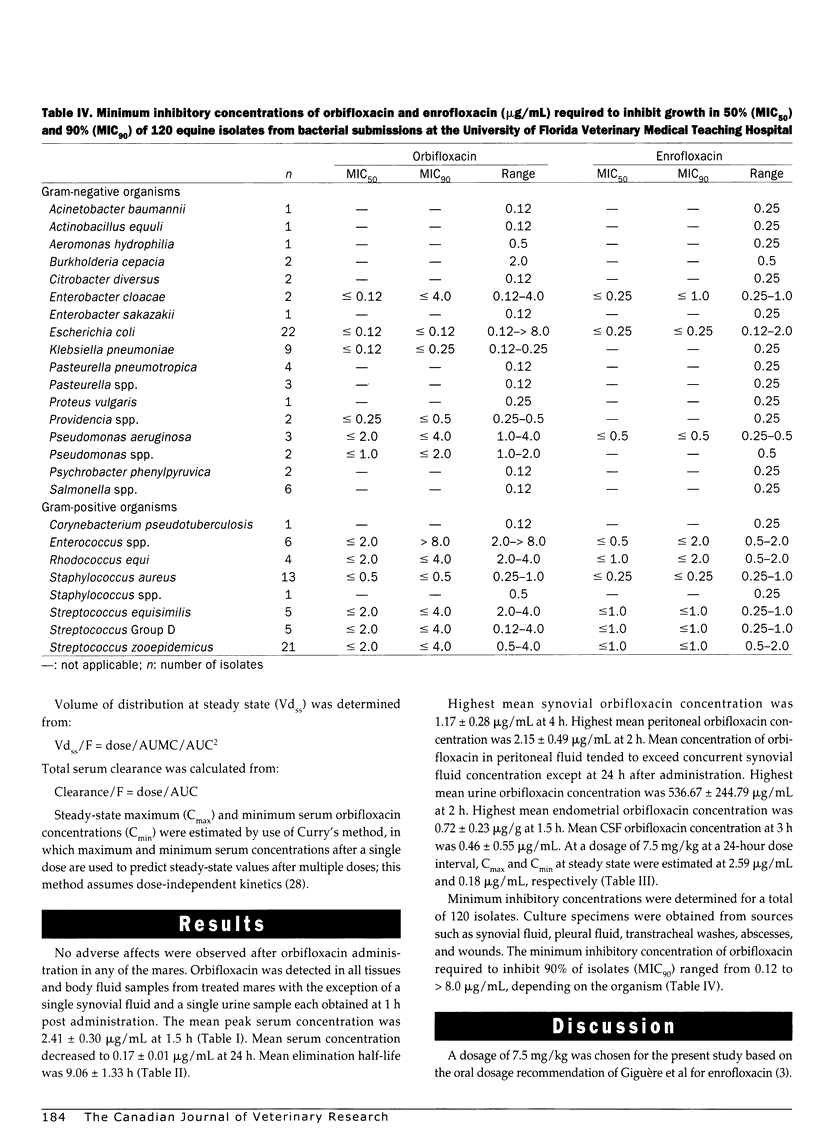
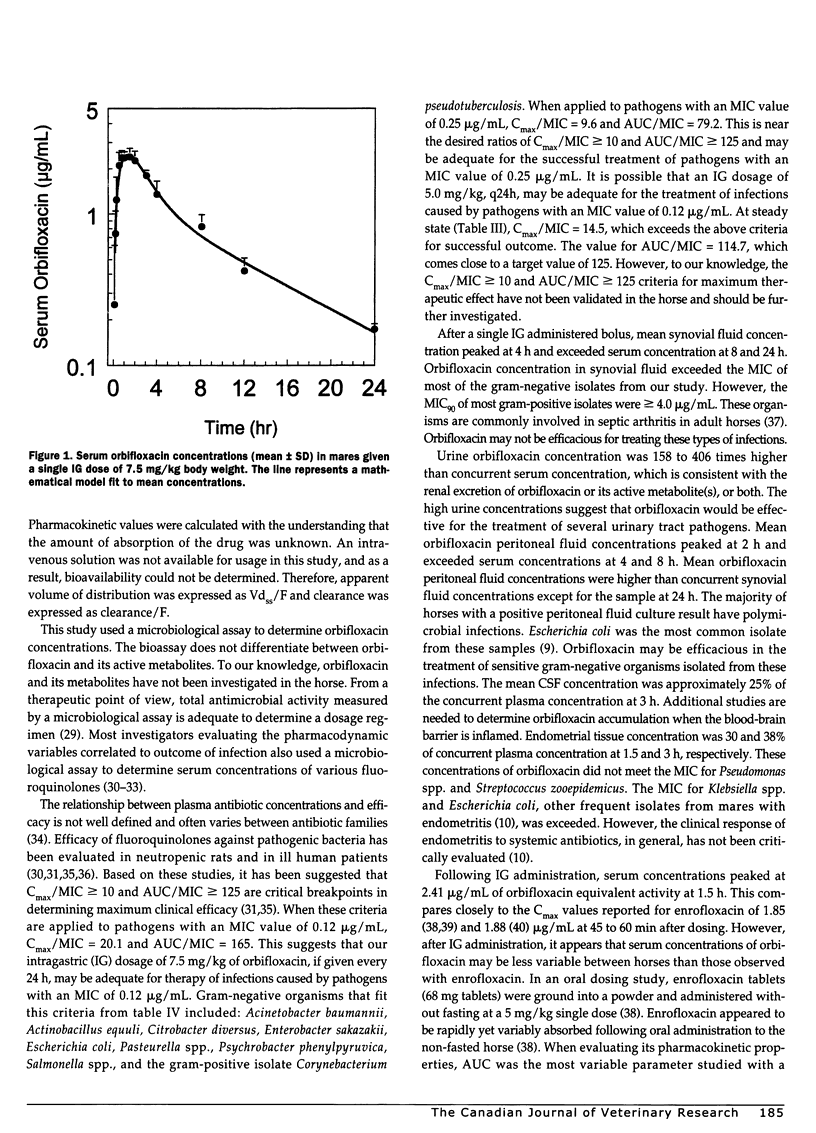
Pharmacokinetics and distribution of orbifloxacin into body fluids and endometrium was studied in 6 mares after intragastric (IG) administration at a single dose rate of 7.5 mg/kg body weight. Orbifloxacin concentrations were serially measured in serum, synovial fluid, peritoneal fluid, urine, cerebrospinal fluid, and endometrial tissues over 24 hours. Minimum inhibitory concentrations of orbifloxacin were determined for 120 equine pathogens over an 11-month period. The mean peak serum concentration (Cmax) was 2.41+/-0.30 microg/mL at 1.5 hours after administration and decreased to 0.17+/-0.01 microg/mL (Cmin) at 24 hours. The mean elimination half-life (t1/2) was 9.06+/-1.33 hours and area under the serum concentration vs time curve (AUC) was 20.54+/-1.70 mg h/L. Highest mean peritoneal fluid concentration was 2.15+/-0.49 microg/mL at 2 hours. Highest mean synovial fluid concentration was 1.17+/-0.28 microg/mL at 4 hours. Highest mean urine concentration was 536.67+/-244.79 microg/mL at 2 hours. Highest mean endometrial concentration was 0.72+/-0.23 microg/g at 1.5 hours. Mean CSF concentration was 0.46+/-0.55 microg/mL at 3 hours. The minimum inhibitory concentration of orbifloxacin required to inhibit 90% of isolates (MIC90) ranged from < or = 0.12 to > 8.0 microg/mL, with gram-negative organisms being more sensitive than gram-positive organisms. Orbifloxacin was uniformly absorbed in the 6 mares and was well distributed into body fluids and endometrial tissue. At a dosage of 7.5 mg/kg once a day, many gram-negative pathogens, such as Actinobacillus equuli, Escherichia coli, Pasteurella spp., and Salmonella spp. would be expected to be susceptible to orbifloxacin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. V., Brodie J. L., Benner E. J., Kirby W. M. Simplified, accurate method for antibiotic assay of clinical specimens. Appl Microbiol. 1966 Mar;14(2):170–177. doi: 10.1128/am.14.2.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham E. C., Papich M. G., Vivrette S. L. Pharmacokinetics of enrofloxacin administered intravenously and orally to foals. Am J Vet Res. 2000 Jun;61(6):706–709. doi: 10.2460/ajvr.2000.61.706. [DOI] [PubMed] [Google Scholar]
- Bertone A. L., Tremaine W. H., Macoris D. G., Simmons E. J., Ewert K. M., Herr L. G., Weisbrode S. E. Effect of long-term administration of an injectable enrofloxacin solution on physical and musculoskeletal variables in adult horses. J Am Vet Med Assoc. 2000 Nov 15;217(10):1514–1521. doi: 10.2460/javma.2000.217.1514. [DOI] [PubMed] [Google Scholar]
- Brown M. P., Gronwall R. R., Houston A. E. Pharmacokinetics and body fluid and endometrial concentrations of ormetoprim-sulfadimethoxine in mares. Can J Vet Res. 1989 Jan;53(1):12–16. [PMC free article] [PubMed] [Google Scholar]
- Burkhardt J. E., Hill M. A., Carlton W. W., Kesterson J. W. Histologic and histochemical changes in articular cartilages of immature beagle dogs dosed with difloxacin, a fluoroquinolone. Vet Pathol. 1990 May;27(3):162–170. doi: 10.1177/030098589002700303. [DOI] [PubMed] [Google Scholar]
- Burkhardt J. E., Hill M. A., Carlton W. W. Morphologic and biochemical changes in articular cartilages of immature beagle dogs dosed with difloxacin. Toxicol Pathol. 1992;20(2):246–252. doi: 10.1177/019262339202000211. [DOI] [PubMed] [Google Scholar]
- Burkhardt J. E., Hill M. A., Turek J. J., Carlton W. W. Ultrastructural changes in articular cartilages of immature beagle dogs dosed with difloxacin, a fluoroquinolone. Vet Pathol. 1992 May;29(3):230–238. doi: 10.1177/030098589202900307. [DOI] [PubMed] [Google Scholar]
- Dowling P. M., Wilson R. C., Tyler J. W., Duran S. H. Pharmacokinetics of ciprofloxacin in ponies. J Vet Pharmacol Ther. 1995 Feb;18(1):7–12. doi: 10.1111/j.1365-2885.1995.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Drusano G. L., Johnson D. E., Rosen M., Standiford H. C. Pharmacodynamics of a fluoroquinolone antimicrobial agent in a neutropenic rat model of Pseudomonas sepsis. Antimicrob Agents Chemother. 1993 Mar;37(3):483–490. doi: 10.1128/aac.37.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest A., Nix D. E., Ballow C. H., Goss T. F., Birmingham M. C., Schentag J. J. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993 May;37(5):1073–1081. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguère S., Sweeney R. W., Bélanger M. Pharmacokinetics of enrofloxacin in adult horses and concentration of the drug in serum, body fluids, and endometrial tissues after repeated intragastrically administered doses. Am J Vet Res. 1996 Jul;57(7):1025–1030. [PubMed] [Google Scholar]
- Gough A. W., Kasali O. B., Sigler R. E., Baragi V. Quinolone arthropathy--acute toxicity to immature articular cartilage. Toxicol Pathol. 1992;20(3 Pt 1):436–450. doi: 10.1177/019262339202000313. [DOI] [PubMed] [Google Scholar]
- Haines G. R., Brown M. P., Gronwall R. R., Merritt K. A. Serum concentrations and pharmacokinetics of enrofloxacin after intravenous and intragastric administration to mares. Can J Vet Res. 2000 Jul;64(3):171–177. [PMC free article] [PubMed] [Google Scholar]
- Hawkins J. F., Bowman K. F., Roberts M. C., Cowen P. Peritonitis in horses: 67 cases (1985-1990). J Am Vet Med Assoc. 1993 Jul 15;203(2):284–288. [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S. Fluoroquinolone antimicrobial agents. N Engl J Med. 1991 Feb 7;324(6):384–394. doi: 10.1056/NEJM199102073240606. [DOI] [PubMed] [Google Scholar]
- Ingerman M. J., Pitsakis P. G., Rosenberg A. F., Levison M. E. The importance of pharmacodynamics in determining the dosing interval in therapy for experimental pseudomonas endocarditis in the rat. J Infect Dis. 1986 Apr;153(4):707–714. doi: 10.1093/infdis/153.4.707. [DOI] [PubMed] [Google Scholar]
- Johnston J. K., Divers T. J., Reef V. B., Acland H. Cholelithiasis in horses: ten cases (1982-1986). J Am Vet Med Assoc. 1989 Feb 1;194(3):405–409. [PubMed] [Google Scholar]
- Kang S. L., Rybak M. J., McGrath B. J., Kaatz G. W., Seo S. M. Pharmacodynamics of levofloxacin, ofloxacin, and ciprofloxacin, alone and in combination with rifampin, against methicillin-susceptible and -resistant Staphylococcus aureus in an in vitro infection model. Antimicrob Agents Chemother. 1994 Dec;38(12):2702–2709. doi: 10.1128/aac.38.12.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston V. C., Sedrish S., Boothe D. M. Disposition of single-dose oral enrofloxacin in the horse. J Vet Pharmacol Ther. 1996 Aug;19(4):316–319. doi: 10.1111/j.1365-2885.1996.tb00057.x. [DOI] [PubMed] [Google Scholar]
- Meinen J. B., McClure J. T., Rosin E. Pharmacokinetics of enrofloxacin in clinically normal dogs and mice and drug pharmacodynamics in neutropenic mice with Escherichia coli and staphylococcal infections. Am J Vet Res. 1995 Sep;56(9):1219–1224. [PubMed] [Google Scholar]
- Moore R. M., Schneider R. K., Kowalski J., Bramlage L. R., Mecklenburg L. M., Kohn C. W. Antimicrobial susceptibility of bacterial isolates from 233 horses with musculoskeletal infection during 1979-1989. Equine Vet J. 1992 Nov;24(6):450–456. doi: 10.1111/j.2042-3306.1992.tb02875.x. [DOI] [PubMed] [Google Scholar]
- Nakamura S. Veterinary use of new quinolones in Japan. Drugs. 1995;49 (Suppl 2):152–158. doi: 10.2165/00003495-199500492-00025. [DOI] [PubMed] [Google Scholar]
- Norrby S. R. Side-effects of quinolones: comparisons between quinolones and other antibiotics. Eur J Clin Microbiol Infect Dis. 1991 Apr;10(4):378–383. doi: 10.1007/BF01967014. [DOI] [PubMed] [Google Scholar]
- Schneider R. K., Bramlage L. R., Moore R. M., Mecklenburg L. M., Kohn C. W., Gabel A. A. A retrospective study of 192 horses affected with septic arthritis/tenosynovitis. Equine Vet J. 1992 Nov;24(6):436–442. doi: 10.1111/j.2042-3306.1992.tb02873.x. [DOI] [PubMed] [Google Scholar]
- Stahlmann R., Förster C., Shakibaei M., Vormann J., Günther T., Merker H. J. Magnesium deficiency induces joint cartilage lesions in juvenile rats which are identical to quinolone-induced arthropathy. Antimicrob Agents Chemother. 1995 Sep;39(9):2013–2018. doi: 10.1128/aac.39.9.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan M. C., Cooper B. W., Nightingale C. H., Quintiliani R., Lawlor M. T. Evaluation of the efficacy of ciprofloxacin against Streptococcus pneumoniae by using a mouse protection model. Antimicrob Agents Chemother. 1993 Feb;37(2):234–239. doi: 10.1128/aac.37.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney C. R., Holcombe S. J., Barningham S. C., Beech J. Aerobic and anaerobic bacterial isolates from horses with pneumonia or pleuropneumonia and antimicrobial susceptibility patterns of the aerobes. J Am Vet Med Assoc. 1991 Mar 1;198(5):839–842. [PubMed] [Google Scholar]
- Takada S., Kato M., Takayama S. Comparison of lesions induced by intra-articular injections of quinolones and compounds damaging cartilage components in rat femoral condyles. J Toxicol Environ Health. 1994 May;42(1):73–88. doi: 10.1080/15287399409531864. [DOI] [PubMed] [Google Scholar]
- Vancutsem P. M., Babish J. G., Schwark W. S. The fluoroquinolone antimicrobials: structure, antimicrobial activity, pharmacokinetics, clinical use in domestic animals and toxicity. Cornell Vet. 1990 Apr;80(2):173–186. [PubMed] [Google Scholar]
- Vogelman B., Gudmundsson S., Leggett J., Turnidge J., Ebert S., Craig W. A. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988 Oct;158(4):831–847. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]


