Abstract
Although most cases of logopenic variant primary progressive aphasia (lvPPA) are caused by Alzheimer’s disease (AD), Lewy body disease (LBD) has also been reported. We assessed brain perfusion, atrophy, dopamine transporter (DAT) uptake, and language function among patients with lvPPA based on beta-amyloid. Thirty-three patients with lvPPA and 28 healthy controls (HCs) underwent MRI, 18F-florbetaben PET, and early- and late-phase DAT PET. All patients completed a language test. General linear models were applied to investigate the association of brain imaging with the aphasia quotient (AQ) and repetition scores. 20 (60.6%) and 13 (39.4%) of the lvPPA patients were amyloid-positive (lvPPAA+) and -negative (lvPPAA−), respectively. Language function was comparable between groups. Compared to HCs, the lvPPAA+ had lower perfusion across widespread brain regions, the lvPPAA− had lower perfusion in the left supramarginal and angular gyri, and both groups had lower DAT in the left caudate and bilateral substantia nigra. In the lvPPAA−, AQ and repetition scores were positively correlated with perfusion in the left temporal and inferior parietal cortices, with perfusion in the left supramarginal gyrus mediating the effect of left substantia nigra DAT. Although AD is the most common underlying pathology of lvPPA, LBD may contribute to the logopenic phenotype.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-90116-x.
Keywords: Logopenic primary progressive aphasia, Alzheimer’s disease, Lewy body disease, Positron emission tomography
Subject terms: Language, Neurological disorders
Introduction
Logopenic variant primary progressive aphasia (lvPPA) is characterized by word-finding difficulties, anomia, and impaired sentence repetition1. The two other types of primary progressive aphasia (PPA), semantic variant and non-fluent agrammatic variant PPA, are usually pathologically explained by frontotemporal lobar degeneration (FTLD)2,3, including TAR DNA-binding protein 43 (TDP-43) type C for semantic variant PPA and FTLD-tau or TDP-43 type A for non-fluent agrammatic variant PPA4. In contrast, the most common finding in the autopsied brains of individuals with lvPPA is Alzheimer’s disease (AD) pathology, with incidences ranging from 54–76%5–7. However, other pathologies, such as FTLD and Lewy body disease (LBD), have also been identified, either alone or coexisting with AD pathologies2,7–11. Considering the existence of previous reports of lvPPA cases with clinically or pathologically diagnosed LBD but no AD pathology7,8,12, it is possible that LBD plays a contributing role in lvPPA phenotype.
Late-phase dopamine transporter (DAT) positron emission tomography (PET) is a useful imaging modality for the clinical evaluation of LBD representing nigrostriatal dopaminergic degeneration13, while early-phase DAT PET can reflect the status of regional brain perfusion14. LBD is characterized by dopaminergic degeneration as well as brain functional changes, both of which are related to clinical symptoms14. In this study, we investigated the language profiles, cortical thickness on brain magnetic resonance imaging (MRI), early- and late-phase DAT PET, and amyloid PET scans in patients with lvPPA. The primary goal of this study was to compare the language profiles and brain changes between amyloid-negative (lvPPAA−) and amyloid-positive (lvPPAA+) subjects with lvPPA. We further explored the relationship between regional brain changes, dopaminergic degeneration, and language performance in each lvPPA group. Our primary hypothesis in this study was that lvPPAA− and lvPPAA+ may exhibit similar language dysfunction profiles. However, in the absence of AD, language dysfunction in patients with lvPPAA− may be more strongly influenced by LBD, either via dopaminergic degeneration on late-phase DAT PET or regional brain perfusion changes on early-phase DAT PET.
Results
Demographics and clinical characteristics of the study participants
The demographic and clinical characteristics of the study participants are shown in Table 1. The healthy control (HC), lvPPAA+, and lvPPAA− groups all had a comparable age, education, and sex distribution. Compared to the HC group, both the lvPPAA+ and lvPPAA− groups had lower mean mini-mental state examination (MMSE) and higher mean clinical dementia rating-sum of boxes (CDR-SOB) scores. The lvPPAA+ and lvPPAA− groups had a comparable apolipoprotein E ε4 carrier frequency. Compared to the lvPPAA+ group, the lvPPAA−group had a lower global18F-florbetaben (FBB) standardized uptake value ratio (SUVR), higher MMSE score, and higher mean neuropsychiatric scores, including delusions, aggression, apathy, and nighttime behavior. However, the prevalence of any reported neuropsychiatric symptoms was comparable between the two groups (Supplementary Table 1). The lvPPAA+ and lvPPAA− groups had comparable unified Parkinson’s disease rating scale (UPDRS) motor score, CDR-SOB, aphasia quotient (AQ), and language quotient (LQ) scores.
Table 1.
Demographic information of the study participants.
| HC (N = 28) | lvPPAA+ (N = 20) | lvPPAA− (N = 13) | P value | |
|---|---|---|---|---|
| Age, year | 69.2 ± 5.9 | 72.2 ± 9.1 | 73.7 ± 6.7 | 0.142 |
| Education, year | 13.1 ± 4.4 | 11.6 ± 5.4 | 9.8 ± 4.4 | 0.126 |
| Female, N (%) | 18 (64.3%) | 13 (65%) | 8 (61.5%) | 0.978 |
| APOE4 carrier, N (%) | - | 5 (25%) | 5 (38.5%) | 0.664 |
| UPDRS | - | 28.1 ± 8.4 | 26.8 ± 10.9 | 0.705 |
| MMSE | 28.9 ± 1.2† | 17.6 ± 7.2* | 24.1 ± 3.1*† | < 0.001 |
| CDR-SOB | 0.0 ± 0.0 | 3.8 ± 2.7* | 2.7 ± 1.8* | < 0.001 |
| NPI | ||||
| Hallucinations | - | 0.0 ± 0.0 | 0.7 ± 2.5 | - |
| Delusions | - | 0.1 ± 0.4 | 0.7 ± 2.5† | 0.044 |
| Aggression | - | 0.3 ± 0.6 | 1.7 ± 2.1† | 0.008 |
| Depression | - | 0.8 ± 1.3 | 1.1 ± 1.9 | 0.155 |
| Anxiety | - | 1.1 ± 2.1 | 1.3 ± 2.4 | 0.515 |
| Apathy | - | 0.8 ± 1.6 | 3.2 ± 3.5† | 0.002 |
| Disinhibition | - | 0.6 ± 1.5 | 1.1 ± 2.6 | 0.585 |
| Irritability | - | 1.3 ± 2.1 | 2.3 ± 2.4 | 0.076 |
| Nighttime behavior | - | 0.5 ± 1.1 | 3.5 ± 4.0† | < 0.001 |
| Appetite changes | - | 0.9 ± 1.8 | 2.2 ± 3.4 | 0.102 |
| Global FBB SUVR | 1.21 ± 0.07 | 2.05 ± 0.30* | 1.27 ± 0.12† | < 0.001 |
| AQ score | - | 73.2 ± 17.2 | 78.9 ± 15.6 | 0.342 |
| LQ score | - | 69.7 ± 19.4 | 74.7 ± 18.8 | 0.509 |
Data are expressed as means ± standard deviations or numbers (%). Group comparisons were performed using the chi-square test or analysis of variance, as appropriate. Comparisons of NPI scores were conducted using generalized linear models with a negative binomial distribution due to data skewness. *Significantly different from HC. †Significantly different from lvPPAA+. Abbreviations: APOE4, apolipoprotein E ε4; AQ, aphasia quotient; CDR-SOB, clinical dementia rating-sum of boxes; FBB, 18F-florbetaben, HC, healthy control; LQ, language quotient, lvPPAA−, amyloid-negative logopenic primary progressive aphasia; lvPPAA+, amyloid-positive logopenic primary progressive aphasia; MMSE, mini-mental state examination; NPI, neuropsychiatric inventory; SUVR, standardized uptake value ratio, UPDRS, unified Parkinson’s disease rating scale.
Comparisons of language function between PPA groups
Compared to the lvPPAA+ group, the lvPPAA− group demonstrated comparable performances across all Korean version of the Western Aphasia Battery (K-WAB) language tests (Table 2). Supplementary Table 2 shows the transformed z-scores of the language function tests based on the means and standard deviations of independent normative data (N = 65). Both the lvPPAA+ and lvPPAA− groups had z scores below − 3.0 in the repetition, fluency, yes/no questions, auditory verbal recognition, object naming, spelled word recognition, and spelling items.
Table 2.
Comparisons of the language function test scores.
| lvPPAA+ (N = 20) | lvPPAA− (N = 13) | P value | |
|---|---|---|---|
| Spontaneous speech | |||
| Content (10) | 7.7 ± 1.6 | 7.8 ± 1.5 | 0.626 |
| Fluency (10) | 7.2 ± 2.0 | 8.0 ± 1.4 | 0.064 |
| Auditory verbal comprehension | |||
| Yes/no questions (60) | 50.2 ± 4.8 | 50.8 ± 9.7 | 0.693 |
| Auditory word recognition (60) | 54.8 ± 6.6 | 56.9 ± 6.2 | 0.125 |
| Sequential commands (80) | 51.7 ± 20.0 | 52.5 ± 17.8 | 0.669 |
| Repetition (100) | 67.6 ± 24.6 | 73.2 ± 22.7 | 0.369 |
| Naming and word finding | |||
| Object naming (60) | 50.0 ± 13.2 | 52.2 ± 14.6 | 0.435 |
| Word fluency (20) | 7.0 ± 4.4 | 8.5 ± 5.3 | 0.154 |
| Sentence completion (10) | 6.5 ± 3.1 | 8.3 ± 2.8 | 0.027 |
| Responsive speech (10) | 7.8 ± 3.3 | 8.7 ± 2.3 | 0.083 |
| Reading | |||
| Comprehension of sentences (40) | 26.0 ± 9.5 | 23.2 ± 9.9 | 0.870 |
| Reading commands (20) | 15.3 ± 5.5 | 16.8 ± 5.2 | 0.092 |
| Written word-object matching (6) | 5.5 ± 0.9 | 7.1 ± 4.8 | 0.235 |
| Written word-picture matching (6) | 5.6 ± 1.0 | 5.6 ± 1.2 | 0.392 |
| Picture-written word matching (6) | 5.4 ± 1.4 | 5.7 ± 0.9 | 0.219 |
| Spoken word-written word matching (4) | 3.5 ± 1.2 | 3.4 ± 1.2 | 0.667 |
| Letter discrimination (6) | 5.1 ± 1.7 | 4.7 ± 2.0 | 0.738 |
| Spelled word recognition (6) | 1.6 ± 2.1 | 1.2 ± 1.0 | 0.853 |
| Spelling (6) | 2.4 ± 2.9 | 1.7 ± 2.0 | 0.424 |
| Writing | |||
| Writing upon request (6) | 5.6 ± 4.9 | 5.3 ± 1.4 | 0.532 |
| Written output (34) | 13.8 ± 11.8 | 20.1 ± 11.3 | 0.013 |
| Writing to dictation (10) | 7.1 ± 7.4 | 6.7 ± 3.1 | 0.452 |
| Writing dictated words (10) | 6.6 ± 3.1 | 6.4 ± 2.4 | 0.116 |
| Alphabets & numbers (22.5) | 16.8 ± 7.9 | 20.0 ± 4.6 | 0.042 |
| Dictated letters & numbers (7.5) | 6.3 ± 2.2 | 6.7 ± 2.2 | 0.256 |
| Copying a sentence (10) | 8.4 ± 3.4 | 9.5 ± 1.0 | 0.047 |
| Speech programming | |||
| 1SP (18) | 16.0 ± 2.9 | 17.6 ± 0.6 | 0.176 |
| 2SP (18) | 15.5 ± 2.9 | 17.4 ± 1.4 | 0.060 |
| 3SP (18) | 16.1 ± 3.2 | 17.6 ± 0.7 | 0.046 |
| 4SP (18) | 13.7 ± 5.2 | 16.6 ± 1.4 | 0.032 |
| Alternating motion rate | |||
| /puh/ rate | 5.4 ± 0.9 | 5.8 ± 1.2 | 0.368 |
| /tuh/ rate | 5.7 ± 0.9 | 5.8 ± 1.0 | 0.748 |
| /kuh/ rate | 5.2 ± 0.9 | 5.3 ± 1.0 | 0.358 |
| Sequential motion rate | |||
| /puh-tuh-kuh/ rate | 1.8 ± 0.7 | 2.5 ± 1.2 | 0.039 |
Data represent the results of general linear models for language function test scores after controlling for age, sex, and education. Significant p-values are shown in boldface after false discovery rate correction for multiple statistical testing across 34 regression analyses. Abbreviations: lvPPAA−, amyloid-negative logopenic primary progressive aphasia; lvPPAA+, amyloid-positive logopenic primary progressive aphasia; SP, speech programming.
In the comparison of language function between the non-fluent agrammatic variant PPA (N = 15) and semantic variant PPA (N = 15) groups, both the lvPPAA+ and lvPPAA− groups demonstrated the worst performance in the repetition task, despite having comparable AQ scores (Supplementary Table 3). The non-fluent agrammatic variant PPA group performed the worst on the fluency subtest and the /tuh/ rate item of the alternating motion rate test, whereas the semantic variant PPA group scored the lowest on all items of the naming and word finding subtest.
Comparisons of regional brain perfusion, cortical thickness, and DAT uptake values
The overall lvPPA group and lvPPAA+ groups both exhibited lower perfusion in widespread cortical regions, except for the perirolandic area and medial occipital cortex, compared to the HC group (Fig. 1). In contrast, the lvPPAA− group showed a lower perfusion in the focal areas including the left supramarginal and angular gyri. No significant differences in regional brain perfusion were observed between the lvPPAA+ and lvPPAA− groups.
Fig. 1.
Group comparison of regional brain perfusion. Results of the general linear models comparing regional early-phase DAT PET uptake after controlling for age, sex, and education. The color scale indicates effect size (r-score) and white line demarcates statistically significant regions after multiple comparisons correction (P < 0.05, false discovery rate). DAT PET, dopamine transporter positron emission tomography; HC, healthy control; lvPPA, logopenic primary progressive aphasia; lvPPAA−, amyloid-negative logopenic primary progressive aphasia; lvPPAA+, amyloid-positive logopenic primary progressive aphasia. The image was generated using the SurfStat toolbox (http://www.math.mcgill.ca/keith/surfstat).
When comparing regional cortical thickness (Supplementary Fig. 1), the overall lvPPA and the lvPPAA+ groups both showed a lower cortical thickness in the widespread areas, excluding the perirolandic area and occipital cortex, while there were no significant differences in regional cortical thickness between the HC and lvPPAA− groups. There were also no significant differences in regional cortical thickness between the lvPPAA+ and lvPPAA− groups.
When comparing DAT uptake values, both the lvPPAA+ and lvPPAA− groups had a lower DAT uptake in the left caudate and bilateral substantia nigra compared to the HC group, while the lvPPAA+ group also had lower DAT uptake in the left putamen, right caudate, and right putamen (Table 3). There were no significant differences in DAT uptake values between the lvPPAA+ and lvPPAA− groups.
Table 3.
Comparisons of DAT uptake values.
| HC (N = 28)1 | lvPPAA− (N = 13)2 | lvPPAA+ (N = 20)3 | P 1vs.2 | P 1vs.3 | P 2vs.3 | |
|---|---|---|---|---|---|---|
| DAT uptake | ||||||
| Left caudate | 8.26 ± 1.01 | 6.95 ± 1.09 | 7.22 ± 1.05 | 0.008 | 0.004 | 0.729 |
| Left putamen | 9.22 ± 0.92 | 8.28 ± 0.84 | 8.26 ± 1.14 | 0.074 | 0.007 | 0.661 |
| Left substantia nigra | 2.12 ± 0.11 | 1.80 ± 0.14 | 1.87 ± 0.14 | < 0.001 | < 0.001 | 0.199 |
| Right caudate | 8.16 ± 0.92 | 7.01 ± 1.19 | 6.87 ± 1.15 | 0.036 | 0.001 | 0.447 |
| Right putamen | 9.26 ± 0.90 | 8.52 ± 0.95 | 8.35 ± 1.24 | 0.297 | 0.017 | 0.361 |
| Right substantia nigra | 2.10 ± 0.11 | 1.82 ± 0.11 | 1.87 ± 0.12 | < 0.001 | < 0.001 | 0.382 |
Data represent the results of general linear models comparing DAT uptake values after controlling for age, sex, and education. Significant p-values are shown in boldface after false discovery rate correction for multiple statistical testing across 18 regression analyses. Abbreviations: DAT, dopamine transporter; HC, healthy control; lvPPAA−, amyloid-negative logopenic primary progressive aphasia; lvPPAA+, amyloid-positive logopenic primary progressive aphasia.
Associations of AQ and repetition scores with regional brain perfusion, cortical thickness, and DAT uptake values
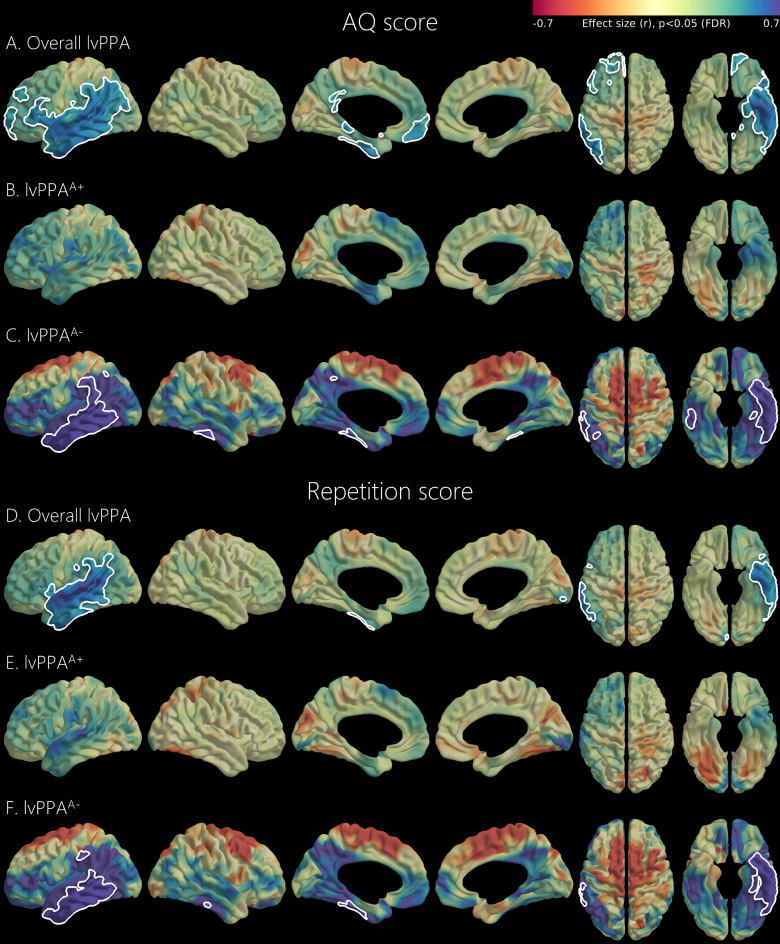
Lower AQ and repetition scores were associated with reduced perfusion in the left temporal and inferior parietal cortices in the overall lvPPA and lvPPAA− groups (Fig. 2). In the overall lvPPA group, lower AQ scores were also associated with reduced perfusion in the left frontal and posterior cingulate cortices. In the lvPPAA− group, lower AQ and repetition scores were additionally associated with decreased perfusion in the right inferior temporal cortex. However, regional brain perfusion was not associated with AQ or repetition scores in the lvPPAA+ group.
Fig. 2.
Association of regional brain perfusion with AQ score and repetition score. Results of the general linear models for regional early-phase DAT PET uptake using AQ score or repetition score as a predictor after controlling for age, sex, and education. Analyses were separately performed in the overall, lvPPAA+, and lvPPAA− groups. The color scale indicates effect size (r-score) and white line demarcates statistically significant regions after multiple comparisons correction (P < 0.05, false discovery rate). AQ, aphasia quotient; DAT, dopamine transporter; lvPPA, logopenic primary progressive aphasia; lvPPAA−, amyloid-negative logopenic primary progressive aphasia; lvPPAA+, amyloid-positive logopenic primary progressive aphasia. The image was generated using the SurfStat toolbox (http://www.math.mcgill.ca/keith/surfstat).
Lower AQ scores were also found to be associated with a reduced cortical thickness in the bilateral temporal, frontal, inferior parietal, and posterior cingulate cortices in the overall lvPPA group and in the lvPPAA− group (Supplementary Fig. 2). However, AQ scores were not associated with the regional cortical thickness in the lvPPAA+ group. Lower repetition scores were associated with lower cortical thickness in the left inferior parietal and bilateral temporal, frontal, and posterior cingulate cortices in the overall lvPPA group, and with lower cortical thickness in the focal region of the left motor cortex in the lvPPAA+ group. However, no association was found between repetition scores and cortical thickness in the lvPPAA− group.
Lower AQ scores were also associated with reduced DAT uptake values in the left substantia nigra in the overall lvPPA, lvPPAA+, and lvPPAA− groups without multiple comparisons correction (Supplementary Table 4). However, this statistical significance was lost after correcting for multiple comparisons. Repetition scores were not associated with DAT uptake.
Relationship between DAT uptake, perfusion, and AQ score
In the overall lvPPA group, DAT uptake in the left substantia nigra was not associated with the perfusion in the left angular gyrus (β = 0.22, standard error (SE) = 0.20, P = 0.275), or that in the left supramarginal gyrus (β = 0.27, SE = 0.15, P = 0.078). In the lvPPAA− group, DAT uptake in the left substantia nigra was associated with perfusion in the left angular gyrus (β = 0.59, SE = 0.20, P = 0.034), as well as that in the left supramarginal gyrus (β = 0.43, SE = 0.13, P = 0.018). As lower DAT uptake in the left substantia nigra and lower perfusions in the left angular gyrus and left supramarginal gyrus were associated with lower AQ scores in the overall lvPPA group and in the lvPPAA− group, multivariable linear regressions were performed for AQ scores using DAT uptake and perfusion as independent predictors, after controlling for age, sex, and education (Table 4). DAT uptake in the left substantia nigra and perfusion in the left angular gyrus were independently associated with AQ scores in the overall lvPPA group, whereas perfusion in the left supramarginal gyrus mediated the effect of left nigral DAT uptake on AQ scores in the lvPPAA− group.
Table 4.
Relationships between the left substantia nigra DAT uptake, perfusion in the left supramarginal or angular gyrus, and AQ scores.
| Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|
| Group | Predictor | B | P value | B | P value | B | P value |
| Overall lvPPA | Left substantia nigra DAT uptake | 0.40 | 0.023 | 0.33 | 0.057 | 0.46 | 0.014 |
| Left supramarginal gyrus perfusion | 0.50 | 0.010 | 0.37 | 0.049 | |||
| Left angular gyrus perfusion | 0.55 | 0.006 | 0.37 | 0.024 | |||
| lvPPAA+ | Left substantia nigra DAT uptake | 0.46 | 0.037 | 0.37 | 0.150 | 0.38 | 0.134 |
| Left supramarginal gyrus perfusion | 0.34 | 0.183 | 0.16 | 0.559 | |||
| Left angular gyrus perfusion | 0.37 | 0.200 | 0.18 | 0.534 | |||
| lvPPAA− | Left substantia nigra DAT uptake | 0.79 | 0.039 | −0.23 | 0.558 | 0.08 | 0.873 |
| Left supramarginal gyrus perfusion | 1.24 | 0.002 | 1.48 | 0.030 | |||
| Left angular gyrus perfusion | 1.02 | 0.012 | 0.96 | 0.119 |
The results are based on the general linear models for AQ scores after controlling for age, sex, and education. Model 1 used left substantia nigra DAT uptake, left supramarginal gyrus perfusion, or left angular gyrus perfusion as a predictor. Model 2 included left substantia nigra DAT uptake and left supramarginal gyrus perfusion as predictors, while Model 3 included left substantia nigra DAT uptake and left angular gyrus perfusion as predictors. Abbreviations: AQ, aphasia quotient; B, standardized beta coefficient; DAT, dopamine transporter; lvPPAA−, amyloid-negative logopenic primary progressive aphasia; lvPPAA+, amyloid-positive logopenic primary progressive aphasia.
Discussion
In the present study, we evaluated the language function profiles of patients with lvPPA according to the presence or absence of amyloid deposition, and further examined their associations with imaging biomarkers, including brain perfusion, cortical thickness, and DAT uptake values. The key findings of this study were as follows: First, out of 33 lvPPA patients, 13 (39.4%) were amyloid-negative, while the lvPPAA− and lvPPAA+ groups showed comparable language deficits with prominent repetition impairment. Second, compared to the HC group, the lvPPAA+ group showed decreased brain perfusion in widespread cortical areas, whereas the lvPPAA− group exhibited decreased perfusion in the focal area involving the left supramarginal and angular gyri. Third, lower AQ and repetition scores were found to be associated with decreased perfusion in the left temporal and inferior parietal cortices in the lvPPA group, which was predominantly driven in the lvPPAA− group. Fourth, decreased left substantia nigra DAT uptake was observed in both the lvPPAA+ and lvPPAA− groups, with perfusion in the left supramarginal gyrus mediating the effect of left substantia nigra DAT uptake on AQ scores in the lvPPAA− group. Taken together, these results suggest that the logopenic PPA phenotype, characterized by hypoperfusion in the left temporal and inferior parietal lobes, can occur in the absence of amyloid deposition with a potential contribution from dopaminergic depletion, a typical feature of LBD.
In our study, 39.4% of subjects with lvPPA were amyloid-negative on FBB PET. Both amyloid-positive and amyloid-negative lvPPA subjects performed comparably on the standardized K-WAB tests, consistent with previous studies7,15–17. Although AD pathologies are the most common underlying pathology in lvPPA, their prevalence varies from 53.8–75.6%.2,6,7,18Other pathologies, such as FTLD and LBD, have also been identified and commonly coexist with AD pathologies8,18, suggesting heterogeneity in the logopenic phenotype. As we excluded patients with unspecified PPA as well as those with clinical or imaging features of behavioral variant frontotemporal dementia (bvFTD), it is possible that our lvPPA cohort included more LBD cases than previously reported. Supporting this speculation, our patients with lvPPA showed decreased DAT uptake in the caudate and substantia nigra. Additionally, decreased substantia nigra DAT uptake was associated with the AQ scores in the overall lvPPA, lvPPAA+, and lvPPAA− groups, although statistical signficance was not reached after multiple comparison corrections. We could not diagnose our lvPPAA−patients as having DLB as they did not exhibit the full clinical presentations of DLB. However, considering that DAT abnormalities in the substantia nigra may precede those in the striatum, and could be associated with the clinical symptoms of DLB19, our results add evidence to the previous literature supporting the potential contribution of LBD to lvPPA.
Our patients with lvPPA showed decreased perfusion throughout widespread cortical regions, a trend which was predominantly driven by lvPPAA+patients, with the left temporoparietal regions showing the most significant changes. These results are consistent with those of previous imaging studies showing that lvPPA is associated with atrophy or hypometabolism in the left temporo-parietal junction20–24. Meanwhile, the lvPPAA−group exhibited decreases of focal perfusion in the left inferior parietal lobe, including the supramarginal and angular gyri, compared to the HC group. Previous imaging studies comparing brain metabolism or perfusion between amyloid-negative lvPPA patients and controls have shown a wider hypo-functional regions than ours, extending to the left temporo-parietal and frontal cortices16,17,22. Different statistical thresholds and methods for patient selection may have contributed to these discrepancies. Overall, our findings suggest that the left supramarginal and angular gyri are key areas for language deficits in lvPPA, regardless of the presence or absence of amyloid deposition.
Our linguistic measures and imaging associations in the overall lvPPA group align with those of previous studies, which highlighted the left temporal20,25–27and inferior parietal lobes20,28,29as the key neural correlates of lvPPA. The most significant hypoperfusion related to repetition deficit was observed in the left superior temporal gyrus and parieto-temporal junction, supporting prior theoretical accounts of phonological working memory30. Notably, the associations between brain perfusion and AQ or repetition score were primarily driven by the lvPPAA− group; however, this may be due to floor effects, as many of these structures were more profoundly affected in the lvPPAA+ group than in the lvPPAA− group.15 16 Nevertheless, to our knowledge, this is the first study to investigate the association between brain perfusion and language measures in amyloid-negative lvPPA.
In the present study, decreased left substantia nigra DAT uptake was observed both in the lvPPAA+ and lvPPAA− groups. DAT uptake in the left substantia nigra and perfusion in the left angular gyrus were further found to be independently associated with AQ scores in the overall lvPPA group. In the lvPPAA− group, perfusion of the left supramarginal gyrus mediated the effect of DAT uptake in the left substantia nigra on AQ scores. Conversely, in the lvPPAA+ group, DAT uptake in the left substantia nigra was associated with AQ scores, but regional perfusion was not. These results support a role of dopaminergic degeneration in the pathogenesis of the lvPPA phenotype, regardless of the presence of AD. Although the exact substrate explaining the correlation between decreased DAT uptake in the substantia nigra and decreased perfusion in the left inferior parietal lobe in the lvPPAA−group is unknown, a higher α-synuclein burden in the inferior parietal cortex, as recently reported in lvPPA brains8, could be a potential candidate.
Although this study provides valuable insights into the association between brain perfusion and language function in amyloid-negative lvPPA patients, it has several limitations that should be acknowledged. First, the underlying causes of neurodegeneration were not confirmed by pathological examinations. Second, the cross-sectional study design limited the ability to interpret causal and temporal relationships. Third, the single-center setting of this study may have introduced selection bias. Fourth, the small sample size necessitated caution when interpreting the results. Despite this, the significant associations in the lvPPAA− group emphasize the role of specific brain regions (i.e., the inferior parietal lobe) in language impairment in amyloid-negative lvPPA. Fifth, late-phase DAT uptake in the striatum, a core imaging biomarker for Parkinson’s disease, was not found to be associated with language measures in either the lvPPAA+ group or lvPPAA−group. However, DAT uptake in the substantia nigra was decreased and correlated with AQ scores in both lvPPA groups. Considering that DAT uptake in the substantia nigra performed better in classifying patients with DLB from control subjects and showed a higher correlation with general cognition compared to DAT uptake in the striatum in our previous study19, the pattern of dopaminergic degeneration may shift in cases with mixed AD and LBD or in those with transitional/diffuse cortical LBD. In a similar vein, striatal and nigral DAT uptake differentially correlated with UPDRS motor score, anxiety, and nighttime behaviors in our lvPPA cohort (Supplementary Table 4). Overall, future studies are warranted to investigate the independent effects of dopaminergic degeneration and cortical perfusion changes on clinical manifestations in patients with pathologically confirmed lvPPA. Sixth, abnormal DAT imaging is not specific to LBD and can be observed in other degenerative diseases, including progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and FTLD. Although we excluded patients with imaging or clinical features suggestive of bvFTD, PSP, or CBD from our study, there is a possibility that patients with these diseases were included in our lvPPA cohort. Future studies are warranted to investigate the independent effects of dopaminergic degeneration and cortical perfusion changes on clinical manifestations in lvPPA patients confirmed using biomarkers with high specificity for LBD, including seed amplification assay.
Conclusions
Overall, our analyses demonstrate the impact of hypoperfusion in the left supramarginal gyrus and DAT uptake in the substantia nigra on language deficits among patients with lvPPA without amyloid pathologies. Widening the focus to investigate the contribution of non-AD pathologies such as LBD will bring us closer to providing a comprehensive understanding of the heterogeneity of logopenic PPA.
Methods
Study participants
This study recruited patients with lvPPA from a prospective cohort study performed to evaluate language function in patients with degenerative diseases including PPA. The cohort enrolled 33 patients with lvPPA, including 15 each with semantic variant and non-fluent agrammatic variant PPA, from November 2018 to May 2023. All patients with PPA were recruited from the dementia clinic of the Yonsei University Severance Hospital, Seoul, South Korea. Speech-language pathologist (H.K.), who was blinded to the neurological, neuropsychological, and neuro-imaging results, reviewed the speech/language evaluations and diagnosed lvPPA based on established criteria31. To diagnose lvPPA, aphasia must be the primary complaint, or the only reason for altered activities of daily living. Additionally, findings from speech/language and neurological examinations had to be characteristic of and consistent with lvPPA, and could not be better explained by any other medical or neurological disorders, including other forms of aphasia. Patients with imaging or clinical features suggestive of bvFTD, or those with an unspecified type of PPA, PSP, or CBD were excluded.
To compare brain perfusion, atrophy, and DAT uptake values in PPA patients with normal aging, HC subjects with normal cognitive function and brain imaging were recruited from a previous independent study of healthy volunteers that did not have any subjective symptoms of cognitive impairment, or any history of neurological or psychiatric illnesses. All HCs further had normal cognitive function and activities of daily living, according to the Korean version of the MMSE and the CDR-SOB.
All participants underwent the UPDRS assessment, MMSE, CDR-SOB, brain MRI, FBB-PET, and early- and late-phase 18F-N-(3-fluoropropyl)-2β-carboxymethoxy-3β-(4-iodophenyl) nortropane (FP-CIT) (FP-CIT)-PET. All patients with lvPPA underwent a detailed language function test (as described below). Amyloid-positivity on FBB-PET was assessed through quantitative analysis and visual ratings by an expert reader (M. Y.) blinded to the clinical diagnoses. Among the 33 subjects with lvPPA, 20 (60.6%) were amyloid-positive and 13 (39.4%) were amyloid-negative.
Caregivers of the patients completed the neuropsychiatric inventory questionnaire32, which consists of 12 behavioral and psychological symptoms over the past 4 weeks, including hallucinations, delusions, aggression, depression, anxiety, euphoria, apathy, disinhibition, irritability, aberrant motor behavior, nighttime behavior, and appetite changes. The severity score for each symptom was rated by calculating the grade and frequency, where higher score indicated a greater burden of the symptoms. The prevalence of each symptom was determined when the score exceeded 0.
Standard protocol approval, registration, and patient consent
The study protocol was approved by the Institutional Review Board of Yonsei University Medical Center (4–2018-1032). Informed consent was obtained from all participants or their caregivers prior to participation. All methods were performed in accordance with the relevant guidelines and regulations, such as Declaration of Helsinki.
Language function evaluation
All subjects with lvPPA underwent the K-WAB, which evaluates four areas of oral language, including spontaneous speech, auditory comprehension, repetition, and naming; two areas of written language including reading and writing; and other cognitive abilities such as praxis, construction, visuo-spatial ability, and calculation33. Each subtest comprises the following components, with the numbers in parenthesis indicating the score range: (1) The spontaneous speech subtest, including conversational questions and picture description, the responses to which are assessed for information content (0–10) and fluency (0–10), grammatical competence, and the presence and forms of paraphasias; (2) The auditory verbal comprehension subtest, including yes/no questions (0–60), auditory word recognition (0–60), and sequential commands (0–80); (3) The repetition subtest (0–100); (4) The naming and word finding subtest, including object naming (0–60), word fluency (0–20), sentence completion (0–10), and responsive speech (0–10); (5) The reading subtest, including comprehension of sentences (0–40), reading commands (0–20), written word-object choice matching (0–6), written word-picture choice matching (range 0–6), picture-written word choice matching (0–6), spoken word-written word choice matching (0–4), letter discrimination (0–6), spelled word recognition (0–6), and spelling (0–6); and (6) The writing subtest, including writing upon request (0–6), written output (0–34), writing to dictation (0–10), writing dictated words (0–10), alphabet and numbers (0–22.5), dictated letters and numbers (0–7.5), and copying a sentence (0–10).
Aphasia quotients (AQ; 0–100) and language quotients (LQ; 0–100) were computed using the following formulae34,35:
 |
 |
Two tasks were conducted to evaluate the apraxia of speech (AOS). First, alternating/sequential motion rates (AMR/SMR) were assessed. Articulatory diadochokinesia was measured using tasks that included rapidly repeating specific syllables for AMR (i.e., /puh/, /tuh/, and/kuh/), and performing three different syllable movements in sequence for SMR (i.e., /puh-tuh-kuh/). The subjects were instructed to repeat the given syllable(s) as quickly and regularly as possible within five seconds. Second, to identify the AOS, which becomes more challenging with increasing word length, words were evaluated in groups of 2–3–4 syllables (i.e., hakgyo-hakgyojang-hakgyojangsil), 3–4–5 syllables (i.e., nangjanggo-nangjanggomoon-nangjanggomoonjak), and 4–5–6 syllables (i.e., haebaragi-haebaragissi-haebaragissiaht). Each word was read aloud, and the subject was asked to repeat it immediately.
Image acquisition of MRI and PET
T1-weighted (T1w) MRIs scans were acquired using a three-dimensional T1w turbo-field echo sequence with the following parameters: axial acquisition matrix, 224 × 224; reconstruction matrix, 256 × 256 with 170 slices; voxel size, 0.859 × 0.859 × 1 mm3; field of view, 220 mm; echo time, 4.6 ms; repetition time, 9.8 ms; and flip angle, 8°.
Dual-phase FP-CIT and FBB PET scans were conducted on separate days using a Discovery 600 (General Electric Healthcare, Milwaukee, MI, USA). Early-phase FP-CIT PET images were captured 10 min following the administration of approximately 185 MBq (5 mCi) FP-CIT. After a 90-minute uptake period, late-phase FP-CIT PET images were acquired for 15 min36. Additionally, FBB PET images were acquired 90 min following the administration of 300 MBq (8 mCi) FBB, with a 20-minute acquisition period. PET images were acquired with a 256 × 256 matrix and reconstructed using an ordered-subset expectation-maximization algorithm, resulting in an iso-0.98-mm voxel size.
Quantification of cortical thickness
Cortical thickness was measured using the CIVET pipeline (http://mcin.ca/civet). T1-weighted images from all subjects were corrected for intensity inhomogeneities and linearly registered to the Alzheimer’s Disease Neuroimaging Initiative (ADNI)-Montreal Neurological Institute (MNI) atlas, a T1-weighted template for older adults37. The images were subsequently tissue-classified38, and the inner and outer cortical surfaces were extracted, yielding 40,962 vertex points per hemisphere39,40. To obtain inter-subject correspondence, the surfaces were registered to an unbiased group template by matching the sulcal folding pattern41. Finally, the cortical thickness was calculated using the three-dimensional Laplace’s Equation between the inner and outer surfaces and smoothed using a 30-mm full width at half maximum (FWHM) surface-based diffusion smoothing kernel42.
Quantification of regional brain perfusion, late-phase DAT uptake, and amyloid burden
To measure the status of regional brain perfusion, early-phase DAT PET images were linearly registered on individual T1-weighted MRI images using rigid-body transformation. SUVR maps were further generated by normalizing PET images to the cerebellar gray matter region as a reference. The SUVR signals were subsequently sampled at 50% of the distance from the inner surface to the outer surface to minimize the partial volume effect. The SUVR values at 40,962 vertex points per hemisphere were spatially blurred using a 10-mm surface-based diffusion smoothing kernel.
Detailed image processing procedures for late-phase DAT PET images have been described in our previous study43. In brief, late-phase DAT PET images were generated using the occipital white matter as a reference region. Median late-phase DAT SUVRs were calculated for three regions of interest: bilateral substantia nigra, caudate, and putamen.
The global amyloid burden was quantified using an automated amyloid PET quantification pipeline, following a detailed methodology published previously44.
Quality assurance for image processing
All MRI and PET images, along with preprocessing outcomes from the automated pipelines, were visually inspected for quality assurance by three researchers (SWK, SJ, and BSY) blinded to the participants’ information.
Statistical analysis
Statistical analyses of demographic and clinical data were performed using the R statistical software (version 4.2.1). Analyses of variance and χ2 tests were performed to compare the clinical features between the lvPPA and HC groups. General linear models (GLMs) were further used to compare language test scores between the lvPPAA+ and lvPPAA− groups, as well as brain perfusion, DAT uptake, and cortical thickness between the HC, lvPPAA+, and lvPPAA− groups. GLMs were applied to identify the relationship between brain perfusion, cortical thickness, DAT uptake, and AQ or repetition scores in the overall lvPPA group, as well as separately in the lvPPAA+ and lvPPAA− groups. All the GLMs were adjusted for age, sex, and education. When the cortical thickness was included in the models, the intracranial volume was additionally adjusted for. Vertex-based statistics for cortical thickness and early-phase DAT PET were performed using the SurfStat toolbox (http://www.math.mcgill.ca/keith/surfstat) with the same model and covariates as described above. To correct for multiple comparisons across the 79,950 cortical vertices excluding the medial filling regions, the false discovery rate (FDR) method was applied, and FDR-corrected p-values < 0.05 were considered statistically significant. Vertex-wise statistical outcomes are displayed on a standard cortical surface according to neurological conventions.
To capture the pattern of language dysfunction in the lvPPAA− and lvPPAA+ groups, language function scores were compared with those of our semantic variant PPA and non-fluent agrammatic variant PPA patients using GLMs after controlling for age, sex, education, and AQ score. Using historical language data from control subjects for the K-WAB, each language function test score in the lvPPAA− and lvPPAA+ groups was displayed as a z-transformed score. Brain imaging evaluations were not performed in these subjects.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are grateful to all of the participants in this study. This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HR22C141101), and by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2022R1C1C2011227), and by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (Project Number: 1711196790, RS-2023-00247272).
Author contributions
Sungwoo Kang: Conceptualization, methodology, Software, Formal analysis, Investigation, Data curation, Writing-Original draft, Visualization. Seun Jeon: Resources, methodology, Neuroimage analysis, Visualization, Writing-Original draft. Young-gun Lee: Investigation, Software. Mijin Yun: Conceptualization, Investigation, Software. HyangHee Kim: Conceptualization, methodology, Software, Formal analysis, Writing-Review & Editing, Visualization, Supervision, Project administration, Funding acquisition. Byoung Seok Ye: Conceptualization, methodology, Software, Formal analysis, Writing-Review & Editing, Visualization, Supervision, Project administration, Funding acquisition.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to sensitive personal information, but are available from the corresponding author on reasonable request and can be shared after anonymization.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Sungwoo Kang and Seun Jeon.
Contributor Information
HyangHee Kim, Email: hkim97.yonsei@gmail.com.
Byoung Seok Ye, Email: romel79@gmail.com.
References
- 1. Gorno-Tempini, M. L. et al. The logopenic/phonological variant of primary progressive aphasia. Neurology71, 1227–1234 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris, J. M. et al. Classification and pathology of primary progressive aphasia. Neurology81, 1832–1839 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Mesulam, M. et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann. Neurol.63, 709–719 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickerson, B. C., Ducharme, S. & Onyike, C. U. Cambridge University Press,. Overviewof clinical assessment of frontotemporal dementia syndromes in Hodges’ Frontotemporal Dementia 2 edn (2016). 10.1017/CBO9781316091586.011 (ed. Dickerson, B. C.) 91–105.
- 5.Spinelli, E. G. et al. Typical and atypical pathology in primary progressive aphasia variants. Ann. Neurol.81, 430–443 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mesulam, M. M. et al. Asymmetry and heterogeneity of Alzheimer’s and frontotemporal pathology in primary progressive aphasia. Brain137, 1176–1192 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giannini, L. A. A. et al. Clinical marker for Alzheimer disease pathology in logopenic primary progressive aphasia. Neurology88, 2276–2284 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buciuc, M. et al. Lewy Body Disease is a contributor to Logopenic Progressive Aphasia phenotype. Ann. Neurol.89, 520–533 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caselli, R. J., Beach, T. G., Sue, L. I., Connor, D. J. & Sabbagh, M. N. Progressive Aphasia with Lewy Bodies. Dement. Geriatr. Cogn. Disord.14, 55–58 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Kakinuma, K. et al. Logopenic aphasia due to Lewy body disease dramatically improved with donepezil. eNeurologicalSci19, 100241 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boes, S. et al. Dementia with Lewy bodies presenting as logopenic variant primary progressive aphasia. Neurocase26, 259–263 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teichmann, M., Migliaccio, R., Kas, A. & Dubois, B. Logopenic progressive aphasia beyond Alzheimer’s–an evolution towards dementia with Lewy bodies. J. Neurol. Neurosurg. Psychiatry. 84, 113–114 (2013). [DOI] [PubMed] [Google Scholar]
- 13.McKeith, I. G. et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology89, 88–100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee, Y. G., Jeon, S., Kang, S. W. & Ye, B. S. Effects of amyloid beta and dopaminergic depletion on perfusion and clinical symptoms. Alzheimers Dement.19, 5719–5729 (2023). [DOI] [PubMed] [Google Scholar]
- 15.Tetzloff, K. A. et al. Quantitative assessment of grammar in amyloid-negative logopenic aphasia. Brain Lang.186, 26–31 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitwell, J. L. et al. Clinical and neuroimaging biomarkers of amyloid-negative logopenic primary progressive aphasia. Brain Lang.142, 45–53 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matías-Guiu, J. A. et al. Amyloid and FDG-PET study of logopenic primary progressive aphasia: evidence for the existence of two subtypes. J. Neurol.262, 1463–1472 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Bergeron, D. et al. Prevalence of amyloid-β pathology in distinct variants of primary progressive aphasia. Ann. Neurol.84, 729–740 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, Y., Jeon, S., Baik, K., Kang, S. W. & Ye, B. S. Substantia Nigral dopamine transporter uptake in dementia with Lewy bodies. Npj Parkinson’s Disease. 9, 88 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leyton, C. E., Piguet, O., Savage, S., Burrell, J. & Hodges, J. R. The neural basis of logopenic progressive aphasia. J. Alzheimers Dis.32, 1051–1059 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Gorno-Tempini, M. L. et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann. Neurol.55, 335–346 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teichmann, M. et al. Deciphering logopenic primary progressive aphasia: a clinical, imaging and biomarker investigation. Brain136, 3474–3488 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Buciuc, M. et al. Clinical, imaging, and pathologic characteristics of patients with right vs left hemisphere–predominant Logopenic Progressive Aphasia. Neurology97, e523–e534 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitwell, J. L.et al. Elevated occipital β-amyloid deposition is associated with widespread cognitive impairment in logopenic progressive aphasia. Journal of Neurology, Neurosurgery & Psychiatry 84, 1357 (2013). [DOI] [PMC free article] [PubMed]
- 25.Foxe, D. et al. The neural correlates of auditory and visuospatial span in logopenic progressive aphasia and Alzheimer’s disease. Cortex83, 39–50 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Owens, T. E. et al. Patterns of neuropsychological dysfunction and cortical volume changes in Logopenic Aphasia. J. Alzheimers Dis.66, 1015–1025 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leyton, C. E., Hodges, J. R., Piguet, O. & Ballard, K. J. Common and divergent neural correlates of anomia in amnestic and logopenic presentations of Alzheimer’s disease. Cortex86, 45–54 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Ash, S. et al. Differentiating primary progressive aphasias in a brief sample of connected speech. Neurology81, 329–336 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petroi, D. et al. Neuroanatomical correlates of phonologic errors in logopenic progressive aphasia. Brain Lang.204, 104773 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller, H. E. et al. Neural substrates of verbal repetition deficits in primary progressive aphasia. Brain Commun.3, fcab015 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorno-Tempini, M. L. et al. Classification of primary progressive aphasia and its variants. Neurology76, 1006–1014 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang, S. J. et al. Caregiver-administered neuropsychiatric inventory (CGA-NPI). J. Geriatr. Psychiatry Neurol.17, 32–35 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Kim, H. & Na, D. L. Normative data on the Korean version of the western Aphasia Battery. J. Clin. Exp. Neuropsychol.26, 1011–1020 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Kertesz, A. & Poole, E. The aphasia quotient: the taxonomic approach to measurement of aphasic disability. Can. J. Neurol. Sci.1, 7–16 (1974). [PubMed] [Google Scholar]
- 35.Shewan, C. M. The Language Quotient (LQ): a new measure for the western Aphasia Battery. J. Commun. Disord. 19, 427–439 (1986). [DOI] [PubMed] [Google Scholar]
- 36.Oh, J. K. et al. Clinical significance of F-18 FP-CIT dual Time Point PET Imaging in Idiopathic Parkinson’s Disease. Nucl. Med. Mol. Imaging. 45, 255–260 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fonov, V., Coupe, P., Eskildsen, S. & Collins, D. Atrophy-specific MRI brain template for Alzheimer’s disease and mild cognitive impairment. Alzheimers Dement.7, S58 (2011). [Google Scholar]
- 38.Zijdenbos, A. P., Forghani, R. & Evans, A. C. Automatic pipeline analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans. Med. Imaging. 21, 1280–1291 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Lorensen, W. & Cline, H. Marching cubes: a high resolution 3D surface construction algorithm. Comput. Graphics. 21, 163–169 (1987). [Google Scholar]
- 40.Kim, J. S. et al. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. NeuroImage27, 210–221 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Lyttelton, O., Boucher, M., Robbins, S. & Evans, A. An unbiased iterative group registration template for cortical surface analysis. NeuroImage34, 1535–1544 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Chung, M. K. et al. Deformation-based surface morphometry applied to gray matter deformation. NeuroImage18, 198–213 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Kang, S., Jeon, S., Lee, Y. G. & Ye, B. S. Striatal dopamine transporter uptake, parkinsonism and cognition in Alzheimer’s disease. Eur. J. Neurol.30, 3105–3113 (2023). [DOI] [PubMed] [Google Scholar]
- 44.Lee, Y. et al. Amyloid-β-related and unrelated cortical thinning in dementia with Lewy bodies. Neurobiol. Aging. 72, 32–39 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to sensitive personal information, but are available from the corresponding author on reasonable request and can be shared after anonymization.